化工学报 ›› 2023, Vol. 74 ›› Issue (1): 342-354.DOI: 10.11949/0438-1157.20221016
收稿日期:2022-07-25
修回日期:2022-09-13
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
苏海佳
作者简介:张秋华(1987—),男,博士研究生,工程师,zqh@brother.com.cn
基金资助:
Qiuhua ZHANG( ), Manlu LIU, Zheng WANG, Yiming ZHANG, Haijia SU(
), Manlu LIU, Zheng WANG, Yiming ZHANG, Haijia SU( )
)
Received:2022-07-25
Revised:2022-09-13
Online:2023-01-05
Published:2023-03-20
Contact:
Haijia SU
摘要:
维生素K2(VK2)作为2-甲基-1,4-萘醌(简称甲萘醌)家族的衍生物,在预防骨质疏松和心血管钙化方面发挥着重要作用,随着其市场需求的日益增加,实现VK2产业化并降低生产成本成为大家关注的焦点。首先概述了VK2传统发酵工艺现状及面临的问题,之后详细分析了VK2生物合成途径及其基因工程改造策略,可以将枯草芽孢杆菌产量提高至1549.6 mg·L-1,产量是传统发酵菌株的7倍以上。此外,还围绕合成途径中经典甲萘醌骨架合成酶的功能及其蛋白结构进行汇总分析,通过对酶的蛋白结构、催化特异性和机理进行分析,揭示各个功能酶在VK2合成途径中发挥的作用及其内在关系。最后从合成生物学技术发展角度对VK2途径设计、构建与应用进行了展望,为提升VK2工业化产量提供理论参考。
中图分类号:
张秋华, 刘曼路, 王峥, 张一鸣, 苏海佳. 维生素K2的生物合成及其甲萘醌基团合成酶的功能分析[J]. 化工学报, 2023, 74(1): 342-354.
Qiuhua ZHANG, Manlu LIU, Zheng WANG, Yiming ZHANG, Haijia SU. Biosynthesis of vitamin K2 and functional analysis of the biosynthetic enzymes involved in its menadione moiety[J]. CIESC Journal, 2023, 74(1): 342-354.
| 菌株 | 发酵形式 | 策略 | 产物 | 发酵水平/(mg·L-1) | 文献 |
|---|---|---|---|---|---|
| Bacillus licheniformis | 液体 | 卡那霉素抗性突变 | MK-7 | 0.25 mg·(g DCW)-1 | [ |
| Bacillus subtilis natto | 固体 | 采用中心复合面设计研究,优化碳源和氮源 | MK-7 | 67 | [ |
| Bacillus subtilis natto | 液体 | 分批补料甘油 | MK-7 | 86.48 | [ |
| Escherichia sp. | 液体 | 添加甜菜碱和不同的表面活性剂 | MK-4 | 47.6 | [ |
| Bacillus subtilis natto | 液体 | 采用响应面分析葡萄糖、酵母提取物和酪蛋白浓度的影响 | MK-7 | 20.5 | [ |
| Bacillus amyloliquefaciens | 液体 | 室温等离子体(ARTP)诱变和碳源、氮源优化 | MK-7 | 61.3 | [ |
| Bacillus subtilis natto | 液体 | 生物反应器设计有网格状时尚 PCS 结构 | MK-7 | 14.7 | [ |
表1 天然VK2生产菌株发酵产量提高的传统策略
Table 1 Traditional strategies for increasing vitamin K2 fermentation titer
| 菌株 | 发酵形式 | 策略 | 产物 | 发酵水平/(mg·L-1) | 文献 |
|---|---|---|---|---|---|
| Bacillus licheniformis | 液体 | 卡那霉素抗性突变 | MK-7 | 0.25 mg·(g DCW)-1 | [ |
| Bacillus subtilis natto | 固体 | 采用中心复合面设计研究,优化碳源和氮源 | MK-7 | 67 | [ |
| Bacillus subtilis natto | 液体 | 分批补料甘油 | MK-7 | 86.48 | [ |
| Escherichia sp. | 液体 | 添加甜菜碱和不同的表面活性剂 | MK-4 | 47.6 | [ |
| Bacillus subtilis natto | 液体 | 采用响应面分析葡萄糖、酵母提取物和酪蛋白浓度的影响 | MK-7 | 20.5 | [ |
| Bacillus amyloliquefaciens | 液体 | 室温等离子体(ARTP)诱变和碳源、氮源优化 | MK-7 | 61.3 | [ |
| Bacillus subtilis natto | 液体 | 生物反应器设计有网格状时尚 PCS 结构 | MK-7 | 14.7 | [ |
| 菌株 | 发酵形式 | 策略 | 产物 | 发酵水平 | 文献 |
|---|---|---|---|---|---|
| Escherichia coli | 液体 | UbiCA基因的缺失,过表达MenA和MenD | MK-8 | 290 mg·(g DCW)-1 | [ |
| 液体 | 优化MVA途径和过表达HepPPS酶 | MK-7 | 8.8 mg·L-1 | [ | |
| Elizabethkingia meningoseptica | 液体 | UbiA突变,Dxr、MenA和UbiE的过表达,以及前体的补充 | MK-7 | 29.63 mg·(g DCW)-1 | [ |
| Bacillus subtilis 168 | 液体 | 使用强启动子P43和过表达 crtE, MenA和MenG酶 | MK-4 | (145.0±2.8) mg·L-1 | [ |
| 液体 | 过表达了MEP途径的5个基因 | MK-7 | 360 mg·L-1 | [ | |
| 液体 | 构建了丙酮酸和丙二酰辅酶A抑制型的基因回路,联级动态调控中心代谢模块、前体IPP反应模块和产物合成模块 | MK-7 | 1549.6 mg·L-1 | [ |
表2 VK2生产菌株产量提升的基因工程策略
Table 2 Genetic engineering strategies for increasing vitamin K2 fermentation titer
| 菌株 | 发酵形式 | 策略 | 产物 | 发酵水平 | 文献 |
|---|---|---|---|---|---|
| Escherichia coli | 液体 | UbiCA基因的缺失,过表达MenA和MenD | MK-8 | 290 mg·(g DCW)-1 | [ |
| 液体 | 优化MVA途径和过表达HepPPS酶 | MK-7 | 8.8 mg·L-1 | [ | |
| Elizabethkingia meningoseptica | 液体 | UbiA突变,Dxr、MenA和UbiE的过表达,以及前体的补充 | MK-7 | 29.63 mg·(g DCW)-1 | [ |
| Bacillus subtilis 168 | 液体 | 使用强启动子P43和过表达 crtE, MenA和MenG酶 | MK-4 | (145.0±2.8) mg·L-1 | [ |
| 液体 | 过表达了MEP途径的5个基因 | MK-7 | 360 mg·L-1 | [ | |
| 液体 | 构建了丙酮酸和丙二酰辅酶A抑制型的基因回路,联级动态调控中心代谢模块、前体IPP反应模块和产物合成模块 | MK-7 | 1549.6 mg·L-1 | [ |
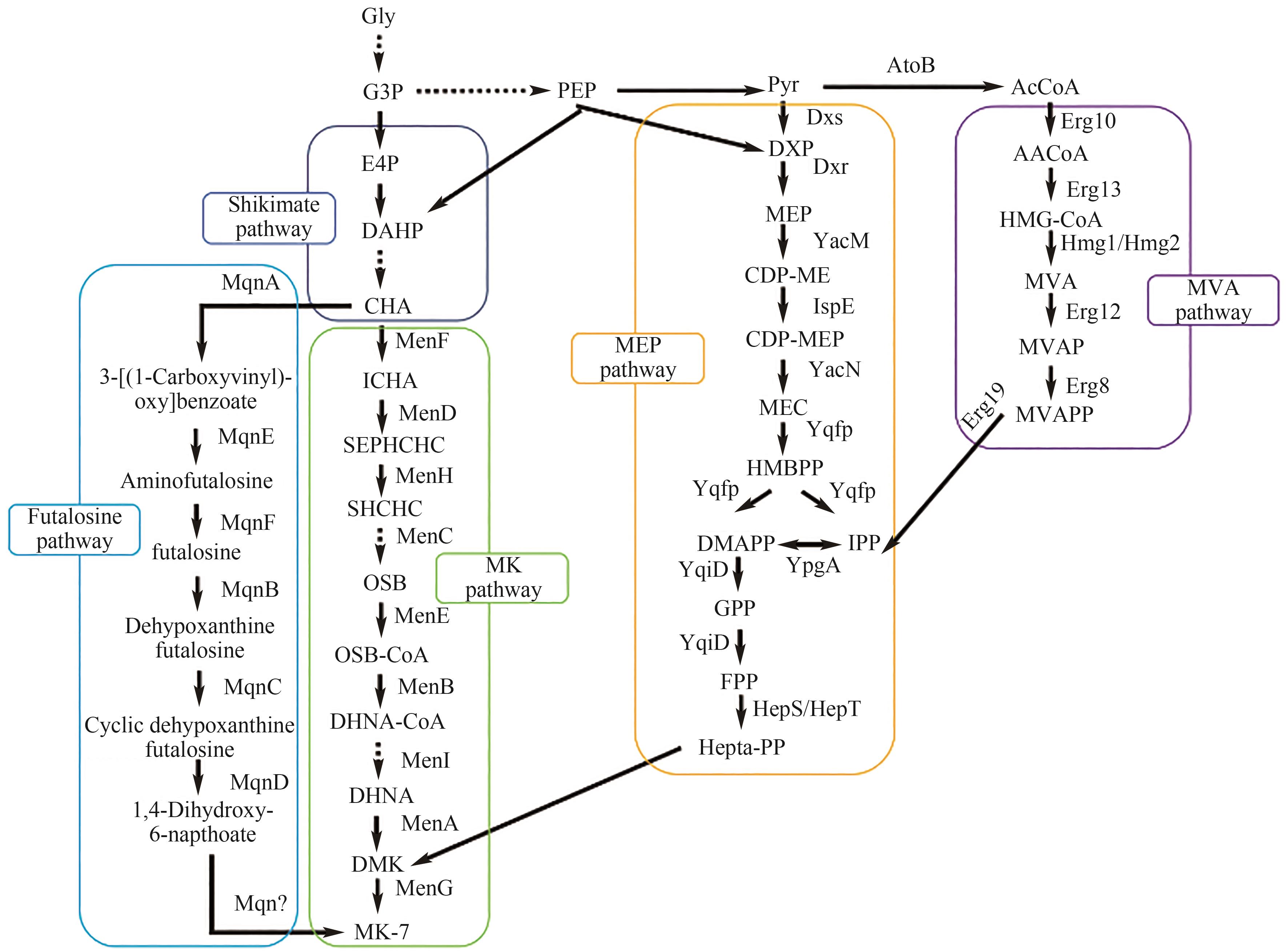
图1 VK2的生物合成途径Shikimate pathway(莽草酸途径):Gly(甘油);G3P(甘油醛-3-磷酸);E4P(赤藓糖-4-磷酸);DAHP(3-脱氧-阿拉伯-庚酮酸酯 7-磷酸盐);CHA(分支酸);MK pathway(甲萘醌途径):ICHA(异分支酸);SEPHCHC(2-琥珀酰基-5-烯醇丙酮酸-6-羟基-3-环己烯-1-羧酸酯);SHCHC[(1R,6R)-2-琥珀酰基-6-羟基-2,4-环己二烯-1-羧酸酯];OSB(2-琥珀酰苯甲酸);OSB-CoA(2-琥珀酰苯甲酸辅酶A);DHNA-CoA(1,4-二羟基-2-萘酰辅酶A); DHNA(1,4-二羟基-2-萘酸);DMK(萘醌);MK-7(甲萘醌);MenF(异分支酸合酶);MenD(2-琥珀酰-5-烯醇丙酮酰-6-羟基-3-环己烯-1-羧酸合酶);MenH(2-琥珀酰-6-羟基-2,4-环己二烯-1-羧酸合酶);MenC(邻琥珀酰苯甲酸合酶);MenE(邻琥珀酰苯甲酸-辅酶A连接酶);MenB(1,4-二羟基-2-萘甲酰-CoA合酶);MenI(1,4-二羟基-2-萘甲酰-CoA水解酶);MenA(1,4-二羟基-2-萘甲酸七异戊二烯转移酶);MenG(去甲基甲萘醌甲基转移酶);MEP pathway(2-C-甲基-D-赤藓糖醇-4-磷酸途径):Pyr(丙酮酸盐);DXP(1-脱氧木酮糖-5-磷酸);MEP(甲基赤藓糖醇-4-二磷酸);CDP-ME(4-二磷酸胞苷-2-C-甲基-D-赤藓糖);CDP-MEP(2-磷酸-4-二磷酸胞苷-2-C-甲基-D-赤藓糖);MEC(2-C-甲基赤藓糖-2,4-环焦磷酸);HMBPP(1-羟基-2-甲基-2-丁烯基-4-焦磷酸);DMAPP(二甲基烯丙基二磷酸);IPP(异戊二烯焦磷酸);GPP(牻牛儿基焦磷酸);FPP(法呢基焦磷酸);Hepta-PP(全反式聚异戊烯二磷酸);Dxs(1-脱氧木酮糖-5-磷酸合酶);Dxr(1-脱氧木酮糖-5-磷酸还原异构酶);YacM(2-C-甲基赤藓糖醇-4-磷酸胞苷酰转移酶);IspE(4-二磷酸胞苷-2-C-甲基赤藓糖醇激酶);YacN(2-C-甲基赤藓糖醇-2,4-环二磷酸合酶);Yqfp(4-羟基-3-甲基丁-2-烯基二磷酸合酶);YpgA(异戊烯基焦磷酸异构酶);YqiD(法呢基焦磷酸合酶);HepS/HepT(七异戊二烯基焦磷酸合成酶);MVA pathway(甲羟戊酸途径): AcCoA(乙酰辅酶A);AtoB(乙酰辅酶A C-乙酰转移酶);AACoA(乙酰乙酰辅酶A); HMG-CoA(3-羟甲基戊二酰辅酶A); MVA(甲羟戊酸); MVAP(甲羟戊酸-5-磷酸);MVAPP(甲羟戊酸-5-焦磷酸);Erg10(乙酰辅酶A乙酰转移酶);Erg13(羟甲基戊二酰辅酶A合酶);Hmg1/Hmg2(羟甲基戊二酰辅酶A还原酶);Erg12(甲羟戊酸激酶);Erg8(磷酸戊酸激酶);Erg19(甲羟戊酸焦磷酸脱羧酶);Futalosine pathway: MqnA(分支酸脱水酶);MqnE(氨基脱氧富他洛辛合酶);MqnF(氨基脱氧氟他嘧啶脱氨酶);MqnB(富他洛辛水解酶);MqnC(环状脱氧黄嘌呤富他罗辛合酶);MqnD(1,4-二羟基-6-萘酸合酶)
Fig.1 Biosynthetic pathway of VK2
| 名称 | 酶编号 | 蛋白大小/kDa | 底物 | (kcat/Km)/(L·s-1·μmol-1) | 辅因子 | 二级结构图 |
|---|---|---|---|---|---|---|
| MenF | 5.4.4.2 | 52.812 | — | — | — |  |
| MenD | 2.2.1.9 | 64.092 | — | — | Mg2+ |  |
| MenH | 4.2.99.20 | 27.128 | — | — | — |  |
| MenC | 4.2.1.113 | 41.629 | (1R,6R)-6-羟基-2-琥珀环己烷基-2,4-二烯-1-羧酸酯 | 2 | — |  |
| MenE | 6.2.1.26 | 50.185 | 2-邻琥珀酰苯甲酸 | 0.77 | Mg2+ |  |
| MenB | 4.1.3.36 | 31.633 | 4-(2'-羧苯基)-4- 氧代丁酰-CoA | 24 | HCO3- |  |
| MenI | 3.1.2.28 | 14.945 | 1-羟基-2-萘甲酰基-CoA | 1.85 | — |  |
| MenA | 2.5.1.74 | 33.838 | — | — | — |  |
| MenG | 2.1.1.163 | 27.128 | — | — | — |  |
表3 Men功能酶的酶学信息
Table 3 Enzymological information on Menase
| 名称 | 酶编号 | 蛋白大小/kDa | 底物 | (kcat/Km)/(L·s-1·μmol-1) | 辅因子 | 二级结构图 |
|---|---|---|---|---|---|---|
| MenF | 5.4.4.2 | 52.812 | — | — | — |  |
| MenD | 2.2.1.9 | 64.092 | — | — | Mg2+ |  |
| MenH | 4.2.99.20 | 27.128 | — | — | — |  |
| MenC | 4.2.1.113 | 41.629 | (1R,6R)-6-羟基-2-琥珀环己烷基-2,4-二烯-1-羧酸酯 | 2 | — |  |
| MenE | 6.2.1.26 | 50.185 | 2-邻琥珀酰苯甲酸 | 0.77 | Mg2+ |  |
| MenB | 4.1.3.36 | 31.633 | 4-(2'-羧苯基)-4- 氧代丁酰-CoA | 24 | HCO3- |  |
| MenI | 3.1.2.28 | 14.945 | 1-羟基-2-萘甲酰基-CoA | 1.85 | — |  |
| MenA | 2.5.1.74 | 33.838 | — | — | — |  |
| MenG | 2.1.1.163 | 27.128 | — | — | — |  |
| Men酶 | 片段 大小/bp | 甘油消耗 速率 | 生物量 | 酶活 | MK-7产量 |
|---|---|---|---|---|---|
| MenA | 936 | +++ | + | +++ | +++ |
| MenB | 819 | — | — | — | — |
| MenC | 1116 | ++ | ++ | + | + |
| MenD | 1737 | + | + | ++ | |
| MenE | 1464 | + | + | ++ | + |
| MenG | 702 | — | — | — | — |
| MenH | 825 | * | ++ | + | * |
表4 Men酶对MK-7表达的影响
Table 4 Effect of Menase on MK-7 expression
| Men酶 | 片段 大小/bp | 甘油消耗 速率 | 生物量 | 酶活 | MK-7产量 |
|---|---|---|---|---|---|
| MenA | 936 | +++ | + | +++ | +++ |
| MenB | 819 | — | — | — | — |
| MenC | 1116 | ++ | ++ | + | + |
| MenD | 1737 | + | + | ++ | |
| MenE | 1464 | + | + | ++ | + |
| MenG | 702 | — | — | — | — |
| MenH | 825 | * | ++ | + | * |
| 1 | 董润锜. 维生素K2的生物学效应及临床意义的研究进展[J]. 河南医学研究, 2021, 30(18): 3451-3454. |
| Dong R Q. Research progress on the biological effects and clinical significance of vitamin K2[J]. Henan Medical Research, 2021, 30(18): 3451-3454. | |
| 2 | 李树壮. 补钙还需补维生素K2[J]. 家庭健康(医学科普), 2020(2): 18. |
| Li S Z. Vitamin K2 is also needed for calcium supplementation [J]. Family Health, 2020(2): 18. | |
| 3 | 李月, 刘艳, 路更, 等. 维生素K对CKD-MBD骨代谢异常以及血管钙化的治疗及作用机制[J]. 中国骨质疏松杂志, 2022, 28(2): 308-312. |
| Li Y, Liu Y, Lu G, et al. Therapeutical action and mechanism of vitamin K in chronic kidney disease-mineral and bone disorder and vascular calcification[J]. Chinese Journal of Osteoporosis, 2022, 28(2): 308-312. | |
| 4 | 朱进伟, 桂王艳, 张安源, 等. 维生素K2的相关合成研究及前景展望[J]. 中国抗生素杂志, 2020, 45(7): 646-654. |
| Zhu J W, Gui W Y, Zhang A Y, et al. Synthetic research and prospects of vitamin K2[J]. Chinese Journal of Antibiotics, 2020, 45(7): 646-654. | |
| 5 | Blume S W, Curtis J R. Medical costs of osteoporosis in the elderly medicare population[J]. Osteoporosis International, 2011, 22(6): 1835-1844. |
| 6 | Ren L J, Peng C, Hu X C, et al. Microbial production of vitamin K2: current status and future prospects[J]. Biotechnology Advances, 2020, 39: 107453. |
| 7 | 原攀红, 吕雪芹, 刘延峰, 等. 调控质膜稳态提高枯草芽孢杆菌积累四烯甲萘醌MK-4[J]. 食品与发酵工业, 2021, 47(18): 1-7. |
| Yuan P H, Lyu X Q, Liu Y F, et al. Regulation of plasma membrane homeostasis to increase the accumulation of menaquinone-4 in Bacillus subtilis [J]. Food and Fermentation Industries, 2021, 47(18): 1-7. | |
| 8 | 尹贵超, 段逸飞,王成,等. 维生素K 2(MK-7)高产菌株及其筛选方法和生产维生素K2(MK-7)的方法: 113755404A[P]. 2021-12-07. |
| Yin G C, Duan Y F, Wang C, et al. High production strain of vitamin K2 (MK-7) and its screening method and production method: 113755404A[P]. 2021-12-07. | |
| 9 | 尉鸿飞. 黄杆菌胞内维生素K2的分离纯化与理化特性研究[D]. 合肥: 中国科学技术大学, 2018. |
| Yu H F. Isolation, purification and physicochemical characterization of vitamin K2 from Flavobacterium [D]. Hefei: University of Science and Technology of China, 2018. | |
| 10 | 刘珍. 纳豆芽孢杆菌产维生素K2(MK-7)的工艺优化及比较代谢组学分析[D]. 无锡: 江南大学, 2021. |
| Liu Z. Process optimization of vitamin K2(MK-7) by Bacillus subtilis natto and comparative metabolomics analysis[D]. Wuxi: Jiangnan University, 2021. | |
| 11 | Kong M K, Lee P C. Metabolic engineering of menaquinone-8 pathway of Escherichia coli as a microbial platform for vitamin K production[J]. Biotechnology and Bioengineering, 2011, 108(8): 1997-2002. |
| 12 | Liu Y, Ding X M, Xue Z L, et al. Site-directed mutagenesis of UbiA to promote menaquinone biosynthesis in Elizabethkingia meningoseptica [J]. Process Biochemistry, 2017, 58: 186-192. |
| 13 | Liu Y, Yang Z M, Xue Z L, et al. Influence of site-directed mutagenesis of UbiA, overexpression of dxr, menA and ubiE, and supplementation with precursors on menaquinone production in Elizabethkingia meningoseptica [J]. Process Biochemistry, 2018, 68: 64-72. |
| 14 | Ma Y W, McClure D D, Somerville M V, et al. Metabolic engineering of the MEP pathway in Bacillus subtilis for increased biosynthesis of menaquinone-7[J]. ACS Synthetic Biology, 2019, 8(7): 1620-1630. |
| 15 | 梁媛. 糖酵解途径和转运系统的改造对大肠杆菌发酵L-苏氨酸的影响[D]. 天津: 天津科技大学, 2014. |
| Liang Y. Effect of manipulation of glycolysis and transport system on L-threonine production in Escherichia coli [D]. Tianjin: Tianjin University of Science & Technology, 2014. | |
| 16 | 谢冲. 利用大肠杆菌莽草酸途径生物合成苯酚、酪醇和羟基酪醇的研究[D]. 北京: 北京化工大学, 2019. |
| Xie C. Biosynthesis of phenol, tyrosol and hydroxytyrosol using the E . coli shikimate pathway[D]. Beijing: Beijing University of Chemical Technology, 2019. | |
| 17 | 顾洋. 重构枯草芽孢杆菌糖转运途径及中心代谢网络高效合成N-乙酰氨基葡萄糖[D]. 无锡: 江南大学, 2020. |
| Gu Y. Rewiring the glucose transportation pathway and central metabolic pathway for overproduction of N-acetylglucosamine in Bacillus subtilis [D]. Wuxi: Jiangnan University, 2020. | |
| 18 | 严为留. 发酵法生产维生素K2的研究[D]. 无锡: 江南大学, 2014. |
| Yan W L. Study on the fermentation of vitamin K2[D]. Wuxi: Jiangnan University, 2014. | |
| 19 | Goodman S R, Marrs B L, Narconis R J, et al. Isolation and description of a menaquinone mutant from Bacillus licheniformis [J]. Journal of Bacteriology, 1976, 125(1): 282-289. |
| 20 | Berenjian A, Mahanama R, Talbot A, et al. Efficient media for high menaquinone-7 production: response surface methodology approach[J]. New Biotechnology, 2011, 28(6): 665-672. |
| 21 | Berenjian A, Mahanama R, Talbot A, et al. Advances in menaquinone-7 production by Bacillus subtilis natto: fed-batch glycerol addition[J]. American Journal of Biochemistry and Biotechnology, 2012, 8(2): 105-110. |
| 22 | Yan L, Zheng Z M, Qiu H W, et al. Surfactant supplementation to enhance the production of vitamin K-2 metabolites in shake flask cultures using Escherichia sp. mutant FM3-1709[J]. Food Technology and Biotechnology, 2014, 52(3): 269-275. |
| 23 | Mahdinia E, Demirci A, Berenjian A. Enhanced vitamin K (menaquinone-7) production by Bacillus subtilis natto in biofilm reactors by optimization of glucose-based medium[J]. Current Pharmaceutical Biotechnology, 2018, 19(11): 917-924. |
| 24 | Xu J Z, Zhang W G. Menaquinone-7 production from maize meal hydrolysate by Bacillus isolates with diphenylamine and analogue resistance[J]. Journal of Zhejiang University-Science B, 2017, 18(6): 462-473. |
| 25 | Mahdinia E, Demirci A, Berenjian A. Biofilm reactors as a promising method for vitamin K (menaquinone-7) production[J]. Applied Microbiology and Biotechnology, 2019, 103(14): 5583-5592. |
| 26 | Berenjian A, Mahanama R, Talbot A, et al. Designing of an intensification process for biosynthesis and recovery of menaquinone-7[J]. Applied Biochemistry and Biotechnology, 2014, 172(3): 1347-1357. |
| 27 | 张晨阳, 武耀康, 徐显皓, 等. 工业微生物代谢网络模型的研究进展及应用[J]. 生物工程学报, 2021, 37(3): 860-873. |
| Zhang C Y, Wu Y K, Xu X H, et al. Current status and future perspectives of metabolic network models of industrial microorganisms[J]. Chinese Journal of Biotechnology, 2021, 37(3): 860-873. | |
| 28 | 曹燕亭, 刘延峰, 李江华, 等. 基于细胞亚群调控提升生物合成效率的研究进展[J]. 生物技术通报, 2020, 36(4): 19-25. |
| Cao Y T, Liu Y F, Li J H, et al. Advances of improving the efficiency of chemical biosynthesis based on cell subpopulation regulation[J]. Biotechnology Bulletin, 2020, 36(4): 19-25. | |
| 29 | 刘艳, 杨自名, 薛正莲, 等. menA过表达菌株构建及两阶段pH控制促进VK2合成[J]. 食品与生物技术学报, 2019, 38(12): 31-38. |
| Liu Y, Yang Z M, Xue Z L, et al. Construction of menA overexpressing strain and two-stage pH control promoting VK2 synthesis [J]. Chinese Journal of Food and Biotechnology, 2019, 38(12): 31-38. | |
| 30 | Gao Q X, Chen H, Wang W Z, et al. Menaquinone-7 production in engineered Escherichia coli [J]. World Journal of Microbiology & Biotechnology, 2020, 36(9): 132. |
| 31 | Yuan P H, Cui S X, Liu Y F, et al. Combinatorial engineering for improved menaquinone-4 biosynthesis in Bacillus subtilis [J]. Enzyme and Microbial Technology, 2020, 141: 109652. |
| 32 | Chen T C, Xia H Z, Cui S X, et al. Combinatorial methylerythritol phosphate pathway engineering and process optimization for increased menaquinone-7 synthesis in Bacillus subtilis [J]. Journal of Microbiology and Biotechnology, 2020, 30(5): 762-769. |
| 33 | 徐显皓. 枯草芽孢杆菌中心代谢级联调控回路的设计、构建与应用[D]. 无锡: 江南大学, 2021. |
| Xu X H. Design, construction and application of the genetic circuits for the layered regulation of central metabolic in Bacillus subtilis [D]. Wuxi: Jiangnan University, 2021. | |
| 34 | 李梦莹, 吕雪芹, 刘延峰, 等. 代谢工程改造大肠杆菌合成L-组氨酸[J]. 食品与发酵工业, 2021, 47(12): 1-12. |
| Li M Y, Lyu X Q, Liu Y F, et al. Metabolic engineering of Escherichia coli for increased synthesis of L-histidine[J]. Food and Fermentation Industries, 2021, 47(12): 1-12. | |
| 35 | 张晓龙, 王晨芸, 刘延峰, 等. 基于合成生物技术构建高效生物制造系统的研究进展[J]. 合成生物学, 2021, 2(6): 863-875. |
| Zhang X L, Wang C Y, Liu Y F, et al. Research progress of constructing efficient biomanufacturing system based on synthetic biotechnology[J]. Synthetic Biology Journal, 2021, 2(6): 863-875. | |
| 36 | 钱蕾, 刘延峰, 李江华, 等. 适应性进化和改造质粒稳定性促进枯草芽孢杆菌合成N-乙酰神经氨酸[J]. 食品与发酵工业, 2021, 47(5): 1-6. |
| Qian L, Liu Y F, Li J H, et al. Regulating the synthesis of N-acetylneuraminic acid based on adaptive evolution and plasmid stability modification in Bacillus subtilis [J]. Food and Fermentation Industries, 2021, 47(5): 1-6. | |
| 37 | 陈泰驰. 枯草芽孢杆菌代谢调控及过程优化发酵生产七烯甲萘醌[D]. 无锡: 江南大学, 2020. |
| Chen T C. Genetic engineering and process optimization of Bacillus subtilis for menaquinone-7 production[D]. Wuxi: Jiangnan University, 2020. | |
| 38 | 徐建中, 王颖妤, 严为留, 等. 维生素K2合成途径中主要酶对MK-7产量的影响[J]. 生物技术通报, 2016, 32(11): 248-254. |
| Xu J Z, Wang Y Y, Yan W L, et al. Effects of major enzymes in the biosynthetic pathway of vitamin K2 on MK-7 production[J]. Biotechnology Bulletin, 2016, 32(11): 248-254. | |
| 39 | 杨绍梅. 枯草芽孢杆菌的模块化路径工程设计促进甲萘醌-7的合成[D]. 天津: 天津大学, 2019. |
| Yang S M. Modular pathway engineering of Bacillus subtilis to promote the biosynthesis of menaquinone-7[D]. Tianjin: Tianjin University, 2019. | |
| 40 | Liu L, Gallagher J, Arevalo E D, et al. Enhancing grain-yield-related traits by CRISPR-Cas9 promoter editing of maize CLE genes[J]. Nature Plants, 2021, 7(3): 287-294. |
| 41 | Grützner R, Martin P, Horn C, et al. High-efficiency genome editing in plants mediated by a Cas9 gene containing multiple introns[J]. Plant Communications, 2020, 2(2): 100135. |
| 42 | McCarty N S, Graham A E, Studená L, et al. Multiplexed CRISPR technologies for gene editing and transcriptional regulation[J]. Nature Communications, 2020, 11: 1281. |
| 43 | Wang M, Chen B Q, Fang Y M, et al. Cofactor engineering for more efficient production of chemicals and biofuels[J]. Biotechnology Advances, 2017, 35(8): 1032-1039. |
| 44 | Dahl R H, Zhang F Z, Alonso-Gutierrez J, et al. Engineering dynamic pathway regulation using stress-response promoters[J]. Nature Biotechnology, 2013, 31(11): 1039-1046. |
| 45 | Kalia V C. Quorum sensing inhibitors: an overview[J]. Biotechnology Advances, 2013, 31(2): 224-245. |
| 46 | Xu J Z, Yan W L, Zhang W G. Enhancing menaquinone-7 production in recombinant Bacillus amyloliquefaciens by metabolic pathway engineering[J]. RSC Advances, 2017, 7(45): 28527-28534. |
| 47 | Jun D, Richardson-Sanchez T, Mahey A, et al. Introduction of the menaquinone biosynthetic pathway into Rhodobacter sphaeroides and de novo synthesis of menaquinone for incorporation into heterologously expressed integral membrane proteins[J]. ACS Synthetic Biology, 2020, 9(5): 1190-1200. |
| 48 | Liao C Y, Ayansola H, Ma Y B, et al. Advances in enhanced menaquinone-7 production from Bacillus subtilis [J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 695526. |
| 49 | Liu Q L, Yu T, Li X W, et al. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals[J]. Nature Communications, 2019, 10: 4976. |
| 50 | Yang S M, Cao Y X, Sun L M, et al. Modular pathway engineering of Bacillus subtilis to promote de novo biosynthesis of menaquinone-7[J]. ACS Synthetic Biology, 2019, 8(1): 70-81. |
| 51 | Cui S X, Lv X Q, Wu Y K, et al. Engineering a bifunctional Phr60-Rap60-Spo0A quorum-sensing molecular switch for dynamic fine-tuning of menaquinone-7 synthesis in Bacillus subtilis [J]. ACS Synthetic Biology, 2019, 8(8): 1826-1837. |
| 52 | Wu J, Li W, Zhao S G, et al. Site-directed mutagenesis of the quorum-sensing transcriptional regulator SinR affects the biosynthesis of menaquinone in Bacillus subtilis [J]. Microbial Cell Factories, 2021, 20(1): 113. |
| 53 | Qin X, Taber H W. T Crystal structure of Escherichia coli enterobactin-specific isochorismate synthase (EntC) bound to its reaction product isochorismate: implications ubtilis menp1 promoter[J]. Journal of Bacteriology, 1996, 178(3): 705-713. |
| 54 | Sridharan S, Howard N, Kerbarh O, et al. Crystal structure of Escherichia coli enterobactin-specific isochorismate synthase (EntC) bound to its reaction product isochorismate: implications for the enzyme mechanism and differential activity of chorismate-utilizing enzymes[J]. Journal of Molecular Biology, 2010, 397(1): 290-300. |
| 55 | Kolappan S, Zwahlen J, Zhou R, et al. Lysine 190 is the catalytic base in MenF, the menaquinone-specific isochorismate synthase from Escherichia coli: implications for an enzyme family[J]. Biochemistry, 2007, 46(4): 946-953. |
| 56 | Dawson A, Fyfe P K, Hunter W N. Specificity and reactivity in menaquinone biosynthesis: the structure of Escherichia coli MenD (2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexadiene-1-carboxylate synthase)[J]. Journal of Molecular Biology, 2008, 384(5): 1353-1368. |
| 57 | Jiang M, Chen X L, Guo Z F, et al. Identification and characterization of (1R, 6R)-2-succinyl-6-hydroxy-2, 4-cyclohexadiene-1-carboxylate synthase in the menaquinone biosynthesis of Escherichia coli [J]. Biochemistry, 2008, 47(11): 3426-3434. |
| 58 | Thompson T B, Garrett J B, Taylor E A, et al. Evolution of enzymatic activity in the enolase superfamily: structure of o-succinylbenzoate synthase from Escherichia coli in complex with Mg2+ and o-succinylbenzoate[J]. Biochemistry, 2000, 39(35): 10662-10676. |
| 59 | Taylor Ringia E A, Garrett J B, Thoden J B, et al. Evolution of enzymatic activity in the enolase superfamily: functional studies of the promiscuous o-succinylbenzoate synthase from Amycolatopsis [J]. Biochemistry, 2004, 43(1): 224-229. |
| 60 | Palmer D R, Garrett J B, Sharma V, et al. Unexpected divergence of enzyme function and sequence: “N-acylamino acid racemase” is o-succinylbenzoate synthase[J]. Biochemistry, 1999, 38(14): 4252-4258. |
| 61 | Klenchin V A, Taylor Ringia E A, Gerlt J A, et al. Evolution of enzymatic activity in the enolase superfamily: structural and mutagenic studies of the mechanism of the reaction catalyzed by o-succinylbenzoate synthase from Escherichia coli [J]. Biochemistry, 2003, 42(49): 14427-14433. |
| 62 | Matarlo J S, Evans C E, Sharma I, et al. Mechanism of MenE inhibition by acyl-adenylate analogues and discovery of novel antibacterial agents[J]. Biochemistry, 2015, 54(42): 6514-6524. |
| 63 | Sun Y R, Song H G, Li J, et al. Structural basis of the induced-fit mechanism of 1, 4-dihydroxy-2-naphthoyl coenzyme A synthase from the crotonase fold superfamily[J]. PLoS One, 2013, 8(4): e63095. |
| 64 | Truglio J J, Theis K, Feng Y G, et al. Crystal structure of Mycobacterium tuberculosis MenB, a key enzyme in vitamin K2 biosynthesis[J]. The Journal of Biological Chemistry, 2003, 278(43): 42352-42360. |
| 65 | Smith H B, Li T L, Liao M K, et al. Listeria monocytogenes MenI encodes a DHNA-CoA thioesterase necessary for menaquinone biosynthesis, cytosolic survival, and virulence[J]. Infection and Immunity, 2021, 89(5): e00792-e00720. |
| 66 | Murad A M, Brognaro H, Falke S, et al. Structure and activity of the DHNA coenzyme-A thioesterase from Staphylococcus aureus providing insights for innovative drug development[J]. Scientific Reports, 2022, 12: 4313. |
| 67 | Suvarna K, Stevenson D, Meganathan R, et al. Menaquinone (vitamin K2) biosynthesis: localization and characterization of the menA gene from Escherichia coli [J]. Journal of Bacteriology, 1998, 180(10): 2782-2787. |
| 68 | Hu L X, Feng J J, Wu J, et al. Identification of six important amino acid residues of MenA from Bacillus subtilis natto for enzyme activity and formation of menaquinone[J]. Enzyme and Microbial Technology, 2020, 138: 109583. |
| 69 | Johnston J M, Arcus V L, Morton C J, et al. Crystal structure of a putative methyltransferase from Mycobacterium tuberculosis: misannotation of a genome clarified by protein structural analysis[J]. Journal of Bacteriology, 2003, 185(14): 4057-4065. |
| 70 | Sakuragi Y, Zybailov B, Shen G Z, et al. Insertional inactivation of the MenG gene, encoding 2-phytyl-1, 4-naphthoquinone methyltransferase of Synechocystis sp. PCC 6803, results in the incorporation of 2-phytyl-1, 4-naphthoquinone into the A(1) site and alteration of the equilibrium constant between A(1) and F(X) in photosystem I[J]. Biochemistry, 2002, 41(1): 394-405. |
| 71 | Xiang M J, Kang Q, Zhang D W. Advances on systems metabolic engineering of Bacillus subtilis as a chassis cell[J]. Synthetic and Systems Biotechnology, 2020, 5(4): 245-251. |
| 72 | 李宏彪, 梁晓琳, 周景文. 酿酒酵母基因编辑技术研究进展[J]. 生物工程学报, 2021, 37(3): 950-965. |
| Li H B, Liang X L, Zhou J W. Progress in gene editing technologies for Saccharomyces cerevisiae [J]. Chinese Journal of Biotechnology, 2021, 37(3): 950-965. |
| [1] | 尹春华, 彭思雨, 马垒珍, 张海洋, 闫海. 纳米氧化锌的生物法合成及固定脂肪酶的研究[J]. 化工学报, 2020, 71(5): 2248-2255. |
| [2] | 黄锦标1,尚龙安2. 聚羟基烷酸酯的生物合成研究进展 [J]. CIESC Journal, 2011, 30(9): 2041-. |
| [3] | 李金娟,赵 林,谭 欣,黄 宇,柳听义. 利用淀粉酸化废水驯化活性污泥合成聚-?-羟基脂肪酸酯及其表征 [J]. CIESC Journal, 2011, 30(7): 1618-. |
| [4] | 王海胜,张晓霞,卢 元,阮志勇,邢新会,姜瑞波. 紫色杆菌素研究进展 [J]. CIESC Journal, 2008, 27(3): 315-. |
| [5] | 李良智,芮新生,万屹东. 微生物及其酶法生产稀有L-戊糖 [J]. CIESC Journal, 2007, 26(5): 750-. |
| [6] | 于慧敏, 史悦, 尹进, 沈忠耀, 杨胜利. 透明颤菌血红蛋白基因和λ噬菌体裂解基因在生产PHB重组大肠杆菌中的同时表达[J]. CIESC Journal, 2001, 9(4): 407-411. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号