化工学报 ›› 2021, Vol. 72 ›› Issue (8): 4204-4214.DOI: 10.11949/0438-1157.20201680
李虎1( ),张自生1,陈久洲2,石好亮2,石永杰2,李洪1,李鑫钢1,高鑫1(
),张自生1,陈久洲2,石好亮2,石永杰2,李洪1,李鑫钢1,高鑫1( )
)
收稿日期:2020-11-23
修回日期:2021-02-08
出版日期:2021-08-05
发布日期:2021-08-05
通讯作者:
高鑫
作者简介:李虎(1996—),男,硕士研究生, 基金资助:
Hu LI1( ),Zisheng ZHANG1,Jiuzhou CHEN2,Haoliang SHI2,Yongjie SHI2,Hong LI1,Xingang LI1,Xin GAO1(
),Zisheng ZHANG1,Jiuzhou CHEN2,Haoliang SHI2,Yongjie SHI2,Hong LI1,Xingang LI1,Xin GAO1( )
)
Received:2020-11-23
Revised:2021-02-08
Online:2021-08-05
Published:2021-08-05
Contact:
Xin GAO
摘要:
相同碳数的正构烯烃与正构烷烃因其结构相似,使其相对挥发度较小、分离难度较大。低共熔溶剂(DES)作为一种可设计的绿色分离介质被广泛应用于该类混合物的分离中,此外过渡金属与烯烃双键之间的化学络合作用是促进正构烯烃/烷烃分离的一个重要方法。鉴于此,开发了新型银基低共熔溶剂(Ag-DES),并将其应用于1-己烯/正己烷的分离,系统探究了原料中烯烃浓度、银离子与烯烃摩尔比、分离温度等对1-己烯分离性能的影响,结果显示Ag-DES具有良好的1-己烯/正己烷分离选择性,选择性在3.5~18之间,并具有出色的循环稳定性。进一步通过FT-Raman表征和量化计算揭示了Ag-DES与烯烃之间的化学络合作用和较强氢键作用是实现其与烷烃分离的本质原因,表明应用Ag-DES的反应萃取分离强化方法可实现从F-T合成油中绿色高效分离C6α-烯烃。
中图分类号:
李虎, 张自生, 陈久洲, 石好亮, 石永杰, 李洪, 李鑫钢, 高鑫. 新型银基低共熔溶剂制备及其在1-己烯/正己烷分离中的应用[J]. 化工学报, 2021, 72(8): 4204-4214.
Hu LI, Zisheng ZHANG, Jiuzhou CHEN, Haoliang SHI, Yongjie SHI, Hong LI, Xingang LI, Xin GAO. Preparation of novel silver-based deep eutectic solvent and its application in separation of 1-hexene/n-hexane[J]. CIESC Journal, 2021, 72(8): 4204-4214.
| 影响因素 | 各因素对1-己烯分离性能影响探究实验条件 | ||||
|---|---|---|---|---|---|
| Ag-DES质量/ g | C6质量/ g | 1-己烯进料质量分数/% | Ag+与1-己烯摩尔比 | 温度/℃ | |
| 原料中烯烃浓度 | 11.106 | 4.48 | 10/30/50/70/90 | — | 25 |
| 银离子与烯烃摩尔比 | 11.106 | 5.04 | 50 | 1∶1/1∶1.5/1∶2/1∶2.5/1∶3 | 25 |
| 分离温度 | 11.106 | 5.04 | 50 | 1∶1.5 | 0/10/20/25/30/40 |
| 循环稳定性 | 11.106 | 5.04 | 50 | 1∶1.5 | 25 |
表1 1-己烯分离性能探究实验条件
Table 1 The experimental conditions for the separation performance of 1-hexene
| 影响因素 | 各因素对1-己烯分离性能影响探究实验条件 | ||||
|---|---|---|---|---|---|
| Ag-DES质量/ g | C6质量/ g | 1-己烯进料质量分数/% | Ag+与1-己烯摩尔比 | 温度/℃ | |
| 原料中烯烃浓度 | 11.106 | 4.48 | 10/30/50/70/90 | — | 25 |
| 银离子与烯烃摩尔比 | 11.106 | 5.04 | 50 | 1∶1/1∶1.5/1∶2/1∶2.5/1∶3 | 25 |
| 分离温度 | 11.106 | 5.04 | 50 | 1∶1.5 | 0/10/20/25/30/40 |
| 循环稳定性 | 11.106 | 5.04 | 50 | 1∶1.5 | 25 |

图2 原料中烯烃浓度对1-己烯、正己烷分配系数及其选择性的影响(在25℃下,11.106 g Ag-DES中加入4.48 g 1-己烯质量分数10%~90%的C6混合物)
Fig.2 Effect of olefin concentration in initial feed on the distribution coefficient and selectivity of 1-hexene to n-hexane(Including 11.106 g Ag-DES and 4.48 g C6 with the mass ratio of 1-hexene in initial feed range 10%—90% at 25℃)
原料中烯烃浓度(质量分数) x0 | 有机相组成(摩尔分数) | 溶剂相组成(摩尔分数) | 分配系数 | 选择性 S1,2 | 1-己烯收率 η1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | D1 | D2 | |||
| 0.1 | 0.037 | 0.962 | 0.000 | 0.126 | 0.180 | 0.693 | 0.706 | 0.039 | 17.967 | 0.686 |
| 0.3 | 0.128 | 0.871 | 0.002 | 0.311 | 0.140 | 0.550 | 0.614 | 0.040 | 15.183 | 0.696 |
| 0.5 | 0.273 | 0.726 | 0.001 | 0.427 | 0.106 | 0.467 | 0.440 | 0.041 | 10.712 | 0.689 |
| 0.7 | 0.513 | 0.486 | 0.001 | 0.535 | 0.068 | 0.397 | 0.328 | 0.044 | 7.454 | 0.697 |
| 0.9 | 0.829 | 0.170 | 0.001 | 0.617 | 0.036 | 0.347 | 0.256 | 0.074 | 3.477 | 0.731 |
表2 1-己烯(1)-正己烷(2)-Ag-DES(3)三元两相体系在298.15 K时的相平衡数据
Table 2 Phase equilibrium data of 1-hexene(1)-n-hexane(2)-Ag-DES(3) in the ternary biphasic system at 298.15 K
原料中烯烃浓度(质量分数) x0 | 有机相组成(摩尔分数) | 溶剂相组成(摩尔分数) | 分配系数 | 选择性 S1,2 | 1-己烯收率 η1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | D1 | D2 | |||
| 0.1 | 0.037 | 0.962 | 0.000 | 0.126 | 0.180 | 0.693 | 0.706 | 0.039 | 17.967 | 0.686 |
| 0.3 | 0.128 | 0.871 | 0.002 | 0.311 | 0.140 | 0.550 | 0.614 | 0.040 | 15.183 | 0.696 |
| 0.5 | 0.273 | 0.726 | 0.001 | 0.427 | 0.106 | 0.467 | 0.440 | 0.041 | 10.712 | 0.689 |
| 0.7 | 0.513 | 0.486 | 0.001 | 0.535 | 0.068 | 0.397 | 0.328 | 0.044 | 7.454 | 0.697 |
| 0.9 | 0.829 | 0.170 | 0.001 | 0.617 | 0.036 | 0.347 | 0.256 | 0.074 | 3.477 | 0.731 |
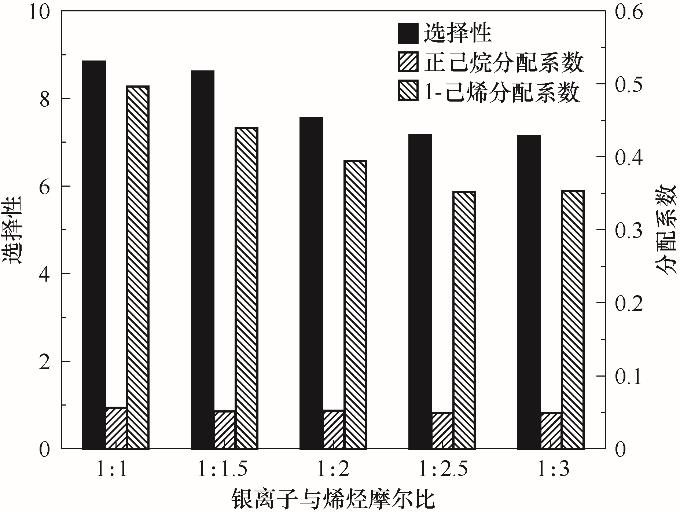
图4 银离子与烯烃摩尔比对1-己烯、正己烷分配系数及其选择性的影响(在25℃下,将11.106 g Ag-DES含烯烃浓度50%的C6混合物按摩尔比(1∶1)~(1∶3)混合)
Fig.4 Effect of molar ratio of silver ion to olefin on the distribution coefficient and selectivity of 1-hexene to n-hexane (Including 11.106 g Ag-DES and C6 mixture with 50% olefin and the molar ratio of Ag-DES to 1-hexene ranging from 1∶1 to 1∶3 at 25℃)
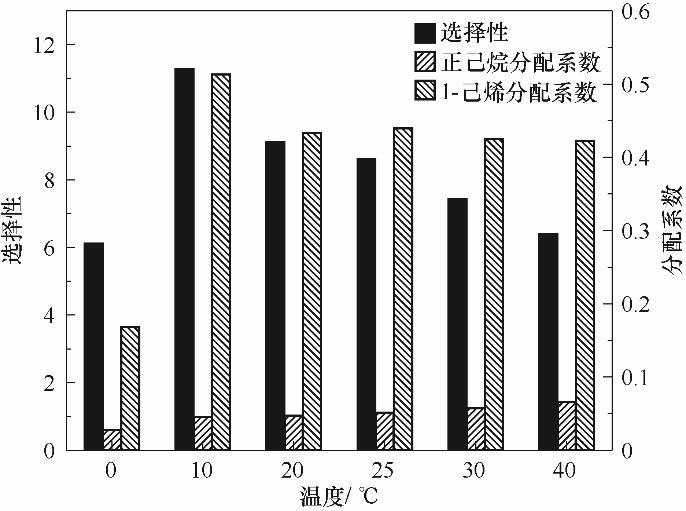
图5 温度对1-己烯、正己烷分配系数及其选择性的影响(将11.106 g Ag-DES与5.04 g烯烃浓度50%的C6混合物分别于0~40℃各温度下混合)
Fig.5 Effect of temperature on the distribution coefficient and selectivity of 1-hexene to n-hexane(Including 11.106 g Ag-DES and 5.04 g C6 mixture with 50% olefin and the operating temperature ranging from 0 to 40℃)
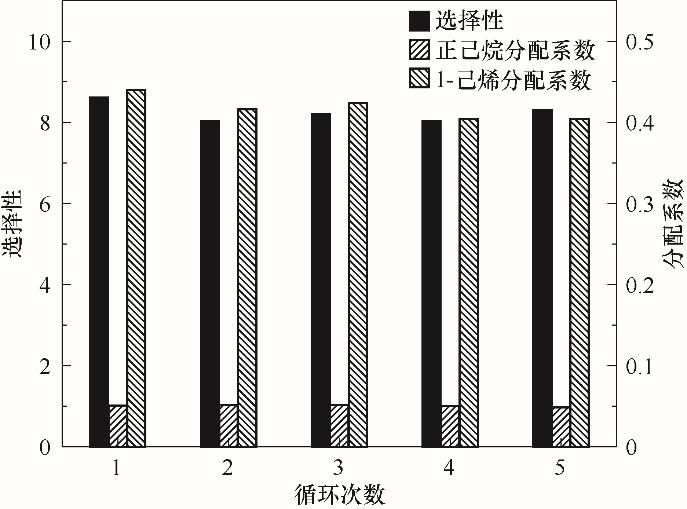
图6 循环次数对1-己烯、正己烷分配系数及其选择性的影响(在25℃下,将11.106 g Ag-DES与5.04 g烯烃浓度50%的C6混合物混合)
Fig.6 Effect of recycle times on the distribution coefficient and selectivity of 1-hexene to n-hexane(Including 11.106 g Ag-DES and 5.04 g C6 mixture with 50% olefin at 25℃)
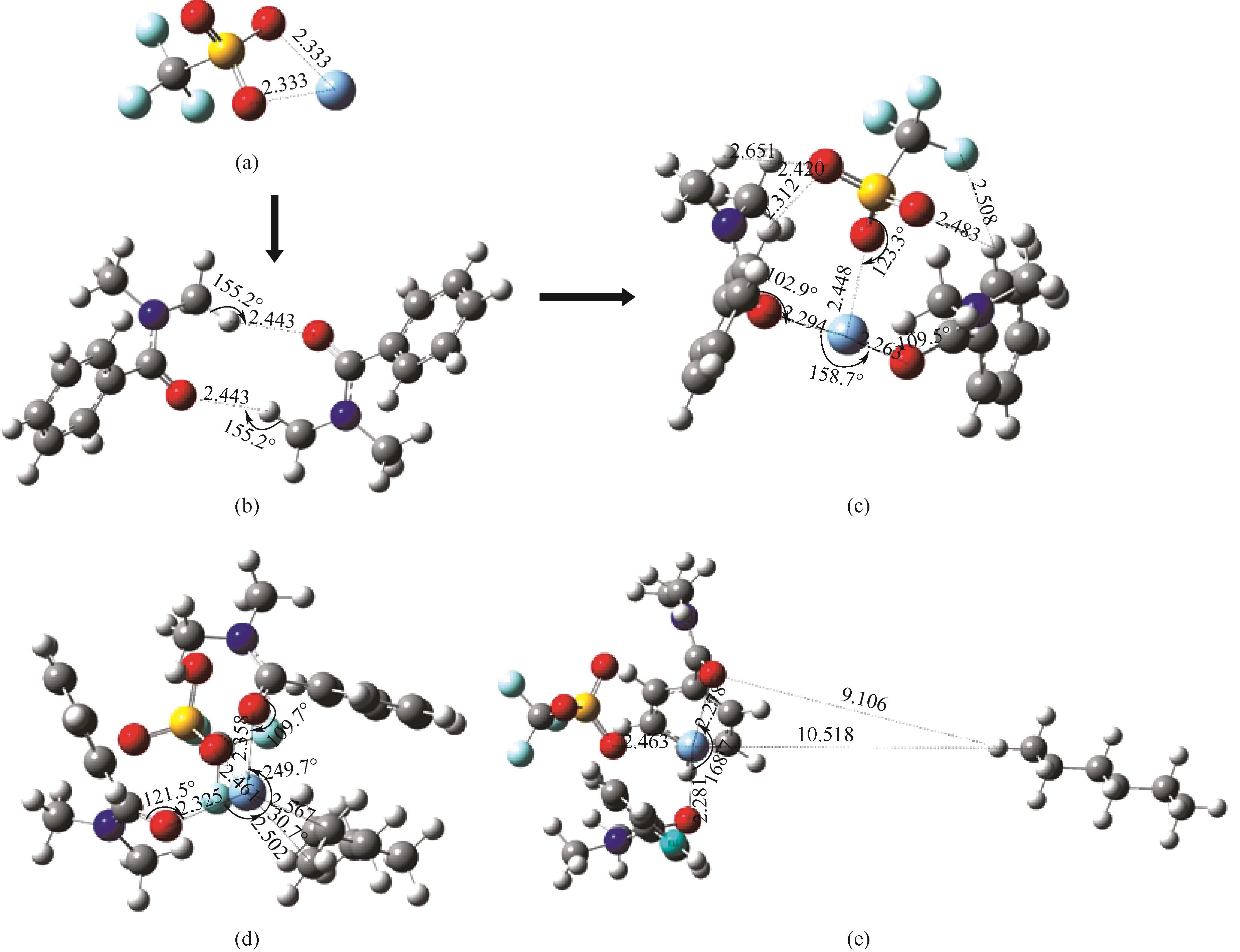
图9 AgCF3SO3(a)、DMBA(b)、Ag-DES (c)、Ag-DES-1-己烯(d)、Ag-DES-正己烷(e)在M062X/def2tzvp级别下的优化构型
Fig.9 The optimized geometry structures for AgCF3SO3 (a), DMBA (b), Ag-DES (c), Ag-DES-1-hexene (d), Ag-DES-n-hexane (e) at the M062X/def2tzvp level
| E | Ag-DES-1-己烯 | Ag-DES-正己烷 |
|---|---|---|
| EA/(kJ/mol) | -5431268.05 | -5431268.05 |
| EB/(kJ/mol) | -619411.70 | -622637.68 |
| EAB/(kJ/mol) | -6050813.59 | -6053982.20 |
| EBSSE/(kJ/mol) | 3.09 | 4.22×10-4 |
| EcintAB/(kJ/mol) | -130.75 | -76.47 |
| ?EcintAB/(kJ/mol) | 54.28 | |
表3 Ag-DES(A)-1-己烯/正己烷(B)在M062X/def2tzvp级别下的相互作用能
Table 3 The interaction energies of the complexes Ag-DES(A)-1-hexene/n-hexane(B) at M062X/def2tzvp level
| E | Ag-DES-1-己烯 | Ag-DES-正己烷 |
|---|---|---|
| EA/(kJ/mol) | -5431268.05 | -5431268.05 |
| EB/(kJ/mol) | -619411.70 | -622637.68 |
| EAB/(kJ/mol) | -6050813.59 | -6053982.20 |
| EBSSE/(kJ/mol) | 3.09 | 4.22×10-4 |
| EcintAB/(kJ/mol) | -130.75 | -76.47 |
| ?EcintAB/(kJ/mol) | 54.28 | |
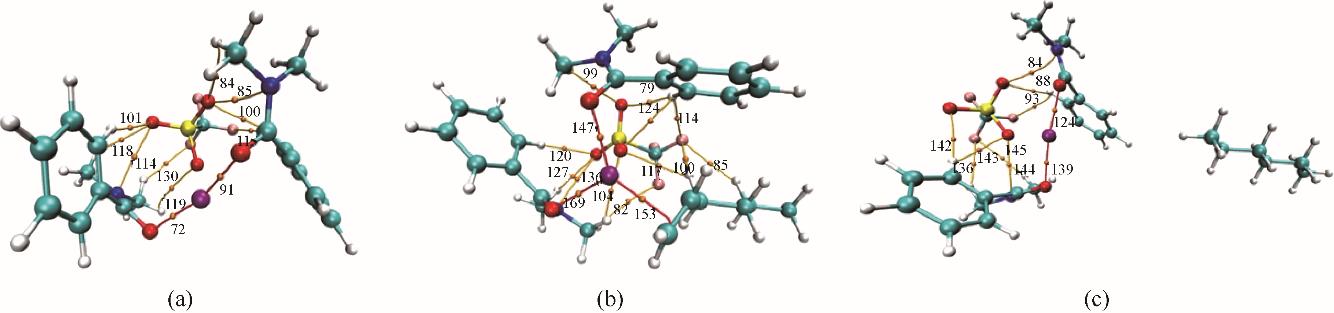
图10 Ag-DES(a)、Ag-DES-1-己烯(b)、Ag-DES-正己烷(c)的AIM分析橘点:BCPs;红线:共价相互作用;橘线:非共价相互作用
Fig.10 The AIM analysis for Ag-DES(a), Ag-DES-1-hexene(b), Ag-DES-n-hexane (c)The BCPs are marked as orange dots, the coordination covalent bonds are linked by red lines and the hydrogen bonds are linked by orange lines
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 72 | Ag9-O44 C43 C43 | 5.52×10-2 | 2.73×10-1 | -4.87×10-3 | -8.82×10-2 |
| 91 | Ag9-O22 C21 C21 | 5.15×10-2 | 2.48×10-1 | -4.47×10-3 | -8.69×10-2 | |
| 氢键 | 119 | C24—H27…O5 | 7.98×10-3 | 3.13×10-2 | 1.44×10-3 | 1.81×10-1 |
| 130 | C24—H26…F7 | 3.67×10-3 | 1.48×10-2 | 8.45×10-4 | 2.30×10-1 | |
| 101 | C12—H18…O3 | 1.22×10-2 | 4.72×10-2 | 2.27×10-3 | 1.87×10-1 | |
| 118 | C28—H29…O3 | 1.01×10-2 | 4.08×10-2 | 1.87×10-3 | 1.86×10-1 | |
| 114 | C21—N23…O3 | 7.50×10-3 | 3.13×10-2 | 1.52×10-3 | 2.03×10-1 | |
| 84 | C46—H48…O4 | 7.34×10-3 | 3.19×10-2 | 1.58×10-3 | 2.16×10-1 | |
| 85 | C43—N45…O4 | 9.86×10-3 | 4.14×10-2 | 1.80×10-3 | 1.83×10-1 | |
| 99 | C34—H40…O4 | 9.88×10-3 | 3.63×10-2 | 1.67×10-3 | 1.69×10-1 | |
| 115 | C34—H40…F6 | 7.00×10-3 | 3.05×10-2 | 1.68×10-3 | 2.40×10-1 |
表4 Ag-DES在BCPs处的拓扑参数
Table 4 Topological parameters of BCPs in Ag-DES
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 72 | Ag9-O44 C43 C43 | 5.52×10-2 | 2.73×10-1 | -4.87×10-3 | -8.82×10-2 |
| 91 | Ag9-O22 C21 C21 | 5.15×10-2 | 2.48×10-1 | -4.47×10-3 | -8.69×10-2 | |
| 氢键 | 119 | C24—H27…O5 | 7.98×10-3 | 3.13×10-2 | 1.44×10-3 | 1.81×10-1 |
| 130 | C24—H26…F7 | 3.67×10-3 | 1.48×10-2 | 8.45×10-4 | 2.30×10-1 | |
| 101 | C12—H18…O3 | 1.22×10-2 | 4.72×10-2 | 2.27×10-3 | 1.87×10-1 | |
| 118 | C28—H29…O3 | 1.01×10-2 | 4.08×10-2 | 1.87×10-3 | 1.86×10-1 | |
| 114 | C21—N23…O3 | 7.50×10-3 | 3.13×10-2 | 1.52×10-3 | 2.03×10-1 | |
| 84 | C46—H48…O4 | 7.34×10-3 | 3.19×10-2 | 1.58×10-3 | 2.16×10-1 | |
| 85 | C43—N45…O4 | 9.86×10-3 | 4.14×10-2 | 1.80×10-3 | 1.83×10-1 | |
| 99 | C34—H40…O4 | 9.88×10-3 | 3.63×10-2 | 1.67×10-3 | 1.69×10-1 | |
| 115 | C34—H40…F6 | 7.00×10-3 | 3.05×10-2 | 1.68×10-3 | 2.40×10-1 |
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 153 | Ag9-C69 C67 C67 | 4.30×10-2 | 1.35×10-1 | -4.83×10-3 | -1.13×10-1 |
| 147 | Ag9-O44 C43 C43 | 4.60×10-2 | 1.73×10-1 | -2.55×10-3 | -5.55×10-2 | |
| 169 | Ag9-O22 C21 C21 | 4.41×10-2 | 2.11×10-1 | -2.90×10-3 | -6.58×10-2 | |
| 氢键 | 104 | C24—H27…O5 | 6.80×10-3 | 2.43×10-2 | 1.15×10-3 | 1.69×10-1 |
| 124 | C34—H40…O5 | 7.08×10-3 | 2.57×10-2 | 1.21×10-3 | 1.72×10-1 | |
| 117 | C64—H66…O5 | 7.04×10-3 | 2.48×10-2 | 1.15×10-3 | 1.63×10-1 | |
| 120 | C12—H18…O3 | 6.47×10-3 | 2.23×10-2 | 1.07×10-3 | 1.66×10-1 | |
| 127 | C28—H29…O3 | 1.06×10-2 | 4.29×10-2 | 1.96×10-3 | 1.85×10-1 | |
| 130 | C21—N23…O3 | 7.21×10-3 | 2.77×10-2 | 1.36×10-3 | 1.88×10-1 | |
| 99 | C46—H49…O4 | 1.05×10-2 | 3.96×10-2 | 1.93×10-3 | 1.84×10-1 | |
| 79 | C34—H40…O4 | 1.03×10-2 | 4.30×10-2 | 1.95×10-3 | 1.89×10-1 | |
| 82 | C24—H26…F7 | 2.52×10-3 | 1.06×10-2 | 6.74×10-4 | 2.68×10-1 | |
| 114 | C34—H40…F6 | 6.24×10-3 | 2.65×10-2 | 1.47×10-3 | 2.35×10-1 | |
| 100 | C64—H66…F6 | 6.13×10-3 | 2.35×10-2 | 1.24×10-3 | 2.02×10-1 | |
| 85 | C61—H63…F6 | 4.38×10-3 | 1.69×10-2 | 9.23×10-4 | 2.11×10-1 |
表5 Ag-DES-1-己烯在BCPs处的拓扑参数
Table 5 Topological parameters of BCPs in Ag-DES-1- hexene
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 153 | Ag9-C69 C67 C67 | 4.30×10-2 | 1.35×10-1 | -4.83×10-3 | -1.13×10-1 |
| 147 | Ag9-O44 C43 C43 | 4.60×10-2 | 1.73×10-1 | -2.55×10-3 | -5.55×10-2 | |
| 169 | Ag9-O22 C21 C21 | 4.41×10-2 | 2.11×10-1 | -2.90×10-3 | -6.58×10-2 | |
| 氢键 | 104 | C24—H27…O5 | 6.80×10-3 | 2.43×10-2 | 1.15×10-3 | 1.69×10-1 |
| 124 | C34—H40…O5 | 7.08×10-3 | 2.57×10-2 | 1.21×10-3 | 1.72×10-1 | |
| 117 | C64—H66…O5 | 7.04×10-3 | 2.48×10-2 | 1.15×10-3 | 1.63×10-1 | |
| 120 | C12—H18…O3 | 6.47×10-3 | 2.23×10-2 | 1.07×10-3 | 1.66×10-1 | |
| 127 | C28—H29…O3 | 1.06×10-2 | 4.29×10-2 | 1.96×10-3 | 1.85×10-1 | |
| 130 | C21—N23…O3 | 7.21×10-3 | 2.77×10-2 | 1.36×10-3 | 1.88×10-1 | |
| 99 | C46—H49…O4 | 1.05×10-2 | 3.96×10-2 | 1.93×10-3 | 1.84×10-1 | |
| 79 | C34—H40…O4 | 1.03×10-2 | 4.30×10-2 | 1.95×10-3 | 1.89×10-1 | |
| 82 | C24—H26…F7 | 2.52×10-3 | 1.06×10-2 | 6.74×10-4 | 2.68×10-1 | |
| 114 | C34—H40…F6 | 6.24×10-3 | 2.65×10-2 | 1.47×10-3 | 2.35×10-1 | |
| 100 | C64—H66…F6 | 6.13×10-3 | 2.35×10-2 | 1.24×10-3 | 2.02×10-1 | |
| 85 | C61—H63…F6 | 4.38×10-3 | 1.69×10-2 | 9.23×10-4 | 2.11×10-1 |
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 124 | Ag9-O44 C43 C43 | 5.38×10-2 | 2.63×10-1 | -4.84×10-3 | -9.01×10-2 |
| 139 | Ag9-O22 C1 C1 | 5.23×10-2 | 2.55×10-1 | -4.23×10-3 | -8.09×10-2 | |
| 氢键 | 143 | C12—H18…O5 | 7.32×10-3 | 2.70×10-2 | 1.26×10-3 | 1.72×10-1 |
| 144 | C21—N23…O5 | 1.06×10-2 | 4.49×10-2 | 1.84×10-3 | 1.72×10-1 | |
| 136 | C28—H29…F7 | 2.24×10-3 | 8.75×10-3 | 5.07×10-4 | 2.26×10-1 | |
| 145 | C24—H26…F7 | 3.43×10-3 | 1.36×10-2 | 7.70×10-4 | 2.25×10-1 | |
| 142 | C12—H18…O3 | 8.03×10-3 | 3.02×10-2 | 1.56×10-3 | 1.94×10-1 | |
| 84 | C43—N45…O4 | 9.56×10-3 | 3.93×10-2 | 1.76×10-3 | 1.84×10-1 | |
| 88 | C34—H40…O4 | 9.70×10-3 | 3.62×10-2 | 1.63×10-3 | 1.68×10-1 | |
| 93 | C34—H40…F6 | 6.11×10-3 | 2.68×10-2 | 1.50×10-3 | 2.46×10-1 |
表6 Ag-DES-正己烷在BCPs处的拓扑参数
Table 6 Topological parameters of BCPs in Ag-DES- n-hexane
| 类型 | BCP | 原子标签 | ρ(BCP) | ?2ρ(BCP) | H(BCP) | H(BCP)/ρ(BCP) |
|---|---|---|---|---|---|---|
| 共价作用 | 124 | Ag9-O44 C43 C43 | 5.38×10-2 | 2.63×10-1 | -4.84×10-3 | -9.01×10-2 |
| 139 | Ag9-O22 C1 C1 | 5.23×10-2 | 2.55×10-1 | -4.23×10-3 | -8.09×10-2 | |
| 氢键 | 143 | C12—H18…O5 | 7.32×10-3 | 2.70×10-2 | 1.26×10-3 | 1.72×10-1 |
| 144 | C21—N23…O5 | 1.06×10-2 | 4.49×10-2 | 1.84×10-3 | 1.72×10-1 | |
| 136 | C28—H29…F7 | 2.24×10-3 | 8.75×10-3 | 5.07×10-4 | 2.26×10-1 | |
| 145 | C24—H26…F7 | 3.43×10-3 | 1.36×10-2 | 7.70×10-4 | 2.25×10-1 | |
| 142 | C12—H18…O3 | 8.03×10-3 | 3.02×10-2 | 1.56×10-3 | 1.94×10-1 | |
| 84 | C43—N45…O4 | 9.56×10-3 | 3.93×10-2 | 1.76×10-3 | 1.84×10-1 | |
| 88 | C34—H40…O4 | 9.70×10-3 | 3.62×10-2 | 1.63×10-3 | 1.68×10-1 | |
| 93 | C34—H40…F6 | 6.11×10-3 | 2.68×10-2 | 1.50×10-3 | 2.46×10-1 |
| 1 | Wentink A E, Kuipers N J M, de Haan A B, et al. Effects of ligand structure on reactive vapor-liquid distribution ratio and selectivity for C6-olefin isomers[J]. Industrial & Engineering Chemistry Research, 2005, 44(24): 9221-9229. |
| 2 | Eagan N M, Moore B M, McClelland D J, et al. Catalytic synthesis of distillate-range ethers and olefins from ethanol through Guerbet coupling and etherification[J]. Green Chemistry, 2019, 21(12): 3300-3318. |
| 3 | Wentink A E, Kuipers N J M, de Haan A B, et al. Olefin isomer separation by reactive extractive distillation: modelling of vapour-liquid equilibria and conceptual design for 1-hexene purification[J]. Chemical Engineering and Processing: Process Intensification, 2007, 46(9): 800-809. |
| 4 | Kuipers N J M, Wentink A E, de Haan A B, et al. Functionalized solvents for olefin isomer purification by reactive extractive distillation[J]. Chemical Engineering Research and Design, 2007, 85(1): 88-99. |
| 5 | 李阳, 屈一新, 王际东. 费托合成油C6馏分中提取烯烃的工艺优化模拟[J]. 北京服装学院学报(自然科学版), 2019, 39(3): 59-65. |
| Li Y, Qu Y X, Wang J D. Process simulation and optimization for separating of C6 olefins from C6-cut of Fischer-Tropsch synthesis oil[J]. Journal of Beijing Institute of Fashion Technology (Natural Science Edition), 2019, 39(3): 59-65. | |
| 6 | Piszczek R, Heins B, Hamilton P, et al. Separating linear alpha olefin involves providing a pre-processed product stream comprising linear alpha olefins to first of series of distillation columns, and element of the series of distillation columns comprising a dividing wall column: WO2020114744-A1[P]. 2020-6-11. |
| 7 | 杨正伟, 孙启文, 张宗森. 萃取精馏脱高温费托合成C6馏分中的含氧化合物[J]. 石油化工, 2016, 45(4): 402-407. |
| Yang Z W, Sun Q W, Zhang Z S. Removing oxygenates from C6 fraction in high-temperature Fisher-Tropsch synthesis products by extractive distillation[J]. Petrochemical Technology, 2016, 45(4): 402-407. | |
| 8 | Yang R H, Gao R M, Qian Z, et al. Batch and fixed bed column selective adsorption of C6, C8 and C10 linear α-olefins from binary liquid olefin/paraffin mixtures onto 5A and 13X microporous molecular sieves[J]. Separation and Purification Technology, 2020, 230: 115884. |
| 9 | Yang R H, Gao R M, Wang Y J, et al. Selective adsorption of C6, C8, and C10 linear α-olefins from binary liquid-phase olefin/paraffin mixtures using zeolite adsorbents: experiment and simulations[J]. Langmuir, 2020, 36(29): 8597-8609. |
| 10 | Safarik D J, Eldridge R B. Olefin/paraffin separations by reactive absorption: a review[J]. Industrial & Engineering Chemistry Research, 1998, 37(7): 2571-2581. |
| 11 | Sun Y L, Bi H R, Dou H Z, et al. A novel copper(I)-based supported ionic liquid membrane with high permeability for ethylene/ethane separation[J]. Industrial & Engineering Chemistry Research, 2017, 56(3): 741-749. |
| 12 | Li R L, Xing H B, Yang Q W, et al. Selective extraction of 1-hexene against n-hexane in ionic liquids with or without silver salt[J]. Industrial & Engineering Chemistry Research, 2012, 51(25): 8588-8597. |
| 13 | Rychlewska K, Kujawski W, Konieczny K. Pervaporative performance of PEBA and PDMS based commercial membranes in thiophene removal from its binary mixtures with hydrocarbons[J]. Fuel Processing Technology, 2017, 165: 9-18. |
| 14 | Smith E L, Abbott A P, Ryder K S. Deep eutectic solvents (DESs) and their applications[J]. Chemical Reviews, 2014, 114(21): 11060-11082. |
| 15 | Esfahani H S, Khoshsima A, Pazuki G. Choline chloride-based deep eutectic solvents as green extractant for the efficient extraction of 1-butanol or 2-butanol from azeotropic n-heptane + butanol mixtures[J]. Journal of Molecular Liquids, 2020, 313: 113524. |
| 16 | Liu F J, Chen W, Mi J X, et al. Thermodynamic and molecular insights into the absorption of H2S, CO2, and CH4 in choline chloride plus urea mixtures[J]. AIChE Journal, 2019, 65(5): e16574. |
| 17 | Ren X Y, Zhu X L, Xu C Y, et al. The electrodeposition of amorphous/nanocrystalline Ni-Cr alloys from ChCl-EG deep eutectic solvent[J]. Journal of The Electrochemical Society, 2020, 167(6): 062502. |
| 18 | Wadekar P H, Khose R V, Pethsangave D A, et al. One-pot synthesis of sulfur and nitrogen co-functionalized graphene material using deep eutectic solvents for supercapacitors[J]. Chem Sus Chem, 2019, 12(14): 3326-3335. |
| 19 | Li H, Zhang Z S, Sun G L, et al. Performance and mechanism of the separation of C8α-olefin from F-T synthesis products using novel Ag-DES[J]. AIChE Journal, 2021: e17252. |
| 20 | Wang Y, Thompson J, Zhou J J, et al. Use of water in aiding olefin/paraffin (liquid + liquid) extraction via complexation with a silver bis(trifluoromethylsulfonyl)imide salt[J]. The Journal of Chemical Thermodynamics, 2014, 77: 230-240. |
| 21 | Zhao Y, Truhlar D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1/2/3): 215-241. |
| 22 | Weigend F, Ahlrichs R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy[J]. Physical Chemistry Chemical Physics, 2005, 7(18): 3297-3305. |
| 23 | Bader R F W. Atoms in molecules[J]. Accounts of Chemical Research, 1985, 18(1): 9-15. |
| 24 | Ahosseini A, Sensenich B, Weatherley L R, et al. Phase equilibrium, volumetric, and interfacial properties of the ionic liquid, 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide and 1-octene[J]. Journal of Chemical & Engineering Data, 2010, 55(4): 1611-1617. |
| 25 | Mortaheb H R, Mafi M, Mokhtarani B, et al. Experimental kinetic analysis of ethylene absorption in ionic liquid [Bmim]NO3 with dissolved AgNO3 by a semi-continuous process[J]. Chemical Engineering Journal, 2010, 158(3): 384-392. |
| 26 | Zhu L, Li F F, Zhu J Q, et al. Liquid-liquid equilibria of ternary systems of 1-hexene/hexane and extraction solvents[J]. Chemical Papers, 2016, 70(5): 585-593. |
| 27 | 李如龙. 离子液体在乙烯/乙烷、1-己烯/正己烷分离中的应用基础研究[D]. 杭州: 浙江大学, 2012. |
| Li R L. Applied fundamental research on the separation of ethylene/ethane and 1-hexene/n-hexane by ionic liquid[D]. Hangzhou: Zhejiang University, 2012. | |
| 28 | Wang Y, Hao W Y, Jacquemin J, et al. Enhancing liquid-phase olefin-paraffin separations using novel silver-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2015, 60(1): 28-36. |
| 29 | Sunderrajan S, Freeman B D, Hall C K. Fourier transform infrared spectroscopic characterization of olefin complexation by silver salts in solution[J]. Industrial & Engineering Chemistry Research, 1999, 38(10): 4051-4059. |
| 30 | Okoshi M, Yamada Y, Komaba S, et al. Theoretical analysis of interactions between potassium ions and organic electrolyte solvents: a comparison with lithium, sodium, and magnesium ions[J]. Journal of the Electrochemical Society, 2016, 164(2): A54-A60. |
| 31 | Lipkowski P, Grabowski S J, Robinson T L, et al. Properties of the C—H···H dihydrogen bond: an ab initio and topological analysis[J]. The Journal of Physical Chemistry A, 2004, 108(49): 10865-10872. |
| 32 | Cremer D, Kraka E. Chemical bonds without bonding electron density—does the difference electron-density analysis suffice for a description of the chemical bond?[J]. Angewandte Chemie International Edition in English, 1984, 23(8): 627-628. |
| [1] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [2] | 王宁, 惠磊, 陈美, 历伟, 周琦. POSS改性负载型Ziegler-Natta催化剂及其乙烯/1-己烯共聚反应[J]. 化工学报, 2021, 72(4): 2102-2112. |
| [3] | 杨冲, 林旭枫, 张金锋, 陈宏, 肖业鹏, 王慧, 程丽华, 欧阳新平. 正己烷–异丙醇共沸体系液液相平衡数据测定及关联[J]. 化工学报, 2020, 71(7): 3009-3017. |
| [4] | 庄志海, 张建强, 刘殿华. 聚甲氧基二甲醚+水+正己烷三元体系的液液相平衡[J]. 化工学报, 2016, 67(9): 3545-3551. |
| [5] | 揭会民, 崔现宝, 彭艳枚, 李晓兵, 徐丽, 林瑞榕. 离子液体反应萃取精馏合成乙酸乙酯[J]. 化工学报, 2016, 67(2): 606-613. |
| [6] | 施梅勤, 郑慧新, 魏爱平, 马淳安. Zn 助剂对WC/HZSM-5催化正己烷芳构化性能影响[J]. 化工学报, 2015, 66(2): 553-560. |
| [7] | 田 静,宋月芹,冯敏超,金亚清,周晓龙. 活化条件对正己烷在Pt/SO42-/ZrO2-Al2O3上的异构化活性影响[J]. 化工进展, 2012, 31(12): 2714-2719. |
| [8] | 张 鸾,朱宏吉,白 鹏. 共沸精馏分离乙醇-异丙醇[J]. 化工进展, 2012, 31(10): 2187-2190. |
| [9] | 亢 宇,张明森,王洪涛,谢伦嘉,姜健准,郭 顺,邱 波,刘长城. 不同形貌介孔材料催化剂的制备及在乙烯聚合中的应用[J]. 化工进展, 2012, 31(05): 1032-1038. |
| [10] | 闫新华1,谷巨明2,石 艳1,李瑛琦1,赵敏坚1,付志峰1. 镍的二亚胺催化剂的负载化及其催化乙烯的聚合 [J]. CIESC Journal, 2011, 30(9): 1977-. |
| [11] | 王雅珍1,2,3,王力博2,陈 洁1,王斯晗2,杨玉林3. 乙烯选择性三聚复配催化体系的设计及评价 [J]. CIESC Journal, 2011, 30(3): 520-. |
| [12] | 王力搏1,陈 洁2,王雅珍2,李 杰3,王斯晗1,张宝军1. 铬(Ⅲ)系1-己烯催化剂的研究进展 [J]. CIESC Journal, 2010, 29(3): 472-. |
| [13] | 孙婧元, 蒋浩, 蒋斌波, 王靖岱, 阳永荣. 乙烯和丙烯在低聚物-正己烷溶液中的溶解度及体积传质系数测定 [J]. 化工学报, 2009, 60(9): 2153-2160. |
| [14] | 王留成;武红旗;李磊;赵建宏;宋成盈. 六甲基二硅氮烷、正己烷、六甲基二硅氧烷二元体系汽液平衡数据的测定与关联 [J]. CIESC Journal, 2008, 59(11): 2701-2705. |
| [15] | 赵晶晶,邓 利,谭天伟,王 芳. 固定化脂肪酶催化菜籽油合成蜡酯 [J]. CIESC Journal, 2007, 26(9): 1311-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号