• •
杨立鹏1,2( ), 顾宇阳1,2, 田十宇1,2, 杨晓光1,2(
), 顾宇阳1,2, 田十宇1,2, 杨晓光1,2( )
)
收稿日期:2025-09-30
修回日期:2025-12-18
出版日期:2025-12-19
通讯作者:
杨晓光
作者简介:杨立鹏(1990—),男,博士研究生,997579110@qq.com
基金资助:
Lipeng YANG1,2( ), Yuyang GU1,2, Shiyu TIAN1,2, Xiao-Guang YANG1,2(
), Yuyang GU1,2, Shiyu TIAN1,2, Xiao-Guang YANG1,2( )
)
Received:2025-09-30
Revised:2025-12-18
Online:2025-12-19
Contact:
Xiao-Guang YANG
摘要:
硫化物全固态电池(ASSBs)因其能量密度高、安全性好,而成为最具前景的下一代电池技术之一。然而其电化学性能仍不尽如人意,特别是在高压正极、高质量负载条件下,混合正极内部存在固-固界面副反应的问题,并引发包括空间电荷层、体积膨胀、电子/离子传输等一系列问题,进而影响全固态电池性能。本文概述了目前对硫化物电池混合正极改性的研究现状,总结了材料表面改性(界面工程)、电解质改性(材料工程) 、电极掺杂改性(部件工程)以及电极结构工程的相关发展现状及应用优势,并为下一步基于硫化物电解质的全固态锂电池混合正极的研发方向提供了指导性建议。
中图分类号:
杨立鹏, 顾宇阳, 田十宇, 杨晓光. 基于硫化物全固态锂离子电池混合正极的研究进展[J]. 化工学报, DOI: 10.11949/0438-1157.20251089.
Lipeng YANG, Yuyang GU, Shiyu TIAN, Xiao-Guang YANG. Recent Advances in Hybrid Cathodes for Sulfide-Based All-Solid-State Lithium-Ion Batteries[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251089.
| 改性策略/涂层类型 | 核心机理 | 优点 | 局限性 | 代表性文章 | |
|---|---|---|---|---|---|
| 传统氧化物涂层 | Li4Ti5O12 | 在活性材料与硫化物电解质之间构筑物理屏障,抑制副反应 | 工艺相对成熟;有效降低界面阻抗;提升循环稳定性与倍率性能 | 涂层离子电导率不足;高电压稳定性有限;难以完全解决体积变化导致的机械接触失效 | Ref. 27 Ref. 28 |
| Li2SiO3 | |||||
| LiNbO3 | |||||
| 先进功能涂层 | Li2O-ZrO2 | 不仅提供物理隔离,更注重涂层材料本身的高离子导、结构稳定或界面亲和性 | 更高的锂离子传输性能;更好的高电压稳定性; 部分涂层(如Li7TaO6)可同时实现体相掺杂,协同改性 | 合成工艺更复杂,对厚度与均匀性控制要求高;成本可能较高 | Ref. 29-34 |
| Li7TaO6 | |||||
| Li3PO4 | |||||
| 非氧化物/梯度涂层 | DLC(类金刚石碳) | 构建热力学更稳定的界面,或形成成分/性能渐变的梯度结构,以完美匹配两侧材料 | 化学/电化学稳定性,从根本上抑制副反应;梯度层能实现应力缓冲和离子传输的平滑过渡;部分涂层(如氟化物)具有优异的高压稳定性 | 制备工艺复杂,难以大规模应用(如原子层沉积);非氧化物涂层的离子电导率需精细调控 | Ref. 9 Ref. 35-36 |
| Li-Ta-O-F | |||||
| Li3P1+xO4S4x | |||||
表1 活性材料界面改性种类、机理以及优缺点
Table 1 Types, Mechanisms, and Advantages/Disadvantages of Interface Modification for Active Materials
| 改性策略/涂层类型 | 核心机理 | 优点 | 局限性 | 代表性文章 | |
|---|---|---|---|---|---|
| 传统氧化物涂层 | Li4Ti5O12 | 在活性材料与硫化物电解质之间构筑物理屏障,抑制副反应 | 工艺相对成熟;有效降低界面阻抗;提升循环稳定性与倍率性能 | 涂层离子电导率不足;高电压稳定性有限;难以完全解决体积变化导致的机械接触失效 | Ref. 27 Ref. 28 |
| Li2SiO3 | |||||
| LiNbO3 | |||||
| 先进功能涂层 | Li2O-ZrO2 | 不仅提供物理隔离,更注重涂层材料本身的高离子导、结构稳定或界面亲和性 | 更高的锂离子传输性能;更好的高电压稳定性; 部分涂层(如Li7TaO6)可同时实现体相掺杂,协同改性 | 合成工艺更复杂,对厚度与均匀性控制要求高;成本可能较高 | Ref. 29-34 |
| Li7TaO6 | |||||
| Li3PO4 | |||||
| 非氧化物/梯度涂层 | DLC(类金刚石碳) | 构建热力学更稳定的界面,或形成成分/性能渐变的梯度结构,以完美匹配两侧材料 | 化学/电化学稳定性,从根本上抑制副反应;梯度层能实现应力缓冲和离子传输的平滑过渡;部分涂层(如氟化物)具有优异的高压稳定性 | 制备工艺复杂,难以大规模应用(如原子层沉积);非氧化物涂层的离子电导率需精细调控 | Ref. 9 Ref. 35-36 |
| Li-Ta-O-F | |||||
| Li3P1+xO4S4x | |||||
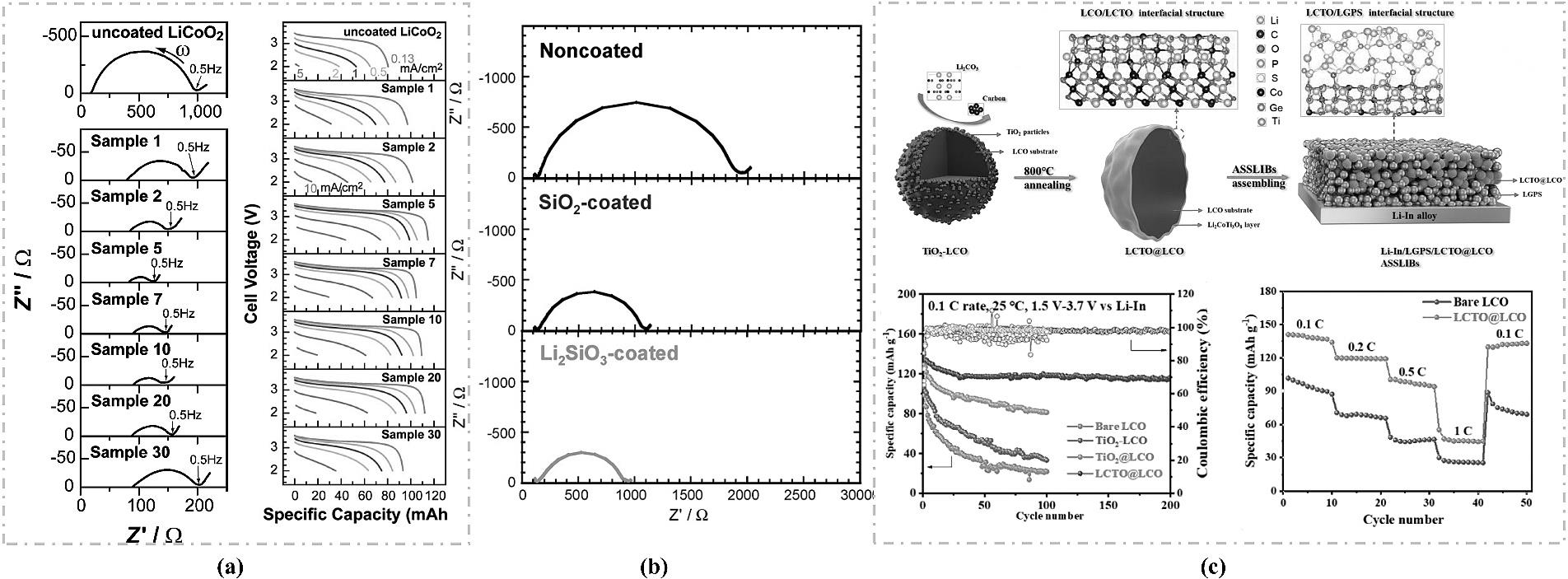
图2 (a) 铟锂/钴酸锂电池的阻抗 (Z) 和充放电曲线[27];(b) 无涂层、SiO2 涂层和 Li2SiO3 涂层钴酸锂全固态电池在第一次充电至 4.6V 后的阻抗曲线(vs. Li) [28];(c) LCO内核上原位形成LCTO涂层的流程示意图以及改性后电池循环与倍率性能[29]。
Fig.2 (a) Impedance (Z) and charge-discharge curves of an In-Li/LCO battery[27]; (b) Impedance curves of uncoated, SiO2-coated, and Li2SiO3-coated LCO all-solid-state batteries after the first charge to 4.6V (vs. Li) [28]; (c) Schematic diagram of the in-situ formation process of an LCTO coating on the LCO core, and the cycling and rate performance of the modified battery[29].
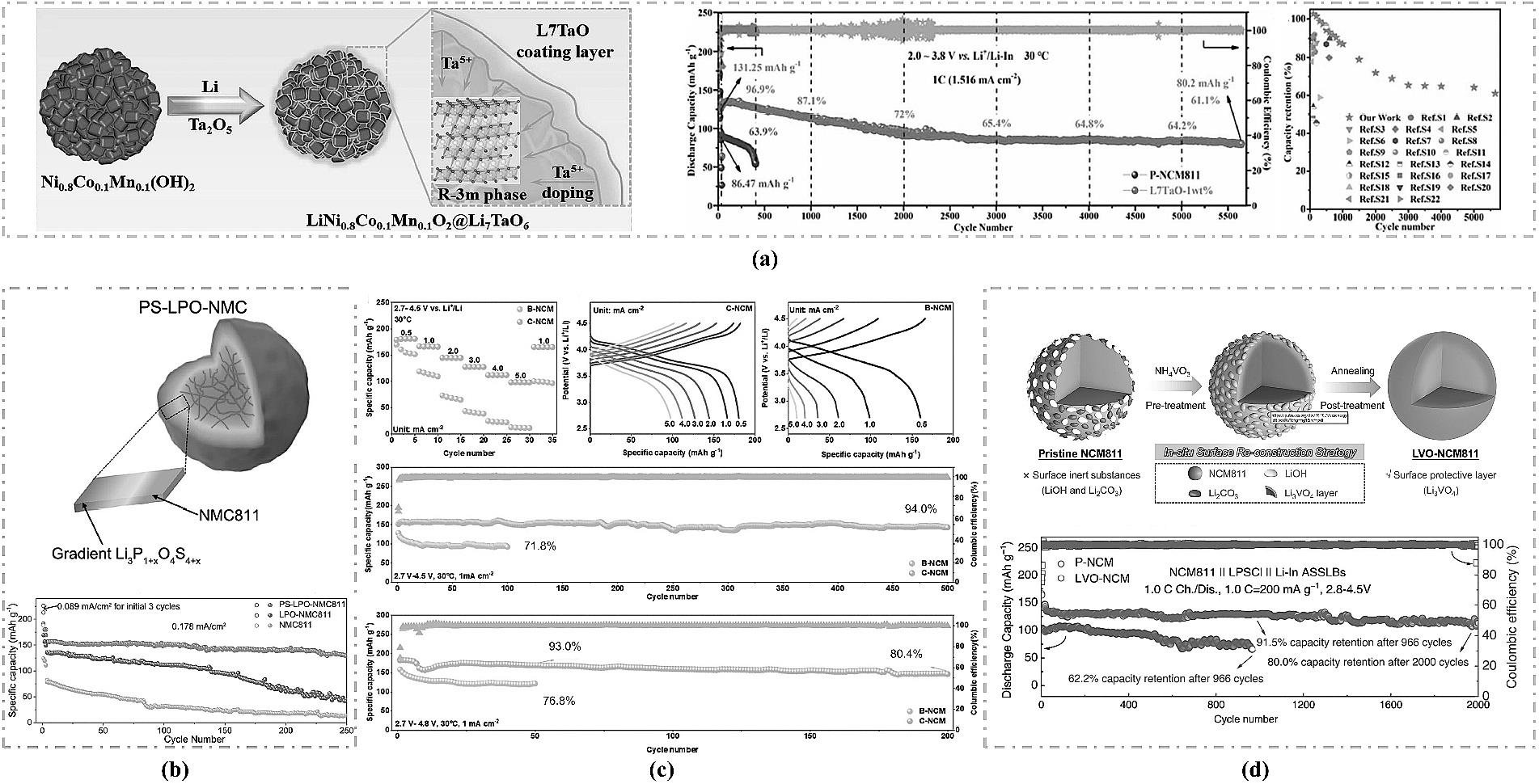
图3 (a) L7TaO包覆NCM811合成示意图、PNCM811 和 L7TaO-1wt% 在 1C注:下的长循环曲线[34];(b) NMC811初级粒子(粉红色)与离子导电和梯度锂梯度Li3P1+xO4S4x涂层示意图及电池循环性能[9];(c) ASSB的倍率性能以及高压循环性能[35];(d) NCM811表面重建过程示意图以及高压循环性能[36]。
Fig.3 (a) Schematic diagram of the L7TaO-coated NCM811 synthesis process and long-term cycling curves of pristine NCM811 and L7TaO-1wt% at 1 C[34]; (b) Schematic diagram of an NMC811 primary particle (pink) with an ion-conductive gradient Li3P1+xO4S4x coating, and the corresponding battery cycling performance[9]; (c) Rate capability and high-voltage cycling performance of the ASSB[35]; (d) Schematic diagram of the surface reconstruction process of NCM811 and its high-voltage cycling performance[36].
| 核心功能 | 涂层材料 | 作用机理 | 实现效果 | 代表性文章 |
|---|---|---|---|---|
| 稳定界面 & 抑制副反应 | Li2ZrF6(LZF)非晶/纳米晶涂层 | 热力学稳定以及抑制界面降解 | 保护层功能更持久,从根本上消除了界面相互作用的动力学障碍,实现超长循环寿命 | Ref. 38 |
| 离子-电子混合导电层(In-cPAN) | 配位稳定;动态稳定界面 | 通过化学键合实现界面强化,并构建动态稳定的电极/电解质界面,显著抑制副反应 | Ref. 41 | |
| 提供快速锂离子传输路径 | Li1.175Nb0.645Ti0.4O3(LNTO)涂层 | 原位形成离子导体 | 不仅降低了初始界面电阻,更在循环过程中构建稳定的高速离子通道,大幅提升倍率性能 | Ref. 37 |
| 铁电体(GClO4)涂层 | 构建内建电场 | 通过电学机制而非传统的化学/物理机制,为界面离子传输提供全新驱动力,突破动力学瓶颈 | Ref. 39 | |
| 表面重构(变害为利) | Li3VO4 (LVO)涂层 | 原位转化 | 清除了表面残锂这一副反应“引发剂”;构建了能抑制副反应和结构滑移的稳定界面层 | Ref. 36 |
| Li-Ta-O-F电解质涂层 | 将有害物质转化为有益功能层,工艺简单,为高电压下稳定工作提供了关键界面保障 | Ref. 35 | ||
| 多机制协同 | LNTO涂层 | 协同:离子传输+界面稳定 | 通过多种元素的不同作用,协同实现了界面的全面稳定与动力学优化 | Ref. 37 |
| TNO包覆+Ti掺杂 | 协同:物理隔绝+体相锚定 | 实现了从“界面”到“体相”的协同增强,是应对高电压、高镍正极复杂挑战的典范策略 | Ref. 40 |
表2 不同功能涂层作用机理及实现效果
Table 2 Mechanisms and Implementation Effects of Different Functional Coatings
| 核心功能 | 涂层材料 | 作用机理 | 实现效果 | 代表性文章 |
|---|---|---|---|---|
| 稳定界面 & 抑制副反应 | Li2ZrF6(LZF)非晶/纳米晶涂层 | 热力学稳定以及抑制界面降解 | 保护层功能更持久,从根本上消除了界面相互作用的动力学障碍,实现超长循环寿命 | Ref. 38 |
| 离子-电子混合导电层(In-cPAN) | 配位稳定;动态稳定界面 | 通过化学键合实现界面强化,并构建动态稳定的电极/电解质界面,显著抑制副反应 | Ref. 41 | |
| 提供快速锂离子传输路径 | Li1.175Nb0.645Ti0.4O3(LNTO)涂层 | 原位形成离子导体 | 不仅降低了初始界面电阻,更在循环过程中构建稳定的高速离子通道,大幅提升倍率性能 | Ref. 37 |
| 铁电体(GClO4)涂层 | 构建内建电场 | 通过电学机制而非传统的化学/物理机制,为界面离子传输提供全新驱动力,突破动力学瓶颈 | Ref. 39 | |
| 表面重构(变害为利) | Li3VO4 (LVO)涂层 | 原位转化 | 清除了表面残锂这一副反应“引发剂”;构建了能抑制副反应和结构滑移的稳定界面层 | Ref. 36 |
| Li-Ta-O-F电解质涂层 | 将有害物质转化为有益功能层,工艺简单,为高电压下稳定工作提供了关键界面保障 | Ref. 35 | ||
| 多机制协同 | LNTO涂层 | 协同:离子传输+界面稳定 | 通过多种元素的不同作用,协同实现了界面的全面稳定与动力学优化 | Ref. 37 |
| TNO包覆+Ti掺杂 | 协同:物理隔绝+体相锚定 | 实现了从“界面”到“体相”的协同增强,是应对高电压、高镍正极复杂挑战的典范策略 | Ref. 40 |
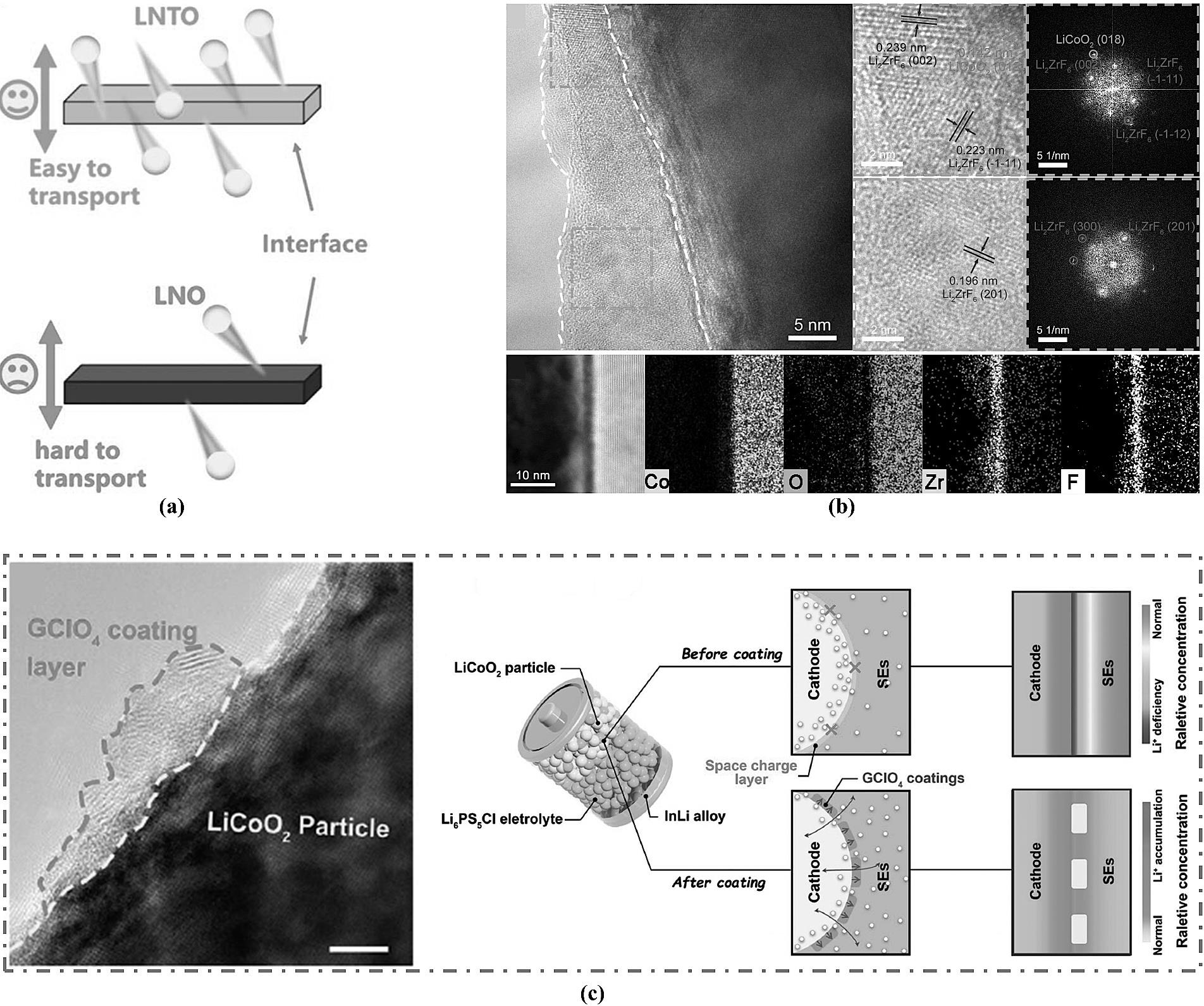
图4 (a) 离子通过 LNO 和 LNTO 夹层的离子传输示意图[37];(b) LZF-LCO 的结构SEM和EDS图谱[38];(c) GClO4包覆结构及全固态电池的示意图[39]。
Fig.4 (a) Illustration of ion transport through LNO and LNTO interlayers at the interface[37]; (b) SEM and EDS Mapping of the LZF-LCO Structure [38]; (c) Schematic diagram of the GClO4 coating structure and the all-solid-state battery[39].
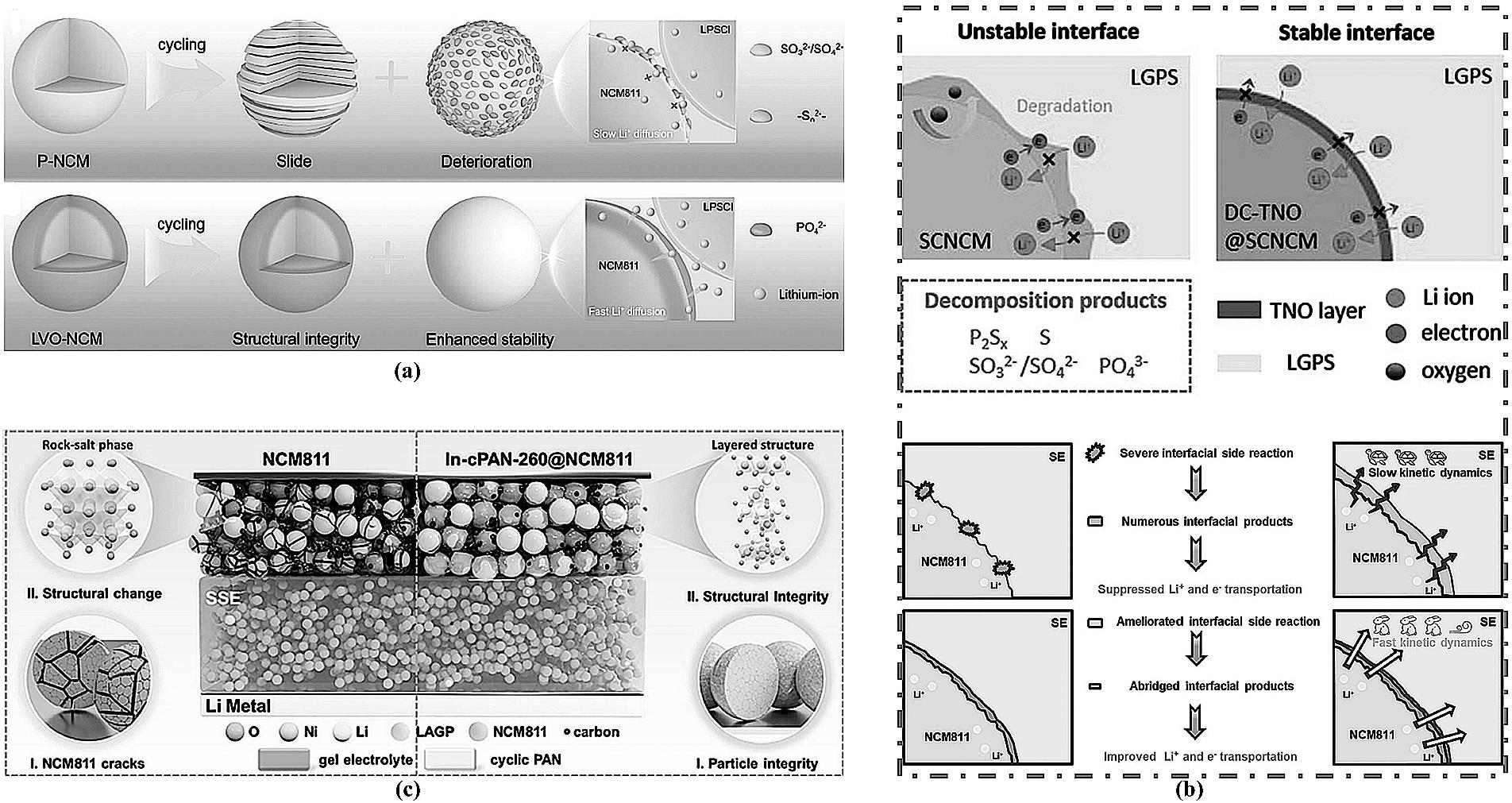
图5 (a) P-NCM 和LVO-NCM在循环过程中的结构演变示意图[36];(b) SCNCM的界面失效机制示意图和 DC-TNO@SCNCM 的协同改性功能机制示意图[40];(c) 多尺度Li||NCM811和Li||In-cPAN@NCM811固态电池模型示意图[41]。
Fig.5 (a) Schematic diagram of the structural evolution of P-NCM and LVO-NCM during cycling[36]; (b) Schematic diagram of the interfacial failure mechanism of SCNCM and the synergistic modification mechanism of DC-TNO@SCNCM[40]; (c) Schematic diagram of multi-scale models for Li||NCM811 and Li||In-cPAN@NCM811 all-solid-state batteries[41].
| 电解质改性策略 | 核心机理 | 优点 | 局限性 | 代表性文章 |
|---|---|---|---|---|
| 单元素掺杂 | 在电解质晶格内部中引入不同的单种元素,改变晶格体积和化学环境,构建富氟界面,增强空气/水稳定性等 | 工艺路线相对简单,易与现有球磨/固相合成放大;可以在保持较高离子电导率的前提下同时提高界面稳定性和空气稳定性;适合针对特定失效机理进行“定向设计” | 掺杂量和占位(阳/阴离子位)存在较窄窗口;含Ag等贵/稀元素时成本和资源可持续性受到限制;掺杂多为体相调控,对高度不均匀的界面/颗粒接触问题缓解有限 | Ref. 45–48 |
| 多元素掺杂 | 引入多种不同种元素进入电解质晶格,调整电解质晶格结构,协同提高电解质电导率、稳定性等 | 多种元素可分工协作,实现导电率、界面稳定性、空气稳定性和机械性能的多目标优化 | 双掺杂的掺杂量比例优化困难 | Ref. 49–55 |
| 无机物表面包覆 | 在硫化物电解质颗粒表面沉积无机薄层,构建电子绝缘/离子导电的界面缓冲层 | 不改变电解质主体相结构,对体相σ影响小;可在成膜之后整体包覆,兼容多种硫化物体系;适配高压 | 工艺复杂度和成本较高;涂层厚度与均匀性窗口窄;部分氧化物/氟化物涂层本身与硫化物存在热力学反应风险 | Ref. 56 Ref. 58 |
| 有机物表面包覆 | 利用有机分子在电解质表面形成致密吸附层,外侧提供疏水有机链段,内侧与硫化物牢固结合;同时可在分子结构中引入含Li+亲和基团,保证界面附近的离子传导能力 | 可显著改善硫化物在潮湿空气中的稳定性;有机层柔性好,能够缓冲压实及循环过程中的应力集中;制备工艺适合复杂形状与大面积电解质膜 | 需要选择与硫化物化学惰性的溶剂和前驱体;有机层本身的电化学窗口相对有限,在高电压/高温下可能分解;若亲离子基团设计不当,会明显降低界面Li+传导 | Ref. 57 |
| 聚合物共混 | 构建“硫化物刚性骨架+聚合物柔性网络”的双连续结构:硫化物提供高速Li+传导主通道,聚合物填充孔隙、提高膜的柔性和韧性,减小界面接触电阻 | 兼具较高σ(10-3–10-4 S·cm-1)与优异机械顺应性,易实现自由支撑薄膜,适配卷绕/叠片工艺 | 制备工艺中所使用溶剂等应与硫化物电解质相适宜;聚合物需要对正负极及电解质稳定,以获得稳定界面 | Ref. 59-60 |
表3 电解质改性策略、机理及优缺点
Table 3 Electrolyte Modification Strategies, Mechanisms, and Advantages/Disadvantages
| 电解质改性策略 | 核心机理 | 优点 | 局限性 | 代表性文章 |
|---|---|---|---|---|
| 单元素掺杂 | 在电解质晶格内部中引入不同的单种元素,改变晶格体积和化学环境,构建富氟界面,增强空气/水稳定性等 | 工艺路线相对简单,易与现有球磨/固相合成放大;可以在保持较高离子电导率的前提下同时提高界面稳定性和空气稳定性;适合针对特定失效机理进行“定向设计” | 掺杂量和占位(阳/阴离子位)存在较窄窗口;含Ag等贵/稀元素时成本和资源可持续性受到限制;掺杂多为体相调控,对高度不均匀的界面/颗粒接触问题缓解有限 | Ref. 45–48 |
| 多元素掺杂 | 引入多种不同种元素进入电解质晶格,调整电解质晶格结构,协同提高电解质电导率、稳定性等 | 多种元素可分工协作,实现导电率、界面稳定性、空气稳定性和机械性能的多目标优化 | 双掺杂的掺杂量比例优化困难 | Ref. 49–55 |
| 无机物表面包覆 | 在硫化物电解质颗粒表面沉积无机薄层,构建电子绝缘/离子导电的界面缓冲层 | 不改变电解质主体相结构,对体相σ影响小;可在成膜之后整体包覆,兼容多种硫化物体系;适配高压 | 工艺复杂度和成本较高;涂层厚度与均匀性窗口窄;部分氧化物/氟化物涂层本身与硫化物存在热力学反应风险 | Ref. 56 Ref. 58 |
| 有机物表面包覆 | 利用有机分子在电解质表面形成致密吸附层,外侧提供疏水有机链段,内侧与硫化物牢固结合;同时可在分子结构中引入含Li+亲和基团,保证界面附近的离子传导能力 | 可显著改善硫化物在潮湿空气中的稳定性;有机层柔性好,能够缓冲压实及循环过程中的应力集中;制备工艺适合复杂形状与大面积电解质膜 | 需要选择与硫化物化学惰性的溶剂和前驱体;有机层本身的电化学窗口相对有限,在高电压/高温下可能分解;若亲离子基团设计不当,会明显降低界面Li+传导 | Ref. 57 |
| 聚合物共混 | 构建“硫化物刚性骨架+聚合物柔性网络”的双连续结构:硫化物提供高速Li+传导主通道,聚合物填充孔隙、提高膜的柔性和韧性,减小界面接触电阻 | 兼具较高σ(10-3–10-4 S·cm-1)与优异机械顺应性,易实现自由支撑薄膜,适配卷绕/叠片工艺 | 制备工艺中所使用溶剂等应与硫化物电解质相适宜;聚合物需要对正负极及电解质稳定,以获得稳定界面 | Ref. 59-60 |
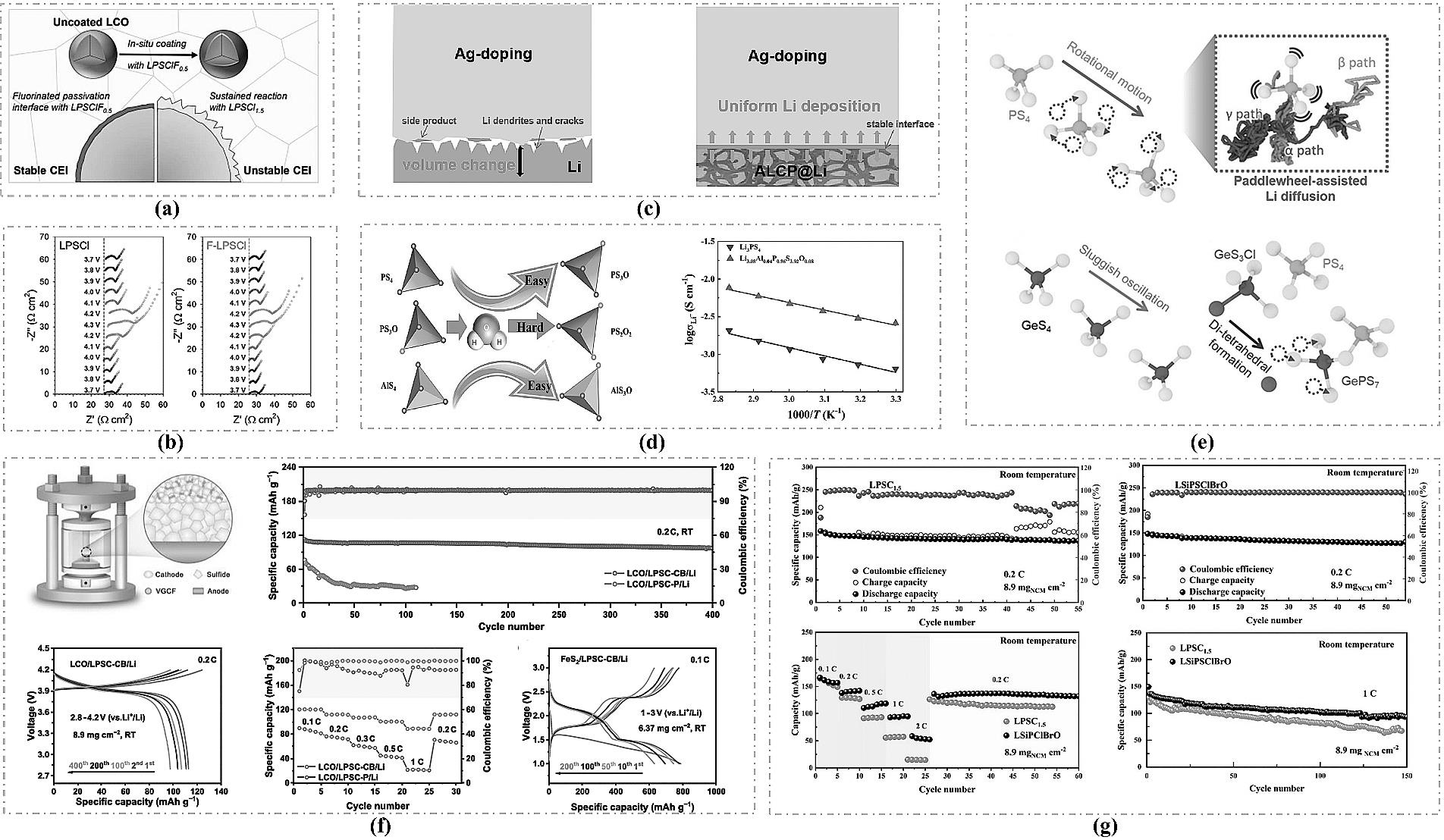
图6 (a) LiF替代LiCl改性LSPCl的机理示意图[45]以及(b)阻抗图[46];(c) Ag掺杂的LPSCl示意图[48];(d) Li2AlO2改性硫化物电解质的机理与性能图[49];(e) Ge、Cl双掺杂的LGPS提升离子跃迁能力[50];(f) Li5.8P0.9Cu0.1S4.5Cl1.3Br0.2全固态电池性能[51];(g) Li-In/ Li5.55Si0.05P0.95S4.4Cl0.75Br0.75O0.1/NCM811@LNO全电池性能[52]。
Fig.6 (a) Schematic diagram of the mechanism for modifying LSPCl with LiF as a substitute for LiCl[45]and (b) the corresponding impedance plot[46]; (c) Schematic diagram of Ag-doped LPSCl[48]; (d) Mechanism and performance plots of Li2AlO2-modified sulfide electrolyte[49]; (e) Enhanced ion migration capability achieved by co-doping LGPS with Ge and Cl[50]; (f) Performance of the Li5.8P0.9Cu0.1S4.5Cl1.3Br0.2 all-solid-state battery[51]; (g) Performance of the Li-In/ Li5.55Si0.05P0.95S4.4Cl0.75Br0.75O0.1/NCM811@LNO full cell[52].
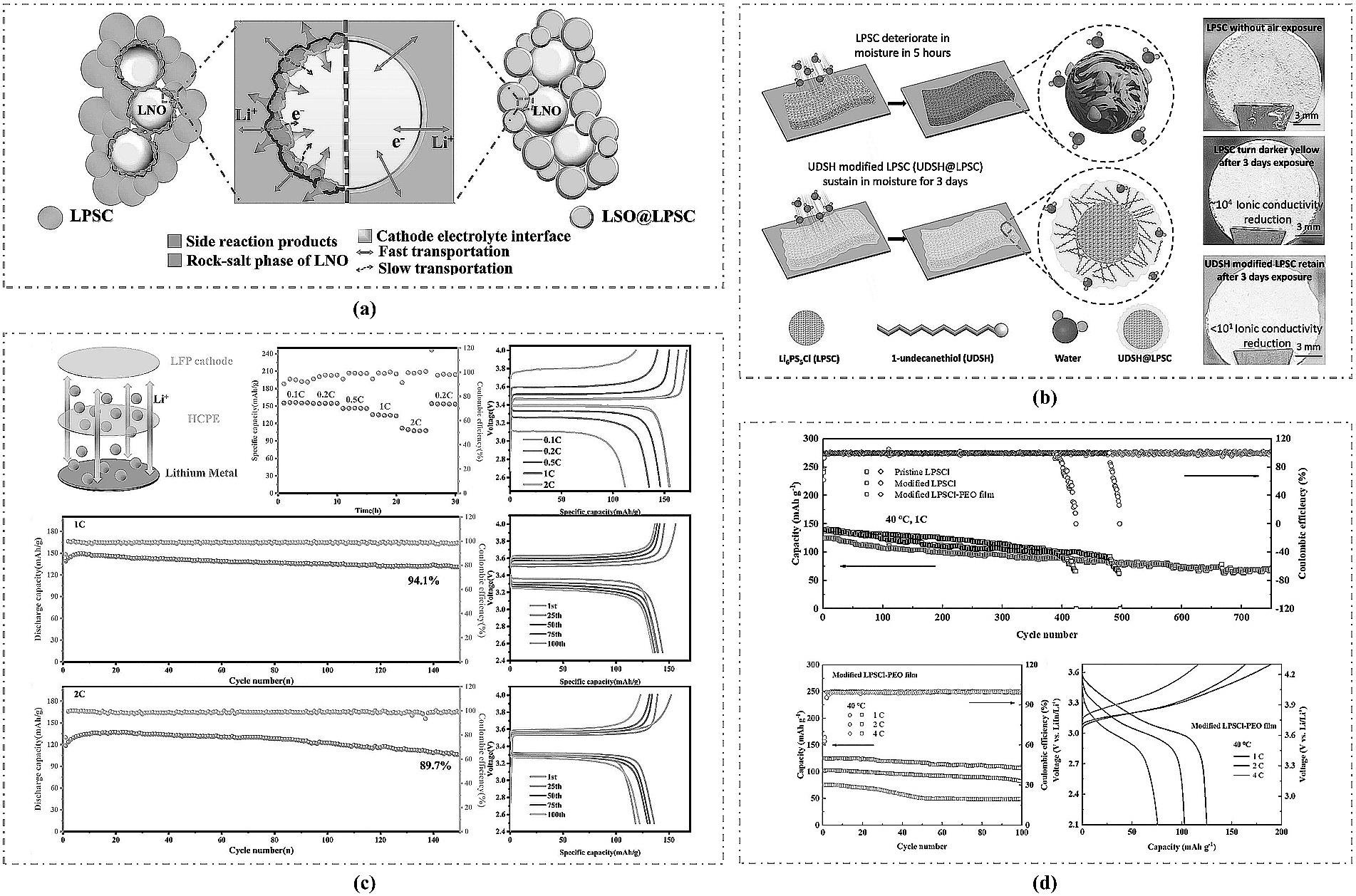
图7 (a) LSO保护层作用机制图[56];(b) UDSH@LPSC复合电解质制备工艺图[57];(c) HCPE电解质体系全固态电池性能[59];(d) 改性LPSCl-PEO与未改性LPSCl电解质全固态电池性能对比[60]。
Fig.7 (a) Mechanism diagram of the protective layer of LSO[56]; (b) Preparation process diagram of the UDSH@LPSC composite electrolyte[57]; (c) Performance of the all-solid-state battery using the HCPE electrolyte system[59]; (d) Performance comparison of all-solid-state batteries using modified LPSCl-PEO versus unmodified LPSCl electrolyte[60].
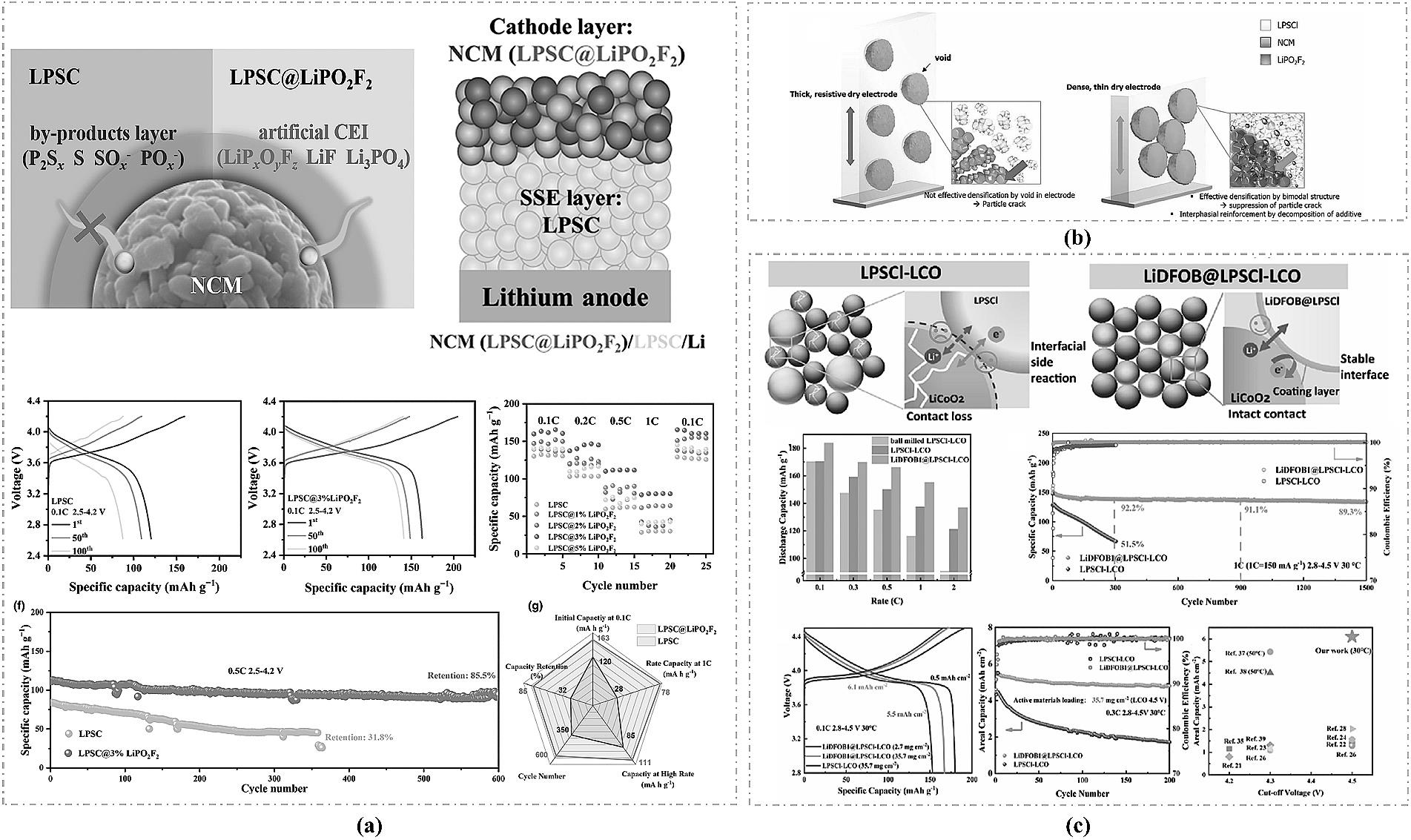
图8 (a) LiPO2F2在正极侧的作用机制及全固态电池性能[65];(b) 掺杂LiPO2F2提升电极的致密性[66];(c) LiDFOB掺杂改性正极的作用机制以及全固态电池性能[67]。
Fig.8 (a) Mechanism of LiPO2F2 at the cathode interface and the corresponding all-solid-state battery performance[65]; (b) Enhanced electrode compactness through LiPO2F2 doping[66]; (c) Modification mechanism of LiDFOB doping on the cathode and the performance of the all-solid-state battery[67].
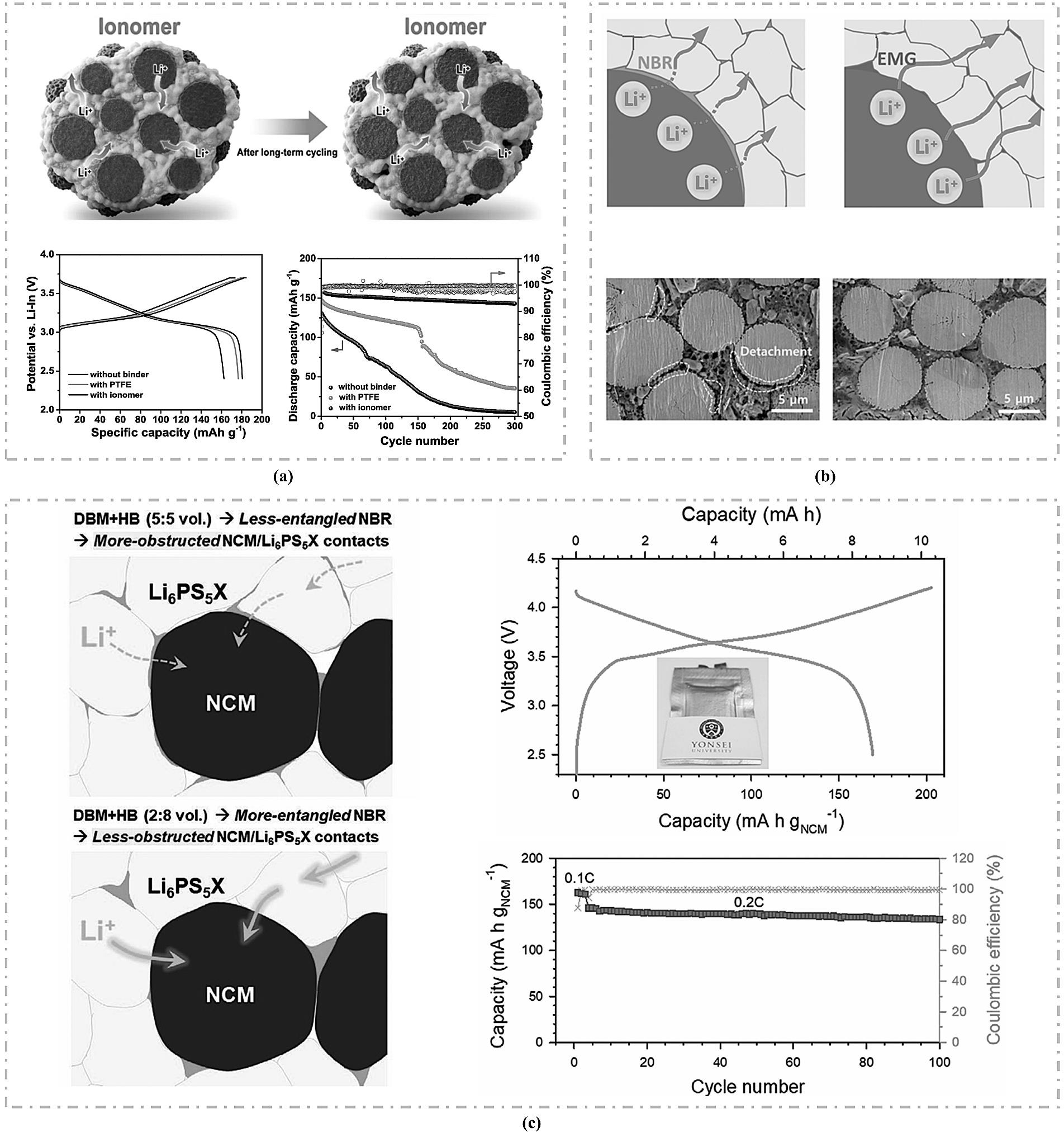
图9 (a) 导电粘结剂PPC-ICP提升电极离子传输示意图及全固态电池性能[71];(b) 共溶NBR和LiTFSI粘结剂提升电极离子传输示意图及全固态电池性能[72];(c) 粘结剂提升电极离子传输及致密性[73]。
Fig.9 (a) Schematic diagram of the conductive binder PPC-ICP enhancing ion transport in the electrode and the corresponding all-solid-state battery performance[71]; (b) Schematic diagram of the co-dissolved NBR and LiTFSI binder improving electrode ion transport and the corresponding all-solid-state battery performance[72]; (c) Enhancement of electrode ion transport and compactness by the binder[73].
| 结构工程方向 | 针对问题 | 核心机理 | 实现效果 | 代表性文章 |
|---|---|---|---|---|
| 活性材料粒径调控 | 厚电极下活性物质未充分活化、CAM-SE接触失效 | 将CAM粒径与SE粒径匹配,在高体积分数CAM条件下形成离子连续相,减小电子输运距离,实现离子/电子双渗流 | 通过调控NCM和LPS颗粒尺寸并改变CAM含量,在70%CAM含量下实现150 mAh/g初始放电比容量 | Ref. 77-78 |
| 活性材料形貌工程 | 循环过程中产生应力集中,二次颗粒易开裂 | 通过采用小尺寸单晶或定向柱状一次颗粒,缩短Li+扩散路径,减少晶界阻力和应力集中 | 通过使用小尺寸单晶 NCM811,在35.67 mg/cm2 的高质量负载下,在 2.72–4.4 V 电压范围内循环500次后稳定性为100% | Ref. 63 Ref. 79-80 |
| 固态电解质粒径/级配 | 离子通道中断、离子迂曲度高,厚电极极化严重 | 使用更小粒径甚至分级SE填充CAM颗粒间的孔隙,降低离子路径的迂曲度 τSE,提升有效离子电导σieff | 通过双步研磨工艺控制电解质粒径,在0.6 C的倍率下,初始放电容量可达179 mAh/g,过电势60 mV | Ref. 16 Ref. 81-83 |
| 导电剂与导电网络设计 | 低碳含量下复合正极内部电子网络不连续 | 通过控制结晶度、采用合适导电剂碳材料搭建长程电子通路,实现电子渗流 | 室温下,使用rGO的全固态电池循环100圈容量保持率为97%,库仑效率为99.8%,电池在60 °C,1 C下循环1000圈容量保持率≈100% | Ref. 87-89 |
| 导电剂界面层设计 | 传统碳材料触发严重的电解质界面副反应,导致阻抗上升 | 在导电剂表面引入导电聚合物,提供电子通路,阻隔碳与电解质的直接接触,抑制副反应 | 将PEDOT半导体界面层引入导电剂表面,在1 C下仍可输出超过100 mAh/g(较未改性提升约10倍) | Ref. 90 |
| 组分配比/结构设计 | 高CAM体积分数、厚电极下离子/电子通路失衡, | 通过传输线模型、电阻网络、梯度设计等方法寻找使 σᵢᵉᶠᶠ 与 σₑᵉᶠᶠ 相当的CAM/SE/碳体积分数窗口,避免单一通道成为瓶颈 | 通过纵向梯度分层设计的电池在3.22 mA/cm2下放电比容量为49±10 mAh/g,显著优于 等组分电极 | Ref. 91-96 |
| 堆压–微结构协同与力学稳定性设计 | 堆叠压力与实际工况矛盾 | 通过多物理场建模以及实验验证,研究复合正极中堆压对微结构、接触面积、反应分布的影响,提出“临界堆压”窗口 | 在理论“临界堆压”17 MPa下,单晶NMC532在0.1 C下初始放电容量为146.94 mAh/g,接近理论容量 | Ref.97 |
表4 结构工程改性机理及实现效果
Table 4 Modification Mechanisms and Implementation Effects of Structural Engineering
| 结构工程方向 | 针对问题 | 核心机理 | 实现效果 | 代表性文章 |
|---|---|---|---|---|
| 活性材料粒径调控 | 厚电极下活性物质未充分活化、CAM-SE接触失效 | 将CAM粒径与SE粒径匹配,在高体积分数CAM条件下形成离子连续相,减小电子输运距离,实现离子/电子双渗流 | 通过调控NCM和LPS颗粒尺寸并改变CAM含量,在70%CAM含量下实现150 mAh/g初始放电比容量 | Ref. 77-78 |
| 活性材料形貌工程 | 循环过程中产生应力集中,二次颗粒易开裂 | 通过采用小尺寸单晶或定向柱状一次颗粒,缩短Li+扩散路径,减少晶界阻力和应力集中 | 通过使用小尺寸单晶 NCM811,在35.67 mg/cm2 的高质量负载下,在 2.72–4.4 V 电压范围内循环500次后稳定性为100% | Ref. 63 Ref. 79-80 |
| 固态电解质粒径/级配 | 离子通道中断、离子迂曲度高,厚电极极化严重 | 使用更小粒径甚至分级SE填充CAM颗粒间的孔隙,降低离子路径的迂曲度 τSE,提升有效离子电导σieff | 通过双步研磨工艺控制电解质粒径,在0.6 C的倍率下,初始放电容量可达179 mAh/g,过电势60 mV | Ref. 16 Ref. 81-83 |
| 导电剂与导电网络设计 | 低碳含量下复合正极内部电子网络不连续 | 通过控制结晶度、采用合适导电剂碳材料搭建长程电子通路,实现电子渗流 | 室温下,使用rGO的全固态电池循环100圈容量保持率为97%,库仑效率为99.8%,电池在60 °C,1 C下循环1000圈容量保持率≈100% | Ref. 87-89 |
| 导电剂界面层设计 | 传统碳材料触发严重的电解质界面副反应,导致阻抗上升 | 在导电剂表面引入导电聚合物,提供电子通路,阻隔碳与电解质的直接接触,抑制副反应 | 将PEDOT半导体界面层引入导电剂表面,在1 C下仍可输出超过100 mAh/g(较未改性提升约10倍) | Ref. 90 |
| 组分配比/结构设计 | 高CAM体积分数、厚电极下离子/电子通路失衡, | 通过传输线模型、电阻网络、梯度设计等方法寻找使 σᵢᵉᶠᶠ 与 σₑᵉᶠᶠ 相当的CAM/SE/碳体积分数窗口,避免单一通道成为瓶颈 | 通过纵向梯度分层设计的电池在3.22 mA/cm2下放电比容量为49±10 mAh/g,显著优于 等组分电极 | Ref. 91-96 |
| 堆压–微结构协同与力学稳定性设计 | 堆叠压力与实际工况矛盾 | 通过多物理场建模以及实验验证,研究复合正极中堆压对微结构、接触面积、反应分布的影响,提出“临界堆压”窗口 | 在理论“临界堆压”17 MPa下,单晶NMC532在0.1 C下初始放电容量为146.94 mAh/g,接近理论容量 | Ref.97 |
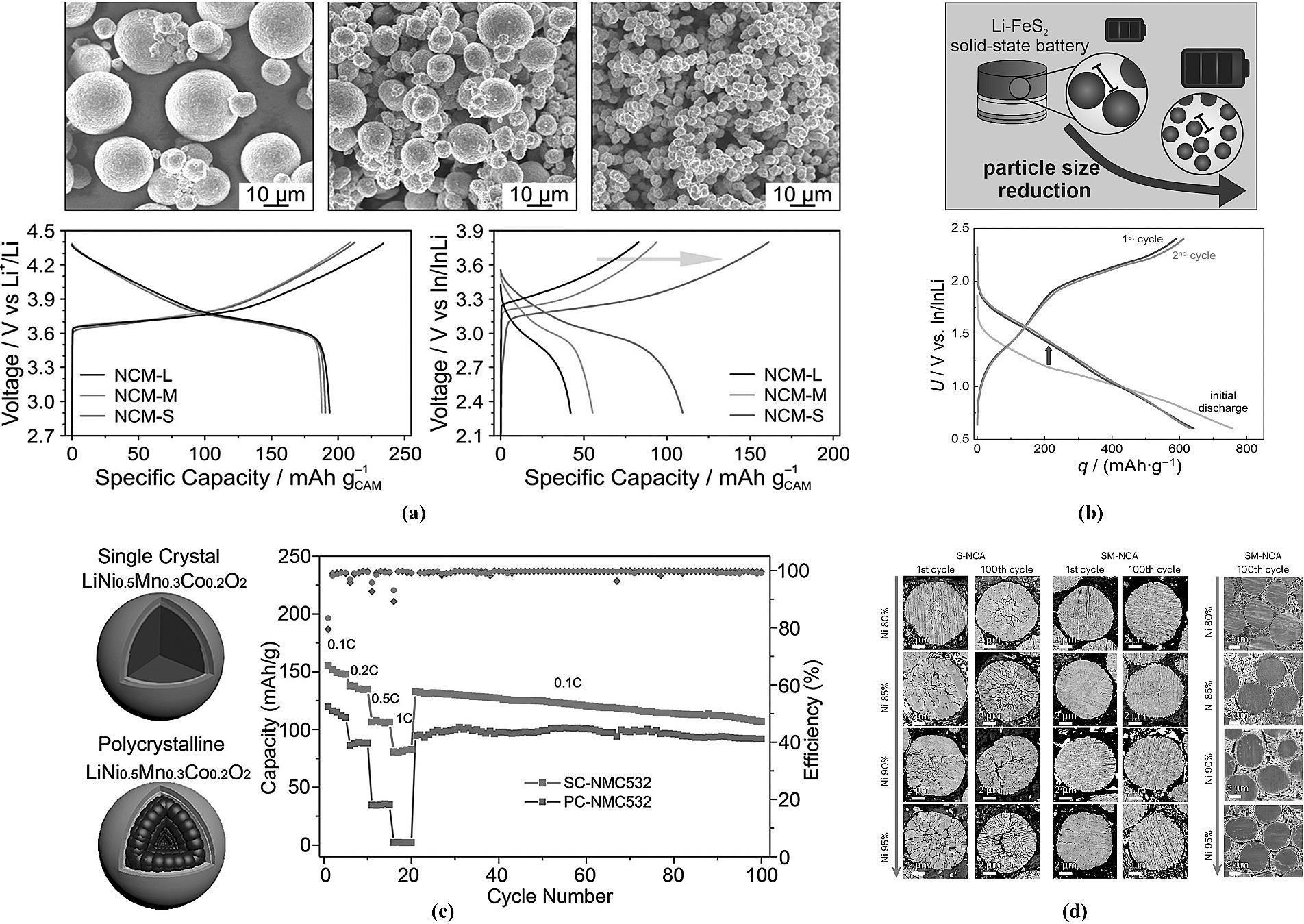
图10 (a) 不同粒径NCM622的全固态电池性能[77];(b) 不同FeS2粒径对全固态电池性能的影响[78];(c) 单晶和多晶NCM532体系全固态电池性能[79];(d) S-NCA和SM-NCA在100圈循环后的SEM图[63]。
Fig.10 (a) Performance of all-solid-state batteries using NCM622 with different particle sizes[77]; (b) Effect of FeS2 particle size on the performance of all-solid-state batteries[78]; (c) Performance comparison of all-solid-state batteries based on single-crystal and polycrystalline NCM532 systems[79]; (d) SEM images of S-NCA and SM-NCA after 100 cycles[63].
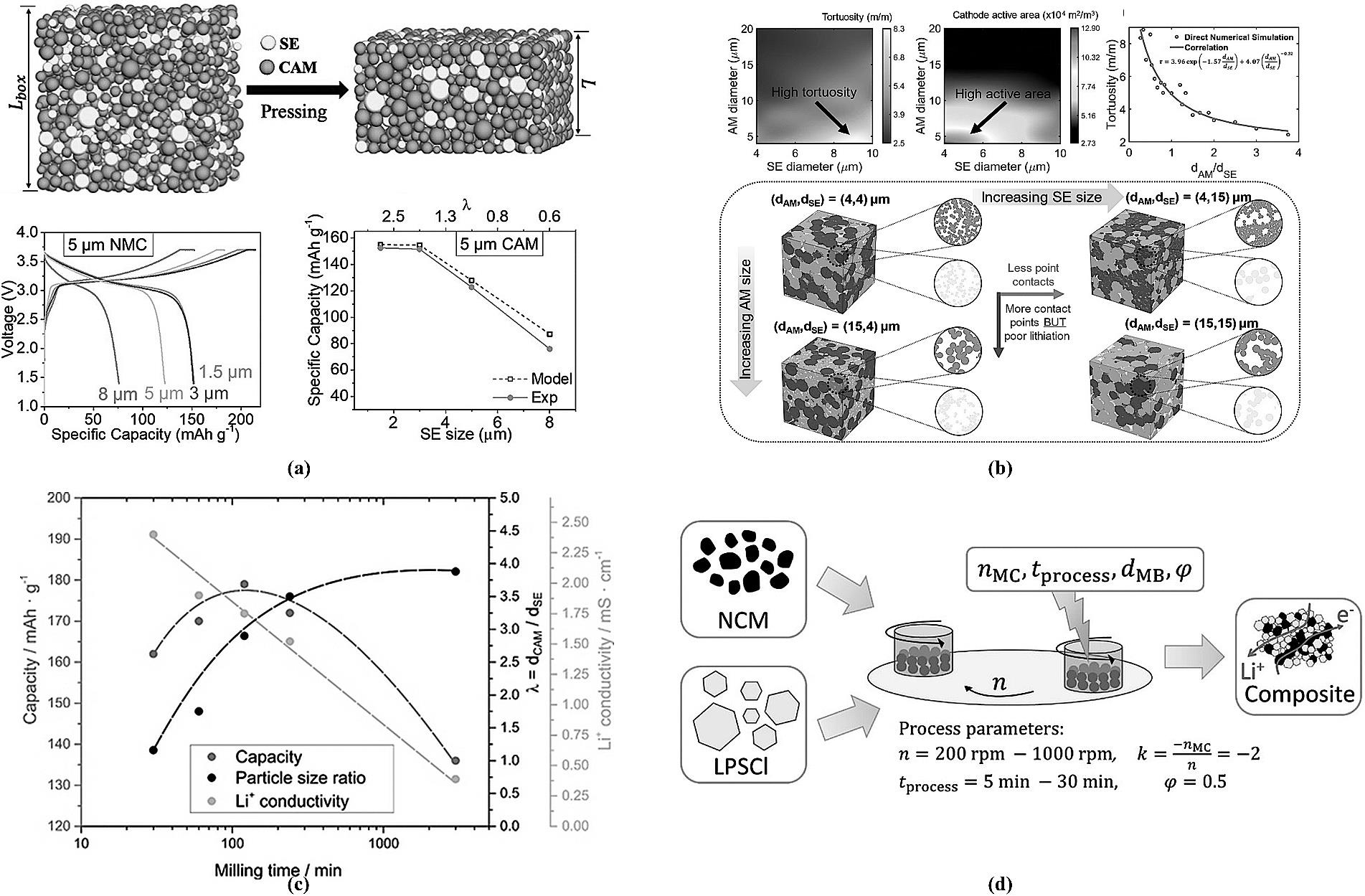
图11 (a) 5 μm NCM匹配不同粒径电解质的性能[16];(b) dAM/dSE粒径匹配影响参数[81];(c) dCAM/dSE与电池放电容量、电导率关系图[82];(d) 高能球磨参数对电极致密度的影响[83]。
Fig.11 (a) Performance of 5μm NCM paired with solid-state electrolytes of different particle sizes[16]; (b) Parameters showing the influence of dAM/dSE particle size matching[81]; (c) Relationship between dCAM/dSE and battery discharge capacity/conductivity[82]; (d) Effect of high-energy ball milling parameters on electrode density[83].
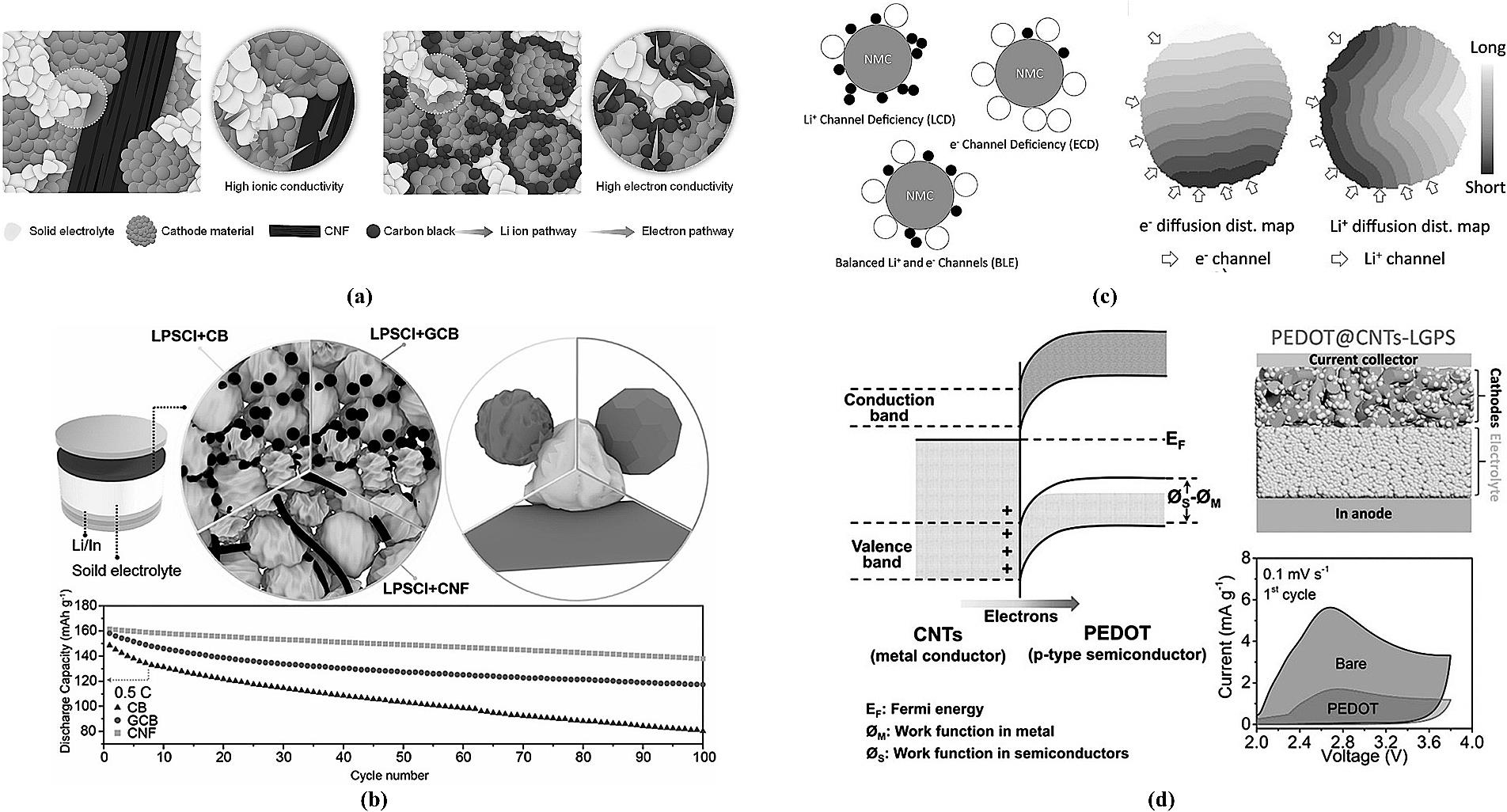
图12 (a) 不同导电剂混合电极结构示意图[86];(b) 不同导电剂电极内部示意图及全固态电池性能对比[87];(c) 电子/离子传输连续性示意图及退化分析图[89];(d) 半导体PEDOT界面层降低界面阻抗示意图[90]。
Fig.12 (a) Schematic diagram of composite electrode structures with different conductive agents[86]; (b) Internal schematic of electrodes with different conductive agents and corresponding all-solid-state battery performance comparison[87]; (c) Schematic of electron/ion transport continuity and corresponding degradation analysis[89]; (d) Schematic illustrating the reduction of interfacial resistance by a semiconducting PEDOT interlayer[90].
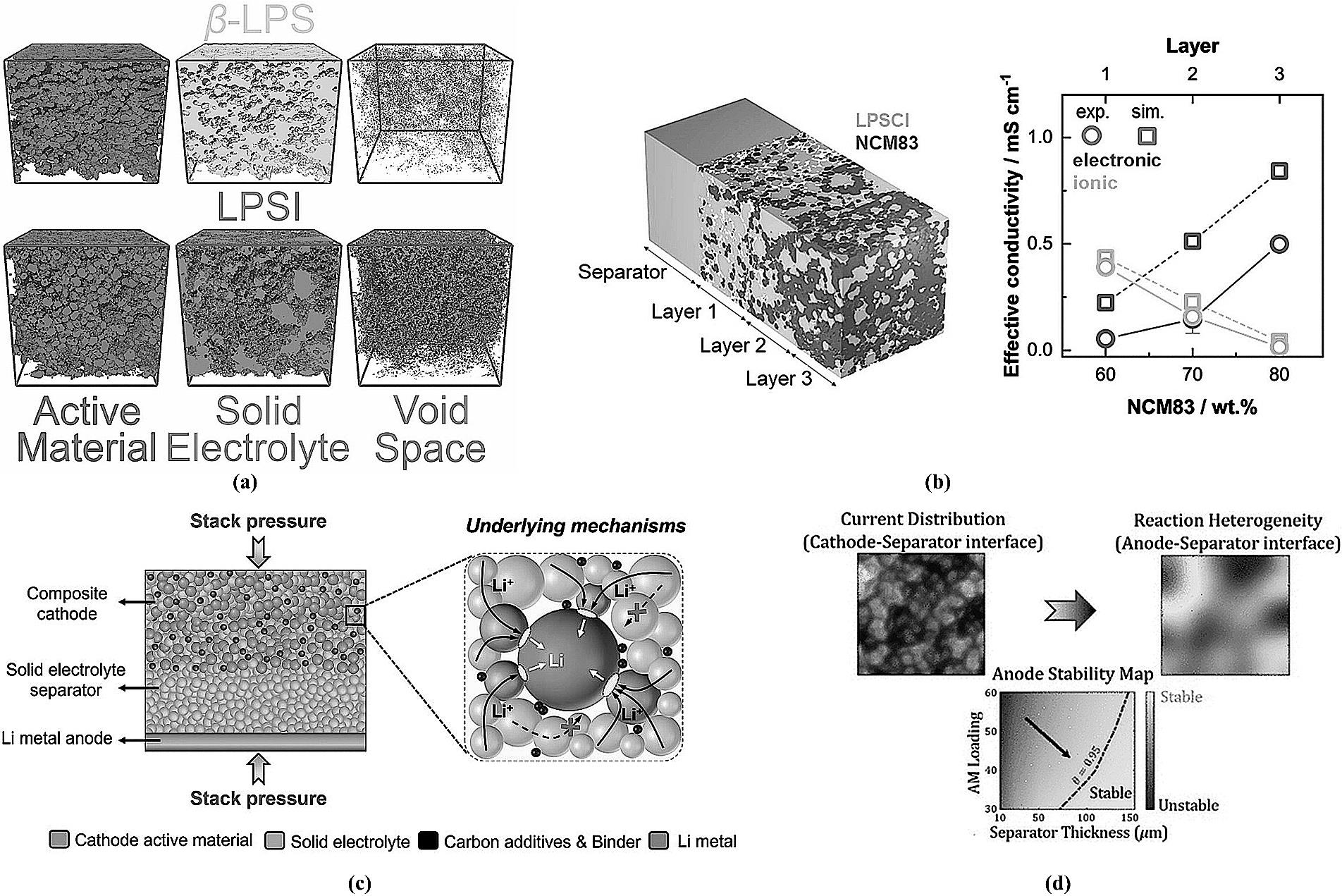
图14 (a) 混合电极内部各组分分布图[95];(b) 三层梯度示意图及组分含量与性能关系[96];(c) 临界堆压对电池内部影响示意图[97];(d) 隔膜厚度-正极架构的协同路线示意图[98]。
Fig.14 (a) Distribution of components within the composite electrode[95]; (b) Schematic of the three-layer gradient structure and the relationship between component content and performance[96]; (c) Schematic illustrating the effect of critical stack pressure on the battery interior[97]; (d) Schematic of the synergistic approach between separator thickness and cathode architecture[98].
| [1] | Xia S X, Wu X S, Zhang Z C, et al. Practical challenges and future perspectives of all-solid-state lithium-metal batteries[J]. Chem, 2019, 5(4): 753-785.[LinkOut] |
| [2] | Wang Q D, Zhao C L, Yao Z P, et al. Entropy-driven liquid electrolytes for lithium batteries[J]. Advanced Materials, 2023, 35(17): 2210677.[LinkOut] |
| [3] | Zhu G R, Zhang Q, Liu Q S, et al. Non-flammable solvent-free liquid polymer electrolyte for lithium metal batteries[J]. Nature Communications, 2023, 14: 4617.[LinkOut] |
| [4] | Liang Z T, Xiao Y, Wang K J, et al. Enabling stable and high areal capacity solid state battery with Ni-rich cathode via failure mechanism study[J]. Energy Storage Materials, 2023, 63: 102987.[LinkOut] |
| [5] | Deng S X, Sun Q, Li M S, et al. Insight into cathode surface to boost the performance of solid-state batteries[J]. Energy Storage Materials, 2021, 35: 661-668.[LinkOut] |
| [6] | Nikodimos Y, Huang C J, Taklu B W, et al. Chemical stability of sulfide solid-state electrolytes: stability toward humid air and compatibility with solvents and binders[J]. Energy & Environmental Science, 2022, 15(3): 991-1033.[LinkOut] |
| [7] | Zhang J X, Fu J M, Lu P S, et al. Challenges and strategies of low-pressure all-solid-state batteries[J]. Advanced Materials, 2025, 37(6): 2413499.[LinkOut] |
| [8] | Huo S D, Sheng L, Xue W D, et al. Challenges of stable ion pathways in cathode electrode for all-solid-state lithium batteries: a review[J]. Advanced Energy Materials, 2023, 13(15): 2204343.[LinkOut] |
| [9] | Liang J W, Zhu Y M, Li X N, et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries[J]. Nature Communications, 2023, 14: 146.[LinkOut] |
| [10] | Chen Y, Huang L, Zhou D L, et al. Elucidating and minimizing the space-charge layer effect between NCM cathode and Li6PS5Cl for sulfide-based solid-state lithium batteries[J]. Advanced Energy Materials, 2024, 14(30): 2304443.[LinkOut] |
| [11] | Ren D S, Lu L G, Hua R, et al. Challenges and opportunities of practical sulfide-based all-solid-state batteries[J]. eTransportation, 2023, 18: 100272.[LinkOut] |
| [12] | Wu Y J, Zhang Z Q, Zhang Q G, et al. Industrialization challenges for sulfide-based all solid state battery[J]. eTransportation, 2024, 22: 100371.[LinkOut] |
| [13] | Wan H L, Wang Z Y, Zhang W R, et al. Interface design for all-solid-state lithium batteries[J]. Nature, 2023, 623(7988): 739-744.[LinkOut] |
| [14] | Lai C, Shu C Y, Li W, et al. Stabilizing a lithium metal battery by an in situ Li2S-modified interfacial layer via amorphous-sulfide composite solid electrolyte[J]. Nano Letters, 2020, 20(11): 8273-8281.[LinkOut] |
| [15] | Oh D Y, Nam Y J, Park K H, et al. Excellent compatibility of solvate ionic liquids with sulfide solid electrolytes: toward favorable ionic contacts in bulk-type all-solid-state lithium-ion batteries[J]. Advanced Energy Materials, 2015, 5(22): 1500865.[LinkOut] |
| [16] | Shi T, Tu Q S, Tian Y S, et al. High active material loading in all-solid-state battery electrode via particle size optimization[J]. Advanced Energy Materials, 2020, 10(1): 1902881.[LinkOut] |
| [17] | Kim J Y, Kim J, Kang S H, et al. Efficient cell design and fabrication of concentration-gradient composite electrodes for high-power and high-energy-density all-solid-state batteries[J]. ETRI Journal, 2020, 42(1): 129-137.[LinkOut] |
| [18] | Yang S Y, Shadike Z, Wang W W, et al. An ultrathin solid-state electrolyte film coated on LiNi0.8Co0.1Mn0.1O2 electrode surface for enhanced performance of lithium-ion batteries[J]. Energy Storage Materials, 2022, 45: 1165-1174.[LinkOut] |
| [19] | Ohta N, Takada K, Sakaguchi I, et al. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries[J]. Electrochemistry Communications, 2007, 9(7): 1486-1490.[LinkOut] |
| [20] | Peng L F, Ren H T, Zhang J Z, et al. LiNbO3-coated LiNi0.7Co0.1Mn0.2O2 and chlorine-rich argyrodite enabling high-performance solid-state batteries under different temperatures[J]. Energy Storage Materials, 2021, 43: 53-61.[LinkOut] |
| [21] | Li X L, Jin L B, Song D W, et al. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery[J]. Journal of Energy Chemistry, 2020, 40: 39-45.[LinkOut] |
| [22] | Liu C J, Yi Z C, Wan J X, et al. Nb5+ ions doping and Li3PO4 artificial layer synergetic strategy to strengthen structure, stabilize interface, and promote ions migration for high-performance Ni-rich cathode[J]. Chemical Engineering Journal, 2025, 503: 158633.[LinkOut] |
| [23] | Okada K, Machida N, Naito M, et al. Preparation and electrochemical properties of LiAlO2-coated Li(Ni1/3Mn1/3Co1/3)O2 for all-solid-state batteries[J]. Solid State Ionics, 2014, 255: 120-127.[LinkOut] |
| [24] | Xu Z H, Wang X H, Wang Z Y, et al. Interface problems, modification strategies and prospects of Ni–rich layered oxide cathode materials in all–solid–state lithium batteries with sulfide electrolytes[J]. Journal of Power Sources, 2023, 571: 233079.[LinkOut] |
| [25] | Ruess R, Schweidler S, Hemmelmann H, et al. Influence of NCM particle cracking on kinetics of lithium-ion batteries with liquid or solid electrolyte[J]. Journal of the Electrochemical Society, 2020, 167(10): 100532.[LinkOut] |
| [26] | Jangid M K, Cho T H, Ma T, et al. Eliminating chemo-mechanical degradation of lithium solid-state battery cathodes during >4.5 V cycling using amorphous Nb2O5 coatings[J]. Nature Communications, 2024, 15: 10233.[LinkOut] |
| [27] | Ohta N, Takada K, Zhang L, et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification[J]. Advanced Materials, 2006, 18(17): 2226-2229.[LinkOut] |
| [28] | Sakuda A, Kitaura H, Hayashi A, et al. All-solid-state lithium secondary batteries with oxide-coated LiCoO2 electrode and Li2S–P2S5 electrolyte[J]. Journal of Power Sources, 2009, 189(1): 527-530.[LinkOut] |
| [29] | Mao J J, Iocozzia J, Huang J Y, et al. Graphene aerogels for efficient energy storage and conversion[J]. Energy & Environmental Science, 2018, 11(4): 772-799.[LinkOut] |
| [30] | Seino Y, Ota T, Takada K. High rate capabilities of all-solid-state lithium secondary batteries using Li4Ti5O12-coated LiNi0.8Co0.15Al0.05O2 and a sulfide-based solid electrolyte[J]. Journal of Power Sources, 2011, 196(15): 6488-6492.[LinkOut] |
| [31] | Ito S, Fujiki S, Yamada T, et al. A rocking chair type all-solid-state lithium ion battery adopting Li2O–ZrO2 coated LiNi0.8Co0.15Al0.05O2 and a sulfide based electrolyte[J]. Journal of Power Sources, 2014, 248: 943-950.[LinkOut] |
| [32] | Visbal H, Fujiki S, Aihara Y, et al. The influence of the carbonate species on LiNi0.8Co0.15Al0.05O2 surfaces for all-solid-state lithium ion battery performance[J]. Journal of Power Sources, 2014, 269: 396-402.[LinkOut] |
| [33] | Visbal H, Aihara Y, Ito S, et al. The effect of diamond-like carbon coating on LiNi0.8Co0.15Al0.05O2 particles for all solid-state lithium-ion batteries based on Li2S–P2S5 glass-ceramics[J]. Journal of Power Sources, 2016, 314: 85-92.[LinkOut] |
| [34] | Shi J, Ma Z H, Han K, et al. Coupling novel Li7TaO6 surface buffering with bulk Ta-doping to achieve long-life sulfide-based all-solid-state lithium batteries[J]. Journal of Materials Chemistry A, 2022, 10(40): 21336-21348.[LinkOut] |
| [35] | Liu Y K, Yu T, Xu S, et al. Constructing an oxyhalide interface for 4.8 V-tolerant high-nickel cathodes in all-solid-state lithium-ion batteries[J]. Angewandte Chemie, 2024, 136(33): e202403617.[LinkOut] |
| [36] | Li Y Y, Li J W, Zeng Z, et al. Surface-reconstructed high-nickel cathodes for ultrastable 4.5 V tolerant sulfide-based all-solid-state batteries[J]. ACS Energy Letters, 2025, 10(5): 2203-2211.[LinkOut] |
| [37] | He W, Ahmad N, Sun S R, et al. Microscopic segregation dominated nano-interlayer boosts 4.5 V cyclability and rate performance for sulfide-based all-solid-state lithium batteries[J]. Advanced Energy Materials, 2023, 13(3): 2203703.[LinkOut] |
| [38] | Zhou X, Chang C Y, Yu D F, et al. Li2ZrF6 protective layer enabled high-voltage LiCoO2 positive electrode in sulfide all-solid-state batteries[J]. Nature Communications, 2025, 16: 112.[LinkOut] |
| [39] | Li W R, Zhang S, Zheng W J, et al. Self-polarized organic–inorganic hybrid ferroelectric cathode coatings assisted high performance all-solid-state lithium battery[J]. Advanced Functional Materials, 2023, 33(27): 2300791.[LinkOut] |
| [40] | Sun N, Song Y J, Liu Q S, et al. Surface-to-bulk synergistic modification of single crystal cathode enables stable cycling of sulfide-based all-solid-state batteries at 4.4 V[J]. Advanced Energy Materials, 2022, 12(29): 2200682.[LinkOut] |
| [41] | Zheng J Y, Jiang H L, Xu X Y, et al. In situ partial-cyclized polymerized acrylonitrile-coated NCM811 cathode for high-Temperature ≥ 100℃ stable solid-state lithium metal batteries[J]. Nano-Micro Letters, 2025, 17(1): 195.[LinkOut] |
| [42] | Schwietert T K, Arszelewska V A, Wang C, et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes[J]. Nature Materials, 2020, 19(4): 428-435.[LinkOut] |
| [43] | Binninger T, Marcolongo A, Mottet M, et al. Comparison of computational methods for the electrochemical stability window of solid-state electrolyte materials[J]. Journal of Materials Chemistry A, 2020, 8(3): 1347-1359.[LinkOut] |
| [44] | Zhu Y Z, He X F, Mo Y F. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations[J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23685-23693.[LinkOut] |
| [45] | Dong C, Bi Z H, Li R, et al. Fluorine-doped argyrodite sulfide electrolyte enables commercial LiCoO2 use for 4.6 V high-voltage all-solid-state batteries[J]. National Science Review, 2025, 12(7): nwaf217.[LinkOut] |
| [46] | Kim K T, Kim J S, Baeck K H, et al. Surface fluorination shielding of sulfide solid electrolytes for enhanced electrochemical stability in all-solid-state batteries[J]. Advanced Materials, 2025, 37(35): 2416816.[LinkOut] |
| [47] | Luan Z Y, Ren P F, Wang X F, et al. Nitrogen doped sulfide solid electrolytes with enhanced air stability and lithium metal compatibility[J]. International Journal of Applied Ceramic Technology, 2024, 21(5): 3370-3377.[LinkOut] |
| [48] | Wu Z K, Yu C, Wei C C, et al. Ag-modification argyrodite electrolytes enable high-performance for all-solid-state lithium metal batteries[J]. Chemical Engineering Journal, 2023, 466: 143304.[LinkOut] |
| [49] | Yu P W, Ahmad N, Yang J, et al. Dual-doping for enhancing chemical stability of functional anionic units in sulfide for high-performance all-solid-state lithium batteries[J]. Journal of Energy Chemistry, 2023, 86: 382-390.[LinkOut] |
| [50] | Choi Y S, Jeong J, Lee Y, et al. Li-ion transport mechanisms in Ge/Cl dual-doped Li10GeP2S12 solid electrolytes: Synergistic insights from experimental structural characterization and machine-learning-assisted atomistic modeling[J]. Carbon Energy, 2024, 6(10): e594.[LinkOut] |
| [51] | Li Y, Wu G, Fan X M, et al. Engineering high-performance argyrodite sulfide electrolytes via metal halide doping for all-solid-state lithium metal batteries[J]. Energy Storage Materials, 2025, 77: 104221.[LinkOut] |
| [52] | Cai C L, Zhu K J, Rao Y, et al. Ternary doping enhances the moisture and electrochemical stability of Li5.5PS4.5Cl1.5 solid-state electrolyte[J]. Journal of Materials Science: Materials in Electronics, 2025, 36(9): 545.[LinkOut] |
| [53] | Lee Y, Jeong J, Lee H J, et al. Lithium argyrodite sulfide electrolytes with high ionic conductivity and air stability for all-solid-state Li-ion batteries[J]. ACS Energy Letters, 2022, 7(1): 171-179.[LinkOut] |
| [54] | Zeng K, Li X B, Zhao C, et al. Experimental and theoretical insights into synergistic Sn-N co-doping enhancing air stability and interfacial compatibility of Li5.5PS4.5Cl1.5 electrolytes for all-solid-state lithium metal batteries[J]. Small, 2025, 21(39): e07465.[LinkOut] |
| [55] | Jiang Z L, Yang J, Liu C, et al. Insights on Bi-O dual-doped Li5.5PS4.5Cl1.5 electrolyte with enhanced electrochemical properties for all-solid-state lithium metal batteries[J]. Nano Energy, 2024, 128: 109926.[LinkOut] |
| [56] | He Z Y, Yang W J, Shi Y, et al. Enhanced electrochemical stability of sulfide electrolytes with surface modification for high-performance LiNiO₂ based all-solid-state lithium batteries[J]. Small, 2025, 21(30): 2503053.[LinkOut] |
| [57] | Liu M C, Hong J J, Sebti E, et al. Surface molecular engineering to enable processing of sulfide solid electrolytes in humid ambient air[J]. Nature Communications, 2025, 16: 213.[LinkOut] |
| [58] | Luo M, Wang C H, Duan Y, et al. Surface coating enabling sulfide solid electrolytes with excellent air stability and lithium compatibility[J]. Energy & Environmental Materials, 2024, 7(6): e12753.[LinkOut] |
| [59] | Zhang J, Bao C S, Jin J, et al. Improve the internal and interface stability of sulfide-based composite electrolytes through high concentration electrolyte and continuous Li+ conductive frameworks[J]. Small Methods, 2025: 2500179.[LinkOut] |
| [60] | Luo S T, Wang Z Y, Fan A R, et al. A high energy and power all-solid-state lithium battery enabled by modified sulfide electrolyte film[J]. Journal of Power Sources, 2021, 485: 229325.[LinkOut] |
| [61] | Janek J, Zeier W G. Challenges in speeding up solid-state battery development[J]. Nature Energy, 2023, 8(3): 230-240.[LinkOut] |
| [62] | Koerver R, Zhang W B, de Biasi L, et al. Chemo-mechanical expansion of lithium electrode materials–on the route to mechanically optimized all-solid-state batteries[J]. Energy & Environmental Science, 2018, 11(8): 2142-2158.[LinkOut] |
| [63] | Park N Y, Lee H U, Yu T Y, et al. High-energy, long-life Ni-rich cathode materials with columnar structures for all-solid-state batteries[J]. Nature Energy, 2025, 10(4): 479-489.[LinkOut] |
| [64] | Krauskopf T, Richter F H, Zeier W G, et al. Physicochemical concepts of the lithium metal anode in solid-state batteries[J]. Chemical Reviews, 2020, 120(15): 7745-7794.[PubMed] |
| [65] | Wu Z, Du L M, Yang T Q, et al. Lithium difluorophosphate additive engineering enabling stable cathodic interface for high-performance sulfide-based all-solid-state lithium battery[J]. Energy & Environmental Materials, 2025, 8(4): e12871.[LinkOut] |
| [66] | Kim H S, Jung J Y, Kim K, et al. Functionalized electrode additive for simultaneously reinforcing chemo-mechanical properties of millimeter-thick dry-electrode for high-energy all-solid-state batteries[J]. Advanced Energy Materials, 2024, 14(14): 2303965.[LinkOut] |
| [67] | Wang K J, Liang Z T, Weng S T, et al. Surface engineering strategy enables 4.5 V sulfide-based all-solid-state batteries with high cathode loading and long cycle life[J]. ACS Energy Letters, 2023, 8(8): 3450-3459.[LinkOut] |
| [68] | Lee K, Lee J, Choi S, et al. Thiol–ene click reaction for fine polarity tuning of polymeric binders in solution-processed all-solid-state batteries[J]. ACS Energy Letters, 2019, 4(1): 94-101.[LinkOut] |
| [69] | Lee J, Lee K, Lee T, et al. In situ deprotection of polymeric binders for solution-processible sulfide-based all-solid-state batteries[J]. Advanced Materials, 2020, 32(37): 2001702.[LinkOut] |
| [70] | Kim J, Choi W, Hwang S J, et al. Incorporation of ionic conductive polymers into sulfide electrolyte-based solid-state batteries to enhance electrochemical stability and cycle life[J]. Energy & Environmental Materials, 2024, 7(6): e12776.[LinkOut] |
| [71] | Hong S B, Lee Y J, Kim U H, et al. All-solid-state lithium batteries: Li+-conducting ionomer binder for dry-processed composite cathodes[J]. ACS Energy Letters, 2022, 7(3): 1092-1100.[LinkOut] |
| [72] | Kim K T, Oh D Y, Jun S, et al. Tailoring slurries using cosolvents and Li salt targeting practical all-solid-state batteries employing sulfide solid electrolytes[J]. Advanced Energy Materials, 2021, 11(17): 2003766.[LinkOut] |
| [73] | Hong S B, Jang Y R, Kim H, et al. Wet-processable binder in composite cathode for high energy density all-solid-state lithium batteries[J]. Advanced Energy Materials, 2024, 14(35): 2400802.[LinkOut] |
| [74] | Hwang J, Matsumoto K, Chen C Y, et al. Pseudo-solid-state electrolytes utilizing the ionic liquid family for rechargeable batteries[J]. Energy & Environmental Science, 2021, 14(11): 5834-5863.[LinkOut] |
| [75] | Jeong S, Ho V C, Kwon O, et al. High-stability room temperature ionic liquids: enabling efficient charge transfer in solid-state batteries by minimizing interfacial resistance[J]. Energy Materials, 2023, 3(6): 300048.[LinkOut] |
| [76] | Kim K, Park J, Jeong G, et al. Rational design of a composite electrode to realize a high-performance all-solid-state battery[J]. ChemSusChem, 2019, 12(12): 2637-2643.[LinkOut] |
| [77] | Strauss F, Bartsch T, de Biasi L, et al. Impact of cathode material particle size on the capacity of bulk-type all-solid-state batteries[J]. ACS Energy Letters, 2018, 3(4): 992-996.[LinkOut] |
| [78] | Dewald G F, Liaqat Z, Lange M A, et al. Influence of iron sulfide nanoparticle sizes in solid-state batteries[J]. Angewandte Chemie International Edition, 2021, 60(33): 17952-17956.[LinkOut] |
| [79] | Wang C H, Yu R Z, Hwang S, et al. Single crystal cathodes enabling high-performance all-solid-state lithium-ion batteries[J]. Energy Storage Materials, 2020, 30: 98-103.[LinkOut] |
| [80] | Tian R Z, Wang Z Y, Liao J G, et al. High-voltage stability of small-size single crystal Ni-rich layered cathode for sulfide-based all-solid-state lithium battery at 4.5 V[J]. Advanced Energy Materials, 2023, 13(26): 2300850.[LinkOut] |
| [81] | Sharma A K, Vishnugopi B S, Ayyaswamy A, et al. Co-design of active material and solid electrolyte particulate phases in solid-state battery composite electrodes[J]. ACS Applied Materials & Interfaces, 2025, 17(35): 49520-49532.[LinkOut] |
| [82] | Cronau M, Duchardt M, Szabo M, et al. Ionic conductivity versus particle size of ball-milled sulfide-based solid electrolytes: strategy towards optimized composite cathode performance in all-solid-state batteries[J]. Batteries & Supercaps, 2022, 5(6): e202200041.[LinkOut] |
| [83] | Frankenberg F, Heck C A, Kissel M, et al. Tailoring composite microstructure through milling for dry-processed sulfide-based solid-state battery cathodes[J]. Small, 2025, 21(41): e07279.[LinkOut] |
| [84] | Kim H S, Park S, Kang S, et al. Accelerated degradation of all-solid-state batteries induced through volumetric occupation of the carbon additive in the solid electrolyte domain[J]. Advanced Functional Materials, 2024, 34(49): 2409318.[LinkOut] |
| [85] | Tan D H S, Chen Y T, Yang H D, et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes[J]. Science, 2021, 373(6562): 1494-1499.[PubMed] |
| [86] | Hong J, Choi S, James T, et al. Analysis of Ni-rich cathode composite electrode performance according to the conductive additive distribution for application in sulfide all-solid-state lithium-ion batteries[J]. Batteries, 2023, 9(12): 590.[LinkOut] |
| [87] | Kim J, Choi W, Jeon Y P, et al. Importance of crystallinity in conductive additives for improving electrochemical performance of sulfide-based solid-state batteries[J]. Journal of Power Sources, 2025, 658: 238306.[LinkOut] |
| [88] | Bhadra A, Brunisholz M, Bonsu J O, et al. Carbon mediated in situ cathode interface stabilization for high rate and highly stable operation of all-solid-state lithium batteries (adv. energy mater. 14/2025)[J]. Advanced Energy Materials, 2025, 15(14): 2570072.[LinkOut] |
| [89] | Deng S M, Wang Y X, Sun T X, et al. Impacts of the conductive networks on solid-state battery operation[J]. Angewandte Chemie International Edition, 2025, 64(39): e202511534.[LinkOut] |
| [90] | Deng S X, Sun Y P, Li X, et al. Eliminating the detrimental effects of conductive agents in sulfide-based solid-state batteries[J]. ACS Energy Letters, 2020, 5(4): 1243-1251.[LinkOut] |
| [91] | Minnmann P, Quillman L, Burkhardt S, et al. Editors' choice: quantifying the impact of charge transport bottlenecks in composite cathodes of all-solid-state batteries[J]. Journal of the Electrochemical Society, 2021, 168(4): 040537.[LinkOut] |
| [92] | Kaiser N, Spannenberger S, Schmitt M, et al. Ion transport limitations in all-solid-state lithium battery electrodes containing a sulfide-based electrolyte[J]. Journal of Power Sources, 2018, 396: 175-181.[LinkOut] |
| [93] | Ketter L, Greb N, Bernges T, et al. Using resistor network models to predict the transport properties of solid-state battery composites[J]. Nature Communications, 2025, 16: 1411.[LinkOut] |
| [94] | Siroma Z, Sato T, Takeuchi T, et al. AC impedance analysis of ionic and electronic conductivities in electrode mixture layers for an all-solid-state lithium-ion battery[J]. Journal of Power Sources, 2016, 316: 215-223.[LinkOut] |
| [95] | Kroll M, Duchardt M, Karstens S L, et al. Sheet-type all-solid-state batteries with sulfidic electrolytes: Analysis of kinetic limitations based on a cathode morphology study[J]. Journal of Power Sources, 2021, 505: 230064.[LinkOut] |
| [96] | Schlautmann E, Drews J, Ketter L, et al. Graded cathode design for enhanced performance of sulfide-based solid-state batteries[J]. ACS Energy Letters, 2025, 10(4): 1664-1670.[LinkOut] |
| [97] | Naik K G, Jangid M K, Vishnugopi B S, et al. Interrogating the role of stack pressure in transport-reaction interaction in the solid-state battery cathode[J]. Advanced Energy Materials, 2025, 15(10): 2403360.[LinkOut] |
| [98] | Naik K G, Chatterjee D, Mukherjee P P. Solid electrolyte–cathode interface dictates reaction heterogeneity and anode stability[J]. ACS Applied Materials & Interfaces, 2022, 14(40): 45308-45319.[LinkOut] |
| [1] | 赵玉海, 罗英武. 可逆失活自由基界面聚合[J]. 化工学报, 2021, 72(2): 653-668. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号