化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3170-3178.DOI: 10.11949/0438-1157.20201690
收稿日期:2020-11-25
修回日期:2021-03-05
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
王士强
作者简介:李栋婵(1982—),女,博士,副教授,基金资助:
LI Dongchan1( ),WANG Jiayu1,WANG Shiqiang1,2(
),WANG Jiayu1,WANG Shiqiang1,2( )
)
Received:2020-11-25
Revised:2021-03-05
Online:2021-06-05
Published:2021-06-05
Contact:
WANG Shiqiang
摘要:
采用等温溶解平衡法,开展四元体系Li+, Mg2+//Cl-, borate–H2O固液相平衡与相图研究,测定平衡溶液的液相组成、密度、折射率和pH。该四元体系相图中存在的盐类矿物为:LiCl·H2O、Li2B4O7·3H2O、 MgCl2·6H2O、Mg2B6O11·15H2O和锂光卤石LiCl·MgCl2·7H2O,其中锂光卤石LiCl·MgCl2·7H2O是异成分复盐,溶液中MgCl2存在下章氏硼镁石(MgB4O7·9H2O)不稳定,转化为多水硼镁石(Mg2B6O11·15H2O)。多水硼镁石结晶区最大,表明镁硼酸盐易于结晶析出,而锂光卤石结晶区最小。采用Pitzer热力学模型对该四元体系的溶解度进行理论预测,计算相图与实验相图吻合较好。该四元体系的稳定相平衡与相图研究,可为含锂硼盐湖老卤中锂、镁、硼产品开发及其综合利用提供理论依据。
中图分类号:
李栋婵, 王嘉宇, 王士强. 四元体系(Li+, Mg2+//Cl-, borate-H2O)308.15 K相平衡与相图研究[J]. 化工学报, 2021, 72(6): 3170-3178.
LI Dongchan, WANG Jiayu, WANG Shiqiang. Phase equilibria and phase diagram of the quaternary system (Li+, Mg2+//Cl-, borate-H2O) at 308.15 K[J]. CIESC Journal, 2021, 72(6): 3170-3178.
| No. | Composition of liquid phase(w)/% | J?necke index(J) | Solid phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Li+ | Mg2+ | Cl- | B4O72- | H2O | J(Mg2+) | J(B4O72-) | J(H2O) | ||
| 1,E1 | 0.28 | 0.01 | 0.00 | 3.20 | 96.51 | 2.00 | 100.15 | 26028.88 | Inderite+Lb |
| 2 | 0.30 | 0.01 | 0.51 | 2.31 | 96.87 | 1.87 | 67.57 | 24416.78 | Inderite+Lb |
| 3 | 0.33 | 0.01 | 0.88 | 1.88 | 96.90 | 1.70 | 50.08 | 22241.73 | Inderite+Lb |
| 4 | 0.44 | 0.01 | 1.55 | 1.65 | 96.35 | 1.28 | 33.10 | 16657.46 | Inderite+Lb |
| 5 | 0.50 | 0.02 | 1.99 | 1.35 | 96.14 | 2.23 | 23.60 | 14485.54 | Inderite+Lb |
| 6 | 0.64 | 0.03 | 2.89 | 1.02 | 95.42 | 2.61 | 13.88 | 11189.12 | Inderite+Lb |
| 7 | 0.86 | 0.05 | 4.12 | 0.92 | 94.05 | 3.21 | 9.26 | 8156.13 | Inderite+Lb |
| 8 | 1.47 | 0.09 | 7.51 | 0.58 | 90.35 | 3.38 | 3.41 | 4576.09 | Inderite+Lb |
| 9 | 2.08 | 0.10 | 10.70 | 0.40 | 86.72 | 2.67 | 1.67 | 3126.82 | Inderite+Lb |
| 10 | 2.88 | 0.10 | 14.88 | 0.27 | 81.87 | 1.94 | 0.82 | 2147.90 | Inderite+Lb |
| 11,F1 | 3.44 | 0.11 | 17.76 | 0.21 | 78.48 | 1.79 | 0.54 | 1726.44 | Inderite+Lb+Lc |
| 12 | 3.35 | 0.11 | 17.31 | 0.24 | 78.99 | 1.84 | 0.63 | 1783.48 | Lb+Lc |
| 13 | 4.04 | 0.10 | 20.83 | 0.18 | 74.85 | 1.39 | 0.39 | 1407.75 | Lb+Lc |
| 14,E2 | 6.55 | 0.00 | 33.36 | 0.18 | 59.91 | 0.00 | 0.25 | 704.81 | Lb+Lc |
| 15,E3 | 5.34 | 2.21 | 33.72 | 0.00 | 58.73 | 19.12 | 0.00 | 685.45 | Bis+Lc |
| 16 | 4.09 | 0.01 | 20.86 | 0.13 | 74.91 | 0.14 | 0.28 | 1409.36 | Inderite+Lc |
| 17 | 4.18 | 0.02 | 21.33 | 0.13 | 74.34 | 0.27 | 0.28 | 1366.70 | Inderite+Lc |
| 18 | 4.10 | 0.10 | 21.18 | 0.18 | 74.44 | 1.37 | 0.39 | 1379.83 | Inderite+Lc |
| 19 | 5.61 | 1.15 | 31.76 | 0.49 | 60.99 | 10.48 | 0.70 | 749.93 | Inderite+Lc |
| 20 | 6.47 | 1.70 | 37.79 | 0.50 | 53.54 | 13.05 | 0.60 | 554.45 | Inderite+Lc |
| 21 | 5.85 | 2.17 | 36.01 | 0.41 | 55.56 | 17.48 | 0.52 | 603.90 | Inderite+Lc |
| 22,F2 | 5.58 | 2.37 | 35.19 | 0.56 | 56.30 | 19.52 | 0.72 | 625.69 | Inderite+Lc+Lcar |
| 23 | 5.26 | 2.41 | 33.63 | 0.59 | 58.11 | 20.74 | 0.79 | 674.72 | Inderite+Lcar |
| 24,E4 | 4.93 | 2.84 | 33.44 | 0.00 | 58.79 | 24.76 | 0.00 | 691.41 | Bis+Lcar |
| 25,F3 | 4.85 | 2.68 | 32.14 | 0.96 | 59.37 | 23.99 | 1.35 | 716.98 | Inderite+Lcar+Bis |
| 26 | 3.99 | 4.12 | 31.84 | 1.19 | 58.86 | 37.10 | 1.68 | 715.03 | Inderite+Bis |
| 27 | 2.54 | 5.65 | 29.22 | 0.55 | 62.04 | 55.96 | 0.85 | 828.95 | Inderite+Bis |
| 28 | 2.07 | 6.34 | 28.75 | 0.70 | 62.14 | 63.63 | 1.10 | 841.36 | Inderite+Bis |
| 29 | 2.10 | 6.15 | 28.40 | 0.57 | 62.78 | 62.58 | 0.91 | 861.92 | Inderite+Bis |
| 30 | 1.37 | 4.96 | 21.23 | 0.51 | 71.93 | 67.40 | 1.09 | 1318.76 | Inderite+Bis |
| 31 | 0.83 | 4.24 | 16.39 | 0.46 | 78.08 | 74.47 | 1.27 | 1850.29 | Inderite+Bis |
| 32 | 0.41 | 3.66 | 12.57 | 0.44 | 82.92 | 83.60 | 1.57 | 2555.37 | Inderite+Bis |
| 33,E5 | 0.00 | 3.19 | 9.12 | 0.42 | 87.27 | 100.00 | 2.06 | 3690.87 | Inderite+Bis |
表1 卤水体系Li+, Mg2+//Cl-, borate–H2O在308.15 K的固液相平衡数据
Table 1 Solid-liquid phase equilibria of brine system (Li+, Mg2+//Cl-, borate–H2O) at 308.15 K
| No. | Composition of liquid phase(w)/% | J?necke index(J) | Solid phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Li+ | Mg2+ | Cl- | B4O72- | H2O | J(Mg2+) | J(B4O72-) | J(H2O) | ||
| 1,E1 | 0.28 | 0.01 | 0.00 | 3.20 | 96.51 | 2.00 | 100.15 | 26028.88 | Inderite+Lb |
| 2 | 0.30 | 0.01 | 0.51 | 2.31 | 96.87 | 1.87 | 67.57 | 24416.78 | Inderite+Lb |
| 3 | 0.33 | 0.01 | 0.88 | 1.88 | 96.90 | 1.70 | 50.08 | 22241.73 | Inderite+Lb |
| 4 | 0.44 | 0.01 | 1.55 | 1.65 | 96.35 | 1.28 | 33.10 | 16657.46 | Inderite+Lb |
| 5 | 0.50 | 0.02 | 1.99 | 1.35 | 96.14 | 2.23 | 23.60 | 14485.54 | Inderite+Lb |
| 6 | 0.64 | 0.03 | 2.89 | 1.02 | 95.42 | 2.61 | 13.88 | 11189.12 | Inderite+Lb |
| 7 | 0.86 | 0.05 | 4.12 | 0.92 | 94.05 | 3.21 | 9.26 | 8156.13 | Inderite+Lb |
| 8 | 1.47 | 0.09 | 7.51 | 0.58 | 90.35 | 3.38 | 3.41 | 4576.09 | Inderite+Lb |
| 9 | 2.08 | 0.10 | 10.70 | 0.40 | 86.72 | 2.67 | 1.67 | 3126.82 | Inderite+Lb |
| 10 | 2.88 | 0.10 | 14.88 | 0.27 | 81.87 | 1.94 | 0.82 | 2147.90 | Inderite+Lb |
| 11,F1 | 3.44 | 0.11 | 17.76 | 0.21 | 78.48 | 1.79 | 0.54 | 1726.44 | Inderite+Lb+Lc |
| 12 | 3.35 | 0.11 | 17.31 | 0.24 | 78.99 | 1.84 | 0.63 | 1783.48 | Lb+Lc |
| 13 | 4.04 | 0.10 | 20.83 | 0.18 | 74.85 | 1.39 | 0.39 | 1407.75 | Lb+Lc |
| 14,E2 | 6.55 | 0.00 | 33.36 | 0.18 | 59.91 | 0.00 | 0.25 | 704.81 | Lb+Lc |
| 15,E3 | 5.34 | 2.21 | 33.72 | 0.00 | 58.73 | 19.12 | 0.00 | 685.45 | Bis+Lc |
| 16 | 4.09 | 0.01 | 20.86 | 0.13 | 74.91 | 0.14 | 0.28 | 1409.36 | Inderite+Lc |
| 17 | 4.18 | 0.02 | 21.33 | 0.13 | 74.34 | 0.27 | 0.28 | 1366.70 | Inderite+Lc |
| 18 | 4.10 | 0.10 | 21.18 | 0.18 | 74.44 | 1.37 | 0.39 | 1379.83 | Inderite+Lc |
| 19 | 5.61 | 1.15 | 31.76 | 0.49 | 60.99 | 10.48 | 0.70 | 749.93 | Inderite+Lc |
| 20 | 6.47 | 1.70 | 37.79 | 0.50 | 53.54 | 13.05 | 0.60 | 554.45 | Inderite+Lc |
| 21 | 5.85 | 2.17 | 36.01 | 0.41 | 55.56 | 17.48 | 0.52 | 603.90 | Inderite+Lc |
| 22,F2 | 5.58 | 2.37 | 35.19 | 0.56 | 56.30 | 19.52 | 0.72 | 625.69 | Inderite+Lc+Lcar |
| 23 | 5.26 | 2.41 | 33.63 | 0.59 | 58.11 | 20.74 | 0.79 | 674.72 | Inderite+Lcar |
| 24,E4 | 4.93 | 2.84 | 33.44 | 0.00 | 58.79 | 24.76 | 0.00 | 691.41 | Bis+Lcar |
| 25,F3 | 4.85 | 2.68 | 32.14 | 0.96 | 59.37 | 23.99 | 1.35 | 716.98 | Inderite+Lcar+Bis |
| 26 | 3.99 | 4.12 | 31.84 | 1.19 | 58.86 | 37.10 | 1.68 | 715.03 | Inderite+Bis |
| 27 | 2.54 | 5.65 | 29.22 | 0.55 | 62.04 | 55.96 | 0.85 | 828.95 | Inderite+Bis |
| 28 | 2.07 | 6.34 | 28.75 | 0.70 | 62.14 | 63.63 | 1.10 | 841.36 | Inderite+Bis |
| 29 | 2.10 | 6.15 | 28.40 | 0.57 | 62.78 | 62.58 | 0.91 | 861.92 | Inderite+Bis |
| 30 | 1.37 | 4.96 | 21.23 | 0.51 | 71.93 | 67.40 | 1.09 | 1318.76 | Inderite+Bis |
| 31 | 0.83 | 4.24 | 16.39 | 0.46 | 78.08 | 74.47 | 1.27 | 1850.29 | Inderite+Bis |
| 32 | 0.41 | 3.66 | 12.57 | 0.44 | 82.92 | 83.60 | 1.57 | 2555.37 | Inderite+Bis |
| 33,E5 | 0.00 | 3.19 | 9.12 | 0.42 | 87.27 | 100.00 | 2.06 | 3690.87 | Inderite+Bis |
| No. | J(Mg2+) | Density, ρ/(g·cm-3) | nD | pH |
|---|---|---|---|---|
| 1,E1 | 2.00 | 1.0262 | 1.3400 | 9.195 |
| 2 | 1.87 | 1.0248 | 1.3421 | 9.173 |
| 3 | 1.70 | 1.022 | 1.3432 | 9.160 |
| 4 | 1.28 | 1.0241 | 1.3503 | 9.036 |
| 5 | 2.23 | 1.0267 | 1.3584 | 9.026 |
| 6 | 2.61 | 1.0281 | 1.3680 | 8.806 |
| 7 | 3.21 | 1.0300 | 1.3768 | 8.524 |
| 8 | 3.38 | 1.0484 | 1.3750 | 8.314 |
| 9 | 2.67 | 1.0694 | 1.3852 | 8.134 |
| 10 | 1.94 | 1.0957 | 1.4213 | 7.961 |
| 11,F1 | 1.79 | 1.1199 | 1.3400 | 7.669 |
| 12 | 1.84 | 1.1256 | 1.3421 | 7.791 |
| 13 | 1.39 | 1.1429 | 1.3432 | 6.826 |
| 14,E2 | 0.00 | 1.2498 | 1.3503 | 4.821 |
| 15,E3 | 19.12 | 1.3121 | 1.4354 | 3.992 |
| 16 | 0.14 | 1.1412 | 1.3849 | 6.066 |
| 17 | 0.27 | 1.1447 | 1.3861 | 5.762 |
| 18 | 1.37 | 1.1483 | 1.3871 | 5.914 |
| 19 | 10.48 | 1.2581 | 1.4217 | 4.409 |
| 20 | 13.05 | 1.3178 | 1.4369 | 4.071 |
| 21 | 17.48 | 1.3190 | 1.438 | 3.961 |
| 22,F2 | 19.52 | 1.3207 | 1.4365 | 3.971 |
| 23 | 20.74 | 1.3191 | 1.4362 | 3.962 |
| 24,E4 | 24.76 | 1.3090 | 1.4348 | 4.007 |
| 25,F3 | 23.99 | 1.3187 | 1.4351 | 3.946 |
| 26 | 37.10 | 1.3180 | 1.4331 | 3.952 |
| 27 | 55.96 | 1.3208 | 1.4353 | 4.021 |
| 28 | 63.63 | 1.3252 | 1.4373 | 3.986 |
| 29 | 62.58 | 1.3258 | 1.4375 | 3.967 |
| 30 | 67.40 | 1.3285 | 1.4389 | 4.001 |
| 31 | 74.47 | 1.3296 | 1.4398 | 4.012 |
| 32 | 83.60 | 1.3311 | 1.4407 | 4.023 |
| 33,E5 | 100.00 | 1.3375 | 1.4428 | 4.112 |
表2 四元体系Li+, Mg2+//Cl-, borate–H2O在308.15 K的物化性质
Table 2 Physiochemical property of the quaternary system (Li+, Mg2+//Cl-, borate–H2O) at 308.15 K
| No. | J(Mg2+) | Density, ρ/(g·cm-3) | nD | pH |
|---|---|---|---|---|
| 1,E1 | 2.00 | 1.0262 | 1.3400 | 9.195 |
| 2 | 1.87 | 1.0248 | 1.3421 | 9.173 |
| 3 | 1.70 | 1.022 | 1.3432 | 9.160 |
| 4 | 1.28 | 1.0241 | 1.3503 | 9.036 |
| 5 | 2.23 | 1.0267 | 1.3584 | 9.026 |
| 6 | 2.61 | 1.0281 | 1.3680 | 8.806 |
| 7 | 3.21 | 1.0300 | 1.3768 | 8.524 |
| 8 | 3.38 | 1.0484 | 1.3750 | 8.314 |
| 9 | 2.67 | 1.0694 | 1.3852 | 8.134 |
| 10 | 1.94 | 1.0957 | 1.4213 | 7.961 |
| 11,F1 | 1.79 | 1.1199 | 1.3400 | 7.669 |
| 12 | 1.84 | 1.1256 | 1.3421 | 7.791 |
| 13 | 1.39 | 1.1429 | 1.3432 | 6.826 |
| 14,E2 | 0.00 | 1.2498 | 1.3503 | 4.821 |
| 15,E3 | 19.12 | 1.3121 | 1.4354 | 3.992 |
| 16 | 0.14 | 1.1412 | 1.3849 | 6.066 |
| 17 | 0.27 | 1.1447 | 1.3861 | 5.762 |
| 18 | 1.37 | 1.1483 | 1.3871 | 5.914 |
| 19 | 10.48 | 1.2581 | 1.4217 | 4.409 |
| 20 | 13.05 | 1.3178 | 1.4369 | 4.071 |
| 21 | 17.48 | 1.3190 | 1.438 | 3.961 |
| 22,F2 | 19.52 | 1.3207 | 1.4365 | 3.971 |
| 23 | 20.74 | 1.3191 | 1.4362 | 3.962 |
| 24,E4 | 24.76 | 1.3090 | 1.4348 | 4.007 |
| 25,F3 | 23.99 | 1.3187 | 1.4351 | 3.946 |
| 26 | 37.10 | 1.3180 | 1.4331 | 3.952 |
| 27 | 55.96 | 1.3208 | 1.4353 | 4.021 |
| 28 | 63.63 | 1.3252 | 1.4373 | 3.986 |
| 29 | 62.58 | 1.3258 | 1.4375 | 3.967 |
| 30 | 67.40 | 1.3285 | 1.4389 | 4.001 |
| 31 | 74.47 | 1.3296 | 1.4398 | 4.012 |
| 32 | 83.60 | 1.3311 | 1.4407 | 4.023 |
| 33,E5 | 100.00 | 1.3375 | 1.4428 | 4.112 |

图4 四元体系(Li+, Mg2+//Cl-, borate–H2O) 308.15 K溶液物化性质
Fig.4 Physiochemical property diagram of the quaternary system (Li+, Mg2+//Cl-, borate–H2O) at 308.15 K
| Species | β(0) | β(1) | β(2) | C? | θ | ψ |
|---|---|---|---|---|---|---|
| LiCl | 0.1469 | 0.3134 | — | -0.00324 | — | — |
| MgCl2 | 0.3455 | 1.6987 | — | 0.05489 | — | — |
| Li2B4O7 | 5.9118 | -37.298 | — | -7.3439 | — | — |
| MgB4O7 | -1.7318 | 6.2356 | -139.94 | -1.4978 | — | — |
| Li+, Mg2+ | — | — | — | — | -0.00743 | — |
| Cl-, B4O72- | — | — | — | — | 0.05252 | — |
| Li+,Mg2+,Cl- | — | — | — | — | — | 0.00 |
| Li+,Mg2+,B4O72- | — | — | — | — | — | 0.00 |
| Li+, Cl-,B4O72- | — | — | — | — | — | 1.0857 |
| Mg2+,Cl-,B4O72- | — | — | — | — | — | 0.7383 |
表3 四元体系Li+, Mg2+//Cl-, borate–H2O在308.15 K模型参数
Table 3 Pitzer parameters of the system (Li+, Mg2+//Cl-, borate–H2O) at 308.15 K
| Species | β(0) | β(1) | β(2) | C? | θ | ψ |
|---|---|---|---|---|---|---|
| LiCl | 0.1469 | 0.3134 | — | -0.00324 | — | — |
| MgCl2 | 0.3455 | 1.6987 | — | 0.05489 | — | — |
| Li2B4O7 | 5.9118 | -37.298 | — | -7.3439 | — | — |
| MgB4O7 | -1.7318 | 6.2356 | -139.94 | -1.4978 | — | — |
| Li+, Mg2+ | — | — | — | — | -0.00743 | — |
| Cl-, B4O72- | — | — | — | — | 0.05252 | — |
| Li+,Mg2+,Cl- | — | — | — | — | — | 0.00 |
| Li+,Mg2+,B4O72- | — | — | — | — | — | 0.00 |
| Li+, Cl-,B4O72- | — | — | — | — | — | 1.0857 |
| Mg2+,Cl-,B4O72- | — | — | — | — | — | 0.7383 |
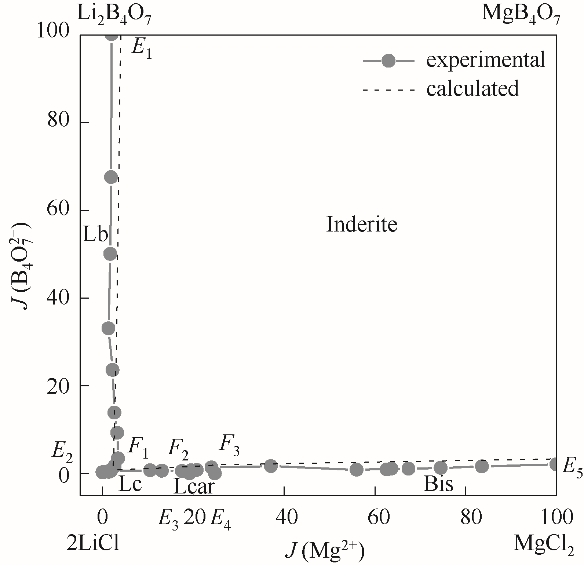
图5 四元体系(Li+, Mg2+//Cl-, borate–H2O) 308.15 K计算与实验相图对比
Fig.5 Comparison of the calculated and experimental phase diagram of the system (Li+, Mg2+//Cl-, borate–H2O) at 308.15 K
| 1 | Choubey P K, Kim M S, Srivastava R R, et al. Advance review on the exploitation of the prominent energy-storage element: lithium(I). From mineral and brine resources[J]. Minerals Engineering, 2016, 89: 119-137. |
| 2 | Yu X P, Fan X B, Guo Y F, et al. Recovery of lithium from underground brine by multistage centrifugal extraction using tri-isobutyl phosphate[J]. Separation and Purification Technology, 2019, 211: 790-798. |
| 3 | 高世杨, 宋彭生, 夏树屏, 等. 盐湖化学:新类型硼锂盐湖[M]. 北京: 科学出版社, 2007. |
| Gao S Y, Song P S, Xia S P, et al. Salt Lake Chemistry: A New Type Brine Containing Lithium and Boron[M]. Beijing: Science Press, 2007. | |
| 4 | 邓天龙, 王士强, 郭亚飞. 柴达木盆地盐湖卤水体系介稳相平衡与相图[M]. 北京: 科学出版社, 2017. |
| Deng T L, Wang S Q, Guo Y F. Metastable Phase Equilibria and Phase Diagram of Brine System in Qaidam Basin[M]. Beijing: Science Press, 2017. | |
| 5 | Liu X H, Chen X Y, He L H, et al. Study on extraction of lithium from salt lake brine by membrane electrolysis[J]. Desalination, 2015, 376: 35-40. |
| 6 | Ji Z Y, Chen Q B, Yuan J S, et al. Preliminary study on recovering lithium from high Mg2+/Li+ ratio brines by electrodialysis[J]. Separation and Purification Technology, 2017, 172: 168-177. |
| 7 | Lee D H, Ryu T, Shin J, et al. Selective lithium recovery from aqueous solution using a modified membrane capacitive deionization system[J]. Hydrometallurgy, 2017, 173: 283-288. |
| 8 | Wang S L, Zheng S L, Wang Z M, et al. Superior lithium adsorption and required magnetic separation behavior of iron-doped lithium ion-sieves[J]. Chemical Engineering Journal, 2018, 332: 160-168. |
| 9 | Liu X H, Zhong M L, Chen X Y, et al. Separating lithium and magnesium in brine by aluminum-based materials[J]. Hydrometallurgy, 2018, 176: 73-77. |
| 10 | Shi D, Zhang L C, Peng X W, et al. Extraction of lithium from salt lake brine containing boron using multistage centrifuge extractors[J]. Desalination, 2018, 441: 44-51. |
| 11 | Gao D L, Yu X P, Guo Y F, et al. Extraction of lithium from salt lake brine with triisobutyl phosphate in ionic liquid and kerosene[J]. Chemical Research in Chinese Universities, 2015, 31(4): 621-626. |
| 12 | 张杰, 史学伟, 赵双良, 等. 水盐体系相平衡研究进展[J]. 化工学报, 2016, 67(2): 379-389. |
| Zhang J, Shi X W, Zhao S L, et al. Progress in study on phase equilibria of salt-water systems[J]. CIESC Journal, 2016, 67(2): 379-389. | |
| 13 | Fu C, Sang S H, Zhou M F, et al. Phase equilibria in the ternary systems Li2B4O7-MgB4O7-H2O and K2B4O7-MgB4O7-H2O at 273 K[J]. Journal of Chemical & Engineering Data, 2016, 61(3): 1071-1077. |
| 14 | Wang S Q, Du X M, Jing Y, et al. Solid-liquid phase equilibrium in the ternary systems (Li2B4O7 + MgB4O7 + H2O) and (Na2B4O7 + MgB4O7 + H2O) at 298.15 K[J]. Journal of Chemical & Engineering Data, 2017, 62(1): 253-258. |
| 15 | 郭智忠, 刘子琴, 陈敬清. Li+, Mg2+/Cl-, SO42--H2O 四元体系(25℃)的介稳平衡[J]. 化学学报, 1991, 49(10): 937-943. |
| Guo Z Z, Liu Z Q, Chen J Q. The metastable phase equilibrium in the system Li+, Mg2+∥Cl-, SO42--H2O at 25℃[J]. Acta Chimica Sinica, 1991, 49(10): 937-943. | |
| 16 | Gao J, Deng T L. Metastable phase equilibrium in the aqueous quaternary system (LiCl + MgCl2 + Li2SO4 + MgSO4 + H2O) at 308.15 K[J]. Journal of Chemical & Engineering Data, 2011, 56(4): 1452-1458. |
| 17 | Meng L Z, Yu X P, Li D, et al. Solid-liquid metastable equilibria of the reciprocal quaternary system (LiCl + MgCl2 + Li2SO4 + MgSO4 + H2O) at 323.15 K[J]. Journal of Chemical & Engineering Data, 2011, 56(12): 4627-4632. |
| 18 | Yu X P, Wang Q, Guo Y F, et al. Metastable phase equilibrium in the reciprocal quaternary system LiCl+MgCl2+Li2SO4+MgSO4+H2O at 348.15 K and 0.1 MPa[J]. Chemical Research in Chinese Universities, 2018, 34(5): 798-802. |
| 19 | Li H X, Zeng D W, Yao Y, et al. Solubility phase diagram of the quaternary system Li+, Mg2+//Cl-, SO42--H2O at 298.15 K: experimental redetermination and model simulation[J]. Industrial & Engineering Chemistry Research, 2014, 53(18): 7579-7590. |
| 20 | 桑世华, 张婷婷, 傅超, 等. 四元体系Li+, K+, Mg2+//B4O72–-H2O 273 K相平衡[J]. 化工学报, 2017, 68(9): 3343-3349. |
| Sang S H, Zhang T T, Fu C, et al. Phase equilibria in quaternary system Li+, K+, Mg2+//B4O72–-H2O at 273 K[J]. CIESC Journal, 2017, 68(9): 3343-3349. | |
| 21 | Tan Q, Zeng Y, Mu P T, et al. Stable phase equilibrium of aqueous quaternary system Li+, K+, Mg2+//borate-H2O at 348 K[J]. Journal of Chemical & Engineering Data, 2014, 59(12): 4173-4178. |
| 22 | Wang S Q, Song Y, Du X M, et al. Solubilities, densities, and refractive indices in the quaternary system (Li2B4O7 + Na2B4O7 + Mg2B6O11 + H2O) at 298.15 K[J]. Russian Journal of Inorganic Chemistry, 2018, 63(1): 116-120. |
| 23 | Wang S Q, Du X M, Jing Y, et al. Phase and physicochemical properties diagrams of quaternary system Li2B4O7 + Na2B4O7 + Mg2B6O11 + H2O[J]. Russian Journal of Physical Chemistry A, 2017, 91(13): 2503-2507. |
| 24 | 宋彭生, 付宏安. 四元交互体系Li+, Mg2+/SO42-, B4O72--H2O 25℃溶解度和溶液物化性质的研究[J]. 无机化学学报, 1991, 7(3): 344-348. |
| Song P S, Fu H A. Solubilities and properties of solution in the reciprocal system Li+, Mg2+/SO42-, B4O72--H2O at 25℃[J]. Chinese Journal of Inorganic Chemistry, 1991, 7(3): 344-348. | |
| 25 | Li H C, Ni S J, Zeng Y. Phase equilibrium of the quaternary system containing lithium, magnesium, sulfate, and borate in aqueous solution at 308 K[J]. Journal of Chemical & Engineering Data, 2014, 59(8): 2523-2529. |
| 26 | 高世扬,许开芬,李刚,等.盐卤硼酸盐化学V:合硼浓缩盐卤稀释过程中硼酸盐的行为[J].化学学报,1986,44(12):1229-1233. |
| Gao S Y, Xu K F, Li G, et al. Chemistry of borate in salt lake brine V. Study on the behaviour of borate in salt lake brine during dilution[J]. Acta Chimica Sinica, 1986, 44(12): 1229-1233. | |
| 27 | 孙柏, 宋彭生. 某些镁硼酸盐溶解及相转化的研究[J]. 盐湖研究, 1999, 7(2): 16-22. |
| Sun B, Song P S. Study on solution and phase transformation of some magnesium borates[J]. Journal of Salt Lake Research, 1999, 7(2): 16-22. | |
| 28 | 景燕. 合成章氏硼镁石的新方法[J]. 海湖盐与化工, 2000, 29(2): 24-25. |
| Jing Y. A new method of synthesis of hungtsaoite [J]. Sea-Lake Salt and Chemical Industry, 2000, 29(2): 24-25. | |
| 29 | 邓天龙, 周桓, 陈侠. 水盐体系相图及应用[M]. 北京: 化学工业出版社, 2013. |
| Deng T L, Zhou H, Chen X. Salt-water System Phase Diagrams and Applications[M]. Beijing: Chemical Industry Press, 2013. | |
| 30 | Wang M M, Guo W T, Wang S Q, et al. Solubilities, densities, and refractive indices of the ternary system (NaBO2 + KBO2 + H2O) at T=(298.15 and 323.15) K and P=0.1 MPa[J]. Journal of Chemical & Engineering Data, 2020, 65(11): 5184-5191. |
| 31 | 袁菲, 宋江涛, 胡佳音, 等. 四元体系CaCl2-CaSO4-CaB6O10-H2O 308.15 K相平衡研究[J]. 化工学报, 2020, 71(1): 209-215. |
| Yuan F, Song J T, Hu J Y, et al. Phase equilibria of quaternary system CaCl2-CaSO4-CaB6O10-H2O at 308.15 K[J]. CIESC Journal, 2020, 71(1): 209-215. | |
| 32 | 中国科学院青海盐湖研究所分析室. 卤水和盐的分析方法[M]. 北京: 科学出版社, 1988. |
| Qinghai Institute of Salt Lakes of CAS. Analytical Methods of Brines and Salts[M]. 2nd ed. Beijing: Science Press, 1988. | |
| 33 | Gao J, Guo Y F, Wang S Q, et al. Interference of lithium in measuring magnesium by complexometry: discussions of the mechanism[J]. Journal of Chemistry, 2013, 2013: 1-4. |
| 34 | Marion G M, Farren R E. Mineral solubilities in the Na-K-Mg-Ca-Cl-SO4-H2O system: a re-evaluation of the sulfate chemistry in the Spencer-Møller-Weare model[J]. Geochimica et Cosmochimica Acta, 1999, 63(9): 1305-1318. |
| 35 | Song P S, Sun B, Zeng D W. Solubility phenomena studies concerning brines in China[J]. Pure and Applied Chemistry, 2013, 85(11): 2097-2116. |
| [1] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [2] | 张学平, 崔瑞芝, 桑世华. NaBr-CaBr2-H2O和KBr-CaBr2-H2O三元体系273.15 K相平衡实验及计算[J]. 化工学报, 2021, 72(9): 4479-4486. |
| [3] | 宋江涛, 袁菲, 余艳, 郭亚飞, 邓天龙. 四元体系LiB5O8 + NaB5O8 + KB5O8 + H2O在298.15 K实验与理论预测相平衡研究[J]. 化工学报, 2021, 72(6): 3179-3187. |
| [4] | 郑秋风,罗军,陈帅,陈念粗,于旭东,曾英. 298.2 K四元体系MgCl2-SrCl2-AlCl3-H2O相平衡实验及溶解度计算[J]. 化工学报, 2020, 71(12): 5443-5451. |
| [5] | 胡锋波,张庆华,詹晓力,陈丰秋. 双(氟代磺酰)亚胺及其盐的制备、性能与应用进展[J]. CIESC Journal, 2011, 30(10): 2097-. |
| [6] | 张国庆,马 莉,倪 佩,刘元刚. 锂离子电池低温电解液的研究进展 [J]. CIESC Journal, 2008, 27(2): 209-. |
| [7] | 韩景立. 应用平均球近似理论计算锂盐有机溶液电导率 [J]. CIESC Journal, 2004, 55(2): 268-270. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号