CIESC Journal ›› 2019, Vol. 70 ›› Issue (9): 3553-3564.DOI: 10.11949/0438-1157.20190179
• Material science and engineering, nanotechnology • Previous Articles Next Articles
Shaofei WU( ),Ting YAN(
),Ting YAN( ),Zihan KUAI,Weiguo PAN(
),Zihan KUAI,Weiguo PAN( )
)
Received:2019-03-03
Revised:2019-05-24
Online:2019-09-05
Published:2019-09-05
Contact:
Ting YAN,Weiguo PAN
通讯作者:
闫霆,潘卫国
作者简介:吴韶飞(1993—),男,硕士研究生,基金资助:CLC Number:
Shaofei WU, Ting YAN, Zihan KUAI, Weiguo PAN. Preparation and thermal energy storage properties of high heat conduction expanded graphite/palmitic acid form-stable phase change materials[J]. CIESC Journal, 2019, 70(9): 3553-3564.
吴韶飞, 闫霆, 蒯子函, 潘卫国. 高导热膨胀石墨/棕榈酸定形复合相变材料的制备及储热性能研究[J]. 化工学报, 2019, 70(9): 3553-3564.
Add to citation manager EndNote|Ris|BibTeX
| 方块密度/(kg/m3) | EG/%(mass) | 质量/g | 密度/(kg/m3) | 样品序号 |
|---|---|---|---|---|
| 600 | 6 | 38.977 | 609 | S1 |
| 12 | 50.940 | 596 | S2 | |
| 18 | 38.076 | 595 | S3 | |
| 24 | 38.339 | 599 | S4 | |
| 30 | 38.001 | 594 | S5 | |
| 700 | 6 | 44.530 | 696 | S6 |
| 12 | 44.539 | 696 | S7 | |
| 18 | 44.596 | 697 | S8 | |
| 24 | 44.679 | 698 | S9 | |
| 30 | 44.730 | 699 | S10 | |
| 800 | 6 | 50.940 | 796 | S11 |
| 12 | 50.840 | 794 | S12 | |
| 18 | 51.095 | 798 | S13 | |
| 24 | 51.098 | 798 | S14 | |
| 30 | 51.138 | 799 | S15 | |
| 900 | 6 | — | — | — |
| 12 | 57.485 | 898 | S16 | |
| 18 | 57.429 | 897 | S17 | |
| 24 | 57.487 | 898 | S18 | |
| 30 | 57.252 | 895 | S19 | |
| 1000 | 6 | — | — | — |
| 12 | — | — | — | |
| 18 | — | — | — | |
| 24 | 63.847 | 998 | S20 | |
| 30 | 63.620 | 994 | S21 |
Table1 PA/EG form-stable composite PCMs sample with different mass fractions of EG and different densities
| 方块密度/(kg/m3) | EG/%(mass) | 质量/g | 密度/(kg/m3) | 样品序号 |
|---|---|---|---|---|
| 600 | 6 | 38.977 | 609 | S1 |
| 12 | 50.940 | 596 | S2 | |
| 18 | 38.076 | 595 | S3 | |
| 24 | 38.339 | 599 | S4 | |
| 30 | 38.001 | 594 | S5 | |
| 700 | 6 | 44.530 | 696 | S6 |
| 12 | 44.539 | 696 | S7 | |
| 18 | 44.596 | 697 | S8 | |
| 24 | 44.679 | 698 | S9 | |
| 30 | 44.730 | 699 | S10 | |
| 800 | 6 | 50.940 | 796 | S11 |
| 12 | 50.840 | 794 | S12 | |
| 18 | 51.095 | 798 | S13 | |
| 24 | 51.098 | 798 | S14 | |
| 30 | 51.138 | 799 | S15 | |
| 900 | 6 | — | — | — |
| 12 | 57.485 | 898 | S16 | |
| 18 | 57.429 | 897 | S17 | |
| 24 | 57.487 | 898 | S18 | |
| 30 | 57.252 | 895 | S19 | |
| 1000 | 6 | — | — | — |
| 12 | — | — | — | |
| 18 | — | — | — | |
| 24 | 63.847 | 998 | S20 | |
| 30 | 63.620 | 994 | S21 |
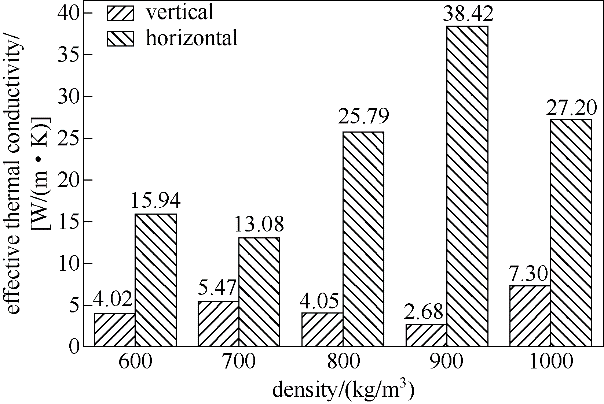
Fig.10 Changes in horizontal and vertical thermal conductivity of PA/EG form-stable composite samples with sample density when content of EG is 30%(mass)
| 1 | Huang X , Alva G , Liu L . et al. Microstructure and thermal properties of cetyl alcohol/high density polyethylene composite phase change materials with carbon fiber as shape-stabilized thermal storage materials [J]. Applied Energy, 2017, 200: 19-27. |
| 2 | Kant K , Shukla A , Sharma A . Advancement in phase change materials for thermal energy storage applications [J]. Solar Energy Materials and Solar Cells, 2017, 172: 82-92. |
| 3 | Chandel S S , Agarwal T . Review of current state of research on energy storage, toxicity, health hazards and commercialization of phase changing materials [J]. Renewable and Sustainable Energy Reviews, 2017, 67: 581-596. |
| 4 | Tang Y , Jia Y , Alva G , et al . Synthesis, characterization and properties of palmitic acid/high density polyethylene/graphene nanoplatelets composites as form-stable phase change materials [J]. Solar Energy Materials and Solar Cells, 2016, 155: 421-429. |
| 5 | Lin Y , Zhu C , Alva G , et al . Palmitic acid/polyvinyl butyral/expanded graphite composites as form-stable phase change materials for solar thermal energy storage [J]. Applied Energy, 2018, 228:1801-1809. |
| 6 | Cárdenas B , León N . High temperature latent heat thermal energy storage: phase change materials, design considerations and performance enhancement techniques [J]. Renewable and Sustainable Energy Reviews, 2013, 27: 724-737. |
| 7 | Atinafu D G , Dong W , Huang X , et al . Introduction of organic-organic eutectic PCM in mesoporous N-doped carbons for enhanced thermal conductivity and energy storage capacity [J]. Applied Energy, 2018, 211: 1203-1215. |
| 8 | Ma G , Liu S , Xie S , et al . Binary eutectic mixtures of stearic acid- n-butyramide/n-octanamide as phase change materials for low temperature solar heat storage [J]. Applied Thermal Engineering, 2017, 111: 1052-1059. |
| 9 | Tay N H S , Liu M , Belusko M , et al . Review on transportable phase change material in thermal energy storage systems [J]. Renewable and Sustainable Energy Reviews, 2017, 75: 264-277. |
| 10 | Lin Y , Jia Y , Alva G , et al . Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage [J]. Renewable and Sustainable Energy Reviews, 2018, 82: 2730-2742. |
| 11 | Khan M A , Saidur R , Al-Sulaiman F A . A review for phase change materials (PCMs) in solar absorption refrigeration systems [J]. Renewable and Sustainable Energy Reviews, 2017, 76: 105-137. |
| 12 | Alva G , Liu L , Huang X , et al . Thermal energy storage materials and systems for solar energy applications[J]. Renewable and Sustainable Energy Reviews, 2017, 68: 693-706. |
| 13 | Souayfane F , Fardoun F , Biwole P H . Phase change materials (PCM) for cooling applications in buildings: a review [J]. Energy and Buildings [J]. 2016, 129: 396-431. |
| 14 | Miró L , Gasia J , Cabeza L F . Thermal energy storage (TES) for industrial waste heat (IWH) recovery: a review [J]. Applied Energy, 2016, 179: 284-301. |
| 15 | Cao R R , Li X , Chen S , et al . Fabrication and characterization of novel shape-stabilized synergistic phase change materials based on PHDA/GO composites [J]. Energy, 2017, 138: 157-166. |
| 16 | Xu Y , Li M J , Zheng Z J , et al . Melting performance enhancement of phase change material by a limited amount of metal foam: configurational optimization and economic assessment [J]. Applied Energy, 2018, 212: 868-880. |
| 17 | Wu S , Li T X , Yan T , et al . High performance form-stable expanded graphite/stearic acid composite phase change material for modular thermal energy storage [J]. International Journal of Heat and Mass Transfer, 2016, 102: 733-744. |
| 18 | Zhang Q , Luo Z , Guo Q , et al . Preparation and thermal properties of short carbon fibers/erythritol phase change materials [J]. Energy Conversion and Management, 2017, 136: 220-228. |
| 19 | Xu T , Chen Q , Huang G , et al . Preparation and thermal energy storage properties of D-mannitol/expanded graphite composite phase change material [J]. Solar Energy Materials and Solar Cells, 2016, 155: 141-146. |
| 20 | Karaipekli A , Biçer A , Sarı A , et al . Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes [J]. Energy Conversion and Management, 2017, 134: 373-381. |
| 21 | Harish S , Orejon D , Takata Y , et al . Thermal conductivity enhancement of lauric acid phase change nanocomposite with graphene nanoplatelets [J]. Applied Thermal Engineering, 2015, 80: 205-211. |
| 22 | Leng G , Qiao G , Xu G , et al . Erythritol-Vermiculite form-stable phase change materials for thermal energy storage [J]. Energy Procedia, 2017, 142: 3363-3368. |
| 23 | Lv P , Liu C , Rao Z . Experiment study on the thermal properties of paraffin/kaolin thermal energy storage form-stable phase change materials [J]. Applied Energy, 2016, 182: 475-487. |
| 24 | Lin C , Rao Z . Thermal conductivity enhancement of paraffin by adding boron nitride nanostructures: a molecular dynamics study [J]. Applied Thermal Engineering, 2017, 110: 1411-1419. |
| 25 | Babapoor A , Karimi G . Thermal properties measurement and heat storage analysis of paraffin nanoparticles composites phase change material: comparison and optimization [J]. Applied Thermal Engineering, 2015, 90: 945-951. |
| 26 | Xiao X , Zhang P , Li M . Preparation and thermal characterization of paraffin/metal foam composite phase change material [J]. Applied Energy, 2013, 112: 1357-1366. |
| 27 | Zheng H , Wang C , Liu Q , et al . Thermal performance of copper foam/paraffin composite phase change material [J]. Energy Conversion and Management, 2018, 157: 372-381. |
| 28 | Zhang H , Gao X , Chen C , et al . A capric-palmitic-stearic acid ternary eutectic mixture/expanded graphite composite phase change material for thermal energy storage [J]. Composites Part A: Applied Science and Manufacturing, 2016, 87: 138-145. |
| 29 | Tang Y , Su D , Huang X , et al . Synthesis and thermal properties of the MA/HDPE composites with nano-additives as form-stable PCM with improved thermal conductivity [J]. Applied Energy, 2016, 180: 116-129. |
| 30 | Li Y L , Li J H , Feng W W , et al . Design and preparation of the phase change materials paraffin/porous Al2O3@graphite foams with enhanced heat storage capacity and thermal conductivity [J]. ACS Sustainable Chemistry & Engineering, 2017, 5: 7594-7603. |
| 31 | Yang J , Qi G Q , Liu Y , et al . Hybrid graphene aerogels/phase change material composites: thermal conductivity, shape-stabilization and light-to-thermal energy storage [J]. Carbon, 2016, 100: 693-702. |
| 32 | Wang J , Huang X , Gao H , et al . Construction of CNT@Cr-MIL-101-NH2 hybrid composite for shape-stabilized phase change materials with enhanced thermal conductivity [J]. Chemical Engineering Journal, 2018, 350: 164-172. |
| 33 | Liang W , Zhang G , Sun H , et al . Graphene-nickel/n -carboxylic acids composites as form-stable phase change materials for thermal energy storage [J]. Solar Energy Materials and Solar Cells, 2015, 132: 425-430. |
| 34 | Zhai T Y , Li T X , Wu S , et al . Preparation and thermal performance of form-stable expanded graphite/stearic acid composite phase change materials with high thermal conductivity [J]. Chinese Science Bulletin, 2017, 63(7): 674-683. |
| 35 | 仵斯, 李廷贤, 闫霆, 等 . 高性能定形复合相变储能材料的制备及热性能[J]. 化工学报, 2015, 66 (12): 5127-5134. |
| Wu S , Li T X , Yan T , et al . Preparation and thermal properties of high performance shape-stabilized phase change composites using stearic acid and expanded graphite [J]. CIESC Journal, 2015, 66 (12): 5127-5134. | |
| 36 | 向欢欢 . 三水合乙酸钠复合相变储热材料的制备与热物性研究 [D]. 广州: 广东工业大学, 2016. |
| Xiang H H . Preparation and thermal performance research of sodium acetate trihydrate composite phase change heat storage material [D]. Guangzhou: Guangdong University of Technology, 2016. | |
| 37 | 秦盟盟 . 碳复合材料的微观结构调控及性能研究 [D]. 天津: 天津大学, 2017. |
| Qin M M . Carbon-based composites with high thermal and mechanical properties by controlling their microstructures [D]. Tianjin: Tianjin University, 2017. | |
| 38 | Wang T , Wang S , Wu W . Experimental study on effective thermal conductivity of microcapsules based phase change composites [J]. International Journal of Heat and Mass Transfer, 2017, 109: 930-937. |
| [1] | Wentao WU, Liangyong CHU, Lingjie ZHANG, Weimin TAN, Liming SHEN, Ningzhong BAO. High-efficient preparation of cardanol-based self-healing microcapsules [J]. CIESC Journal, 2023, 74(7): 3103-3115. |
| [2] | Zhilong WANG, Ye YANG, Zhenzhen ZHAO, Tao TIAN, Tong ZHAO, Yahui CUI. Influence of mixing time and sequence on the dispersion properties of the cathode slurry of lithium-ion battery [J]. CIESC Journal, 2023, 74(7): 3127-3138. |
| [3] | Zhen LI, Bo ZHANG, Liwei WANG. Development and properties of PEG-EG solid-solid phase change materials [J]. CIESC Journal, 2023, 74(6): 2680-2688. |
| [4] | Feng ZHU, Kailin CHEN, Xiaofeng HUANG, Yinzhu BAO, Wenbin LI, Jiaxin LIU, Weiqiang WU, Wangwei GAO. Performance study of KOH modified carbide slag for removal of carbonyl sulfide [J]. CIESC Journal, 2023, 74(6): 2668-2679. |
| [5] | Xueting ZHANG, Jijiang HU, Jing ZHAO, Bogeng LI. Preparation of high molecular weight polypropylene glycol in microchannel reactor [J]. CIESC Journal, 2023, 74(3): 1343-1351. |
| [6] | Zhiyuan JIN, Guorong SHAN, Pengju PAN. Preparation and heat and salt resistance of AM/AMPS/SSS terpolymer [J]. CIESC Journal, 2023, 74(2): 916-923. |
| [7] | Chengwei LI, Huayong LUO, Mingxuan ZHANG, Peng LIAO, Qian FANG, Hongwei RONG, Jingyin WANG. Microfludically-generated lanthanum hydroxide cross-linked chitosan microspheres for phosphate removal [J]. CIESC Journal, 2022, 73(9): 3929-3939. |
| [8] | Jianing LIU, Jiahao MA, Junying ZHANG, Jue CHENG. Construction and properties of sequential dual thermal curing thiol-acrylate-epoxy 3D network [J]. CIESC Journal, 2022, 73(9): 4173-4186. |
| [9] | Lei ZHONG, Xueqing QIU, Wenli ZHANG. Advances in lignin-derived carbon anodes for alkali metal ion batteries [J]. CIESC Journal, 2022, 73(8): 3369-3380. |
| [10] | Duanhui GAO, Weiqiang XIAO, Feng GAO, Qian XIA, Manqiu WANG, Xinbo LU, Xiaoli ZHAN, Qinghua ZHANG. Preparation and application of polyimide-based aerogels [J]. CIESC Journal, 2022, 73(7): 2757-2773. |
| [11] | Chaoyu SONG, Yaxuan XIONG, Jinhua ZHANG, Yuhe JIN, Chenhua YAO, Huixiang WANG, Yulong DING. Preparation and performance study of incinerated slag based shape-stable phase change composites [J]. CIESC Journal, 2022, 73(5): 2279-2287. |
| [12] | Hang GUO, Wenli HAN, Xiaoling DONG, Wencui LI. Adjusting carbonization process to optimize sodium storage performance of coal-based hard carbon anode [J]. CIESC Journal, 2022, 73(4): 1794-1806. |
| [13] | Chaoqun XU, Juan YU, Yimin FAN, Jifu WANG, Fuxiang CHU. Chemical modification of nanocellulose via atom transfer radical polymerization: strategy, applications and challenges [J]. CIESC Journal, 2022, 73(3): 1022-1043. |
| [14] | ZHOU Dongyi, XIAO Xianghua, XIAO Biao, LIU Yicai. Method of determining optimum mass ratio of fatty acids in composite phase change materials for thermal energy storage [J]. CIESC Journal, 2021, 72(S1): 560-566. |
| [15] | Kang YAN, Song YANG, Shoujun LIU, Chao YANG, Huiling FAN, Ju SHANGGUAN. In-situ preparation of ZnO-based activated carbon desulfurizer from low-rank coal [J]. CIESC Journal, 2021, 72(9): 4921-4930. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||