化工学报 ›› 2022, Vol. 73 ›› Issue (7): 3240-3250.DOI: 10.11949/0438-1157.20220316
收稿日期:2022-03-02
修回日期:2022-04-01
出版日期:2022-07-05
发布日期:2022-08-01
通讯作者:
钟海
作者简介:李文涛(1996—),男,硕士研究生,基金资助:
Wentao LI( ),Huijuan LIN,Hai ZHONG(
),Huijuan LIN,Hai ZHONG( )
)
Received:2022-03-02
Revised:2022-04-01
Online:2022-07-05
Published:2022-08-01
Contact:
Hai ZHONG
摘要:
以六氟磷酸锂(LiPF6)为四氢呋喃的聚合引发剂制备凝胶电解质,同时作为氟源在金属锂负极表面原位构建富含LiF的固态电解质界面层(solid electrolyte interface,SEI)来抑制锂枝晶的生长以及金属锂/电解液之间的副反应。所制备的凝胶电解质具有较高的室温离子电导率(1.33 mS·cm-1)和较宽的电化学稳定窗口(4.5 V)。原位聚合方式组装金属锂对称电池循环后,锂负极表面没有明显的锂枝晶和被损毁的形貌出现;XPS结果表明锂负极表面生成了富含LiF的SEI。组装的LiFePO4全电池在1 C的电流密度下,稳定循环400周后仍保持118.7 mAh·g-1的放电比容量。得益于四氢呋喃在开环聚合反应过程中,促进了LiPF6分解反应平衡的正向移动,在锂负极表面形成稳定的富含LiF的SEI,能够抑制锂枝晶的生长并防止其被持续性的腐蚀破坏。
中图分类号:
李文涛, 林慧娟, 钟海. 原位构建富氟SEI的凝胶电解质用于金属锂二次电池[J]. 化工学报, 2022, 73(7): 3240-3250.
Wentao LI, Huijuan LIN, Hai ZHONG. LiF-rich SEI generated by in-situ gel polymer electrolyte process for lithium metal rechargeable batteries[J]. CIESC Journal, 2022, 73(7): 3240-3250.
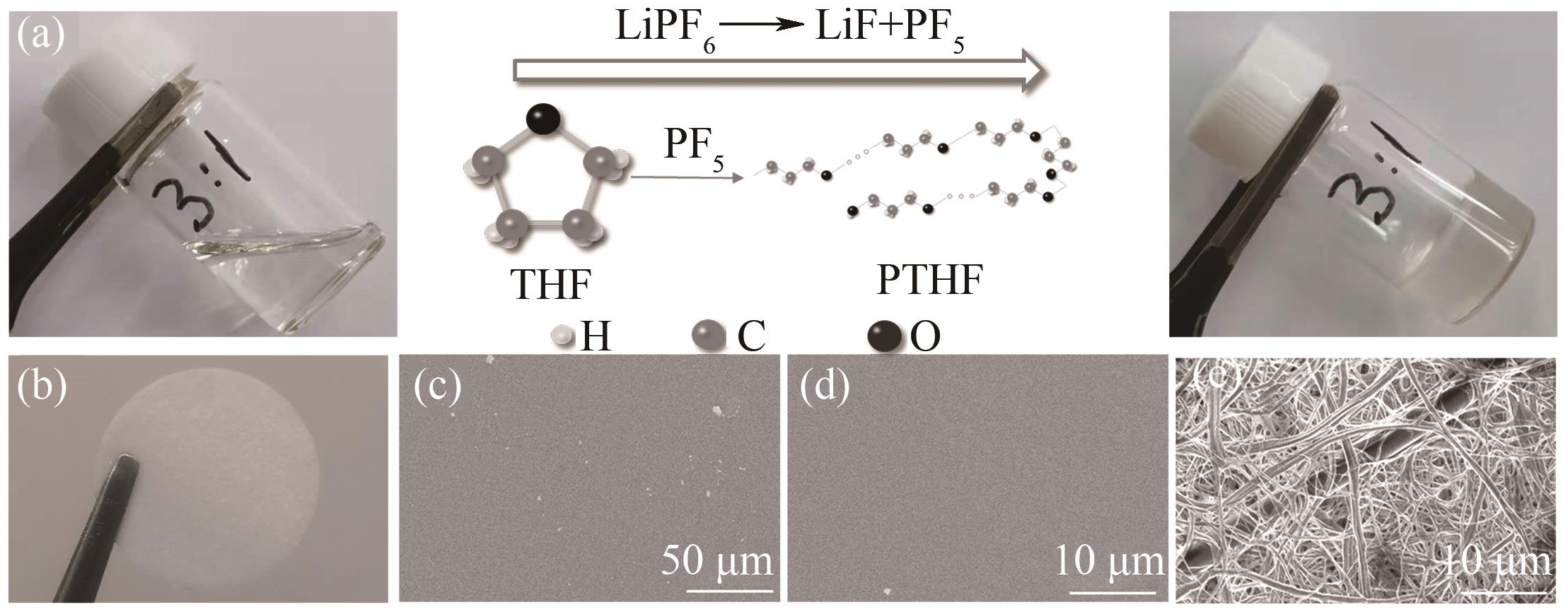
图1 (a) THF开环聚合机理示意图; (b) 纤维素隔膜支撑的GPE光学照片; (c),( d)不同放大倍数的GPE的SEM图;(e) 纤维素隔膜的SEM图
Fig.1 (a) Illustration of polymerization mechanism; (b) Photograph of GPE with cellulose film; (c), (d) SEM images of GPE under different magnifications; (e) SEM image of cellulose film
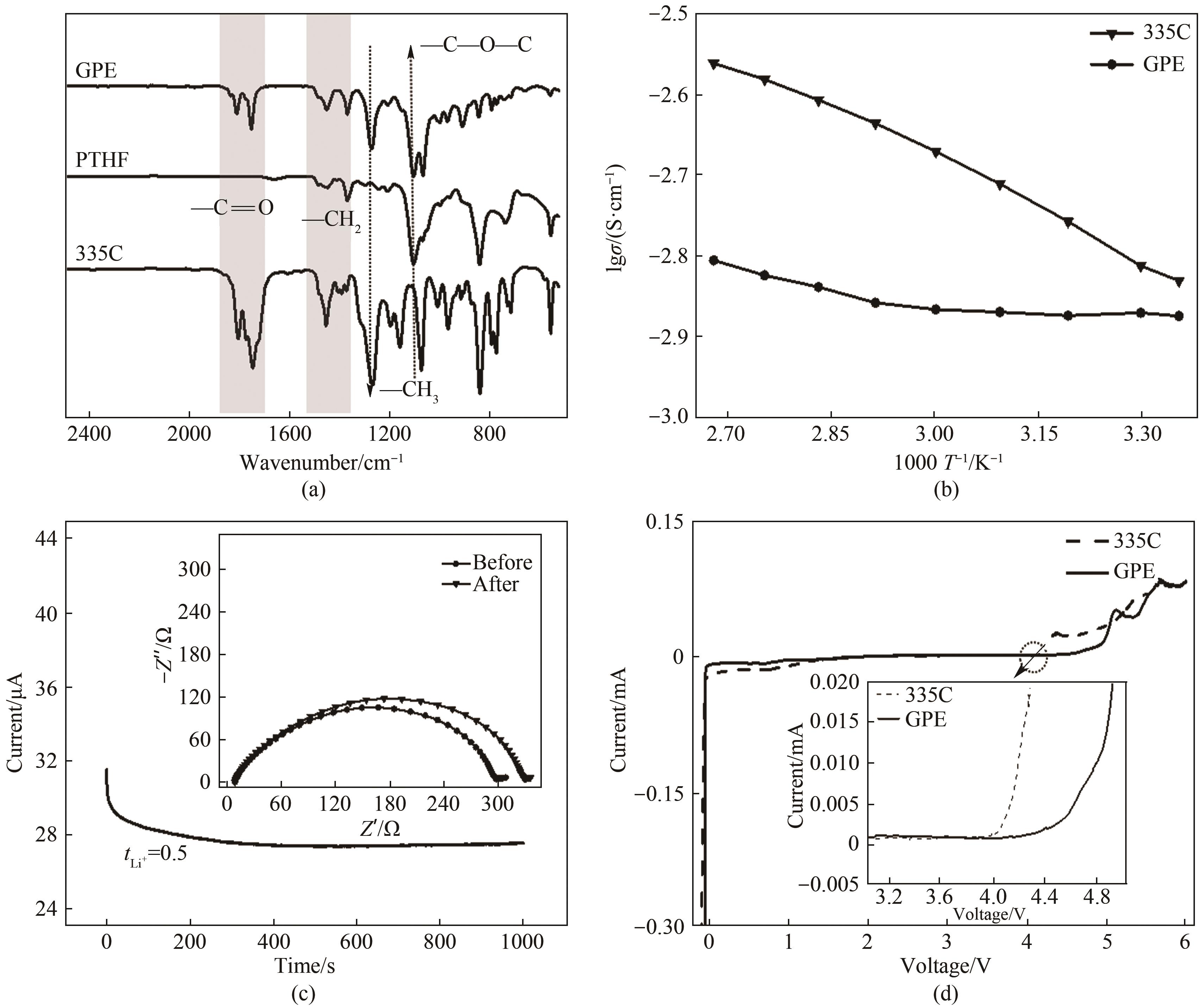
图2 (a) GPE, PTHF以及335C电解液的FTIR光谱; (b) GPE与335C电解液的离子电导率-温度变化曲线; (c) Li|GPE|Li对称电池的计时安培曲线(插图为对称电池极化前后的交流阻抗谱); (d) GPE与335C电解液的LSV曲线
Fig.2 (a) FTIR spectra of GPE, PTHF and 335C electrolyte; (b) Temperature dependent ionic conductivity of GPE and 335C electrolyte; (c) The chronoamperometry profile of Li|GPE|Li symmetric cell(the inset displays the impedance spectra before and after chronoamperometry); (d) Linear sweep curves of GPE and 335C electrolyte

图3 (a) 室温下Li|GPE|Li对称电池在不同静置时间的交流阻抗谱图; (b) 采用GPE、 335C电解液组装的Li|Li电池在电流密度为0.5 mA·cm-2, 面容量为0.5 mAh·cm-2条件下的循环稳定性能图;(c) 335C电解液及(d) GPE组装的锂负极经过20次循环后表面的SEM图
Fig.3 (a) AC impedance spectra of Li|GPE|Li symmetrical cells for different time at room temperature; (b) Cyclic performance of Li symmetric cells with GPE, 335C electrolyte at a current density of 0.5 mA·cm-2 with area capacity of 0.5 mAh·cm-2; Typical SEM images of the Li anode with (c) 335Celectrolyte, and (d) GPE after 20 cycles
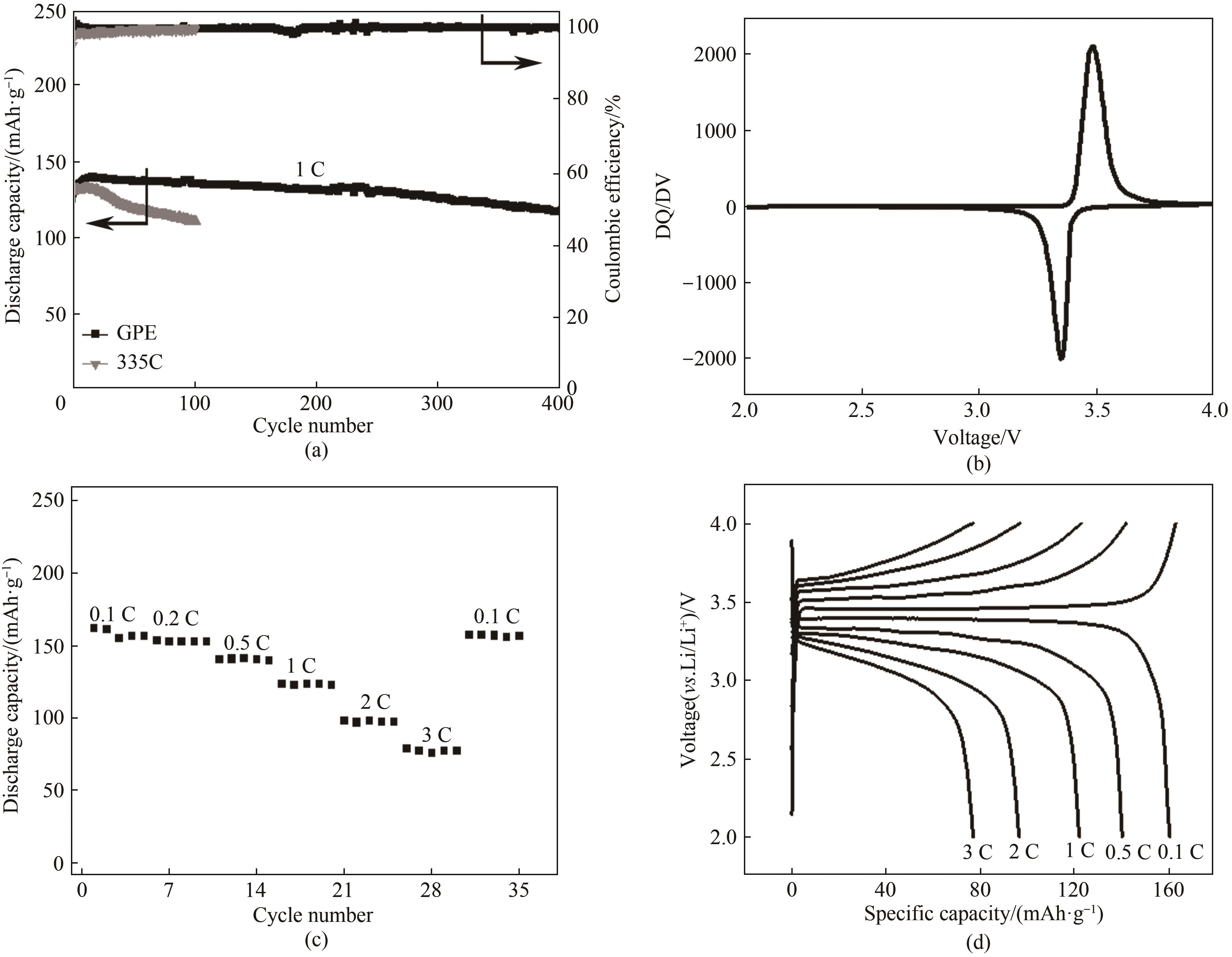
图4 (a) Li|LiFePO4电池在充放电电压为2.0 ~ 4.0 V以及电流密度为1 C条件下分别采用GPE和335C电解液的长期循环性能;(b) Li|GPE|LiFePO4电池放电曲线所对应的DQ/DV图; (c) Li|GPE|LiFePO4电池在不同电流密度下的倍率性能及其(d)对应的电压-比容量
Fig.4 (a) Long-term cycling performance of Li|GPE|LiFePO4 cells(the cells were charged and discharged between 2.0—4.0 V at a current rate of 1 C); (b) DQ/DV diagram corresponding to charge and discharge curves of LMBs; (c) Rate performance of Li|GPE|LiFePO4 at various current rate; (d) Corresponding voltage profiles at various current rates

图5 GPE和335C电解液中循环50周后锂负极表面XPS的(a) F 1s谱图和(b) Li 1s谱图; (c) 金属锂表面LiF物质生成反应路径示意图
Fig.5 XPS spectra of Li metal (a) F 1s spectra, (b) Li 1s spectra in GPE and 335C electrolyte after 50 cycle;(c) Schematic diagram of LiF produce reaction
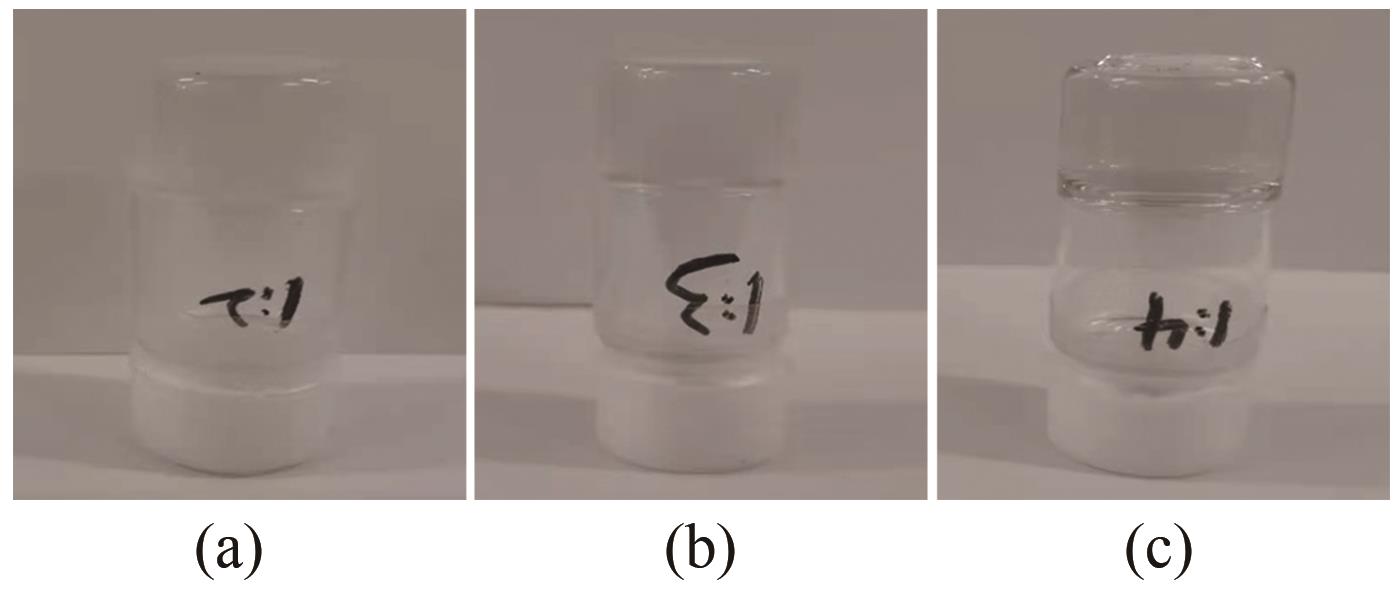
图A1 THF与335C前体混合溶液质量比为1∶2 (a), 1∶3 (b), 1∶4 (c)引发聚合后倒置状态的照片
Fig.A1 The photograph of THF/335C precursor solution with mass ratios of 1∶2 (a), 1∶3 (b), 1∶4 (c) after completion of polymerization
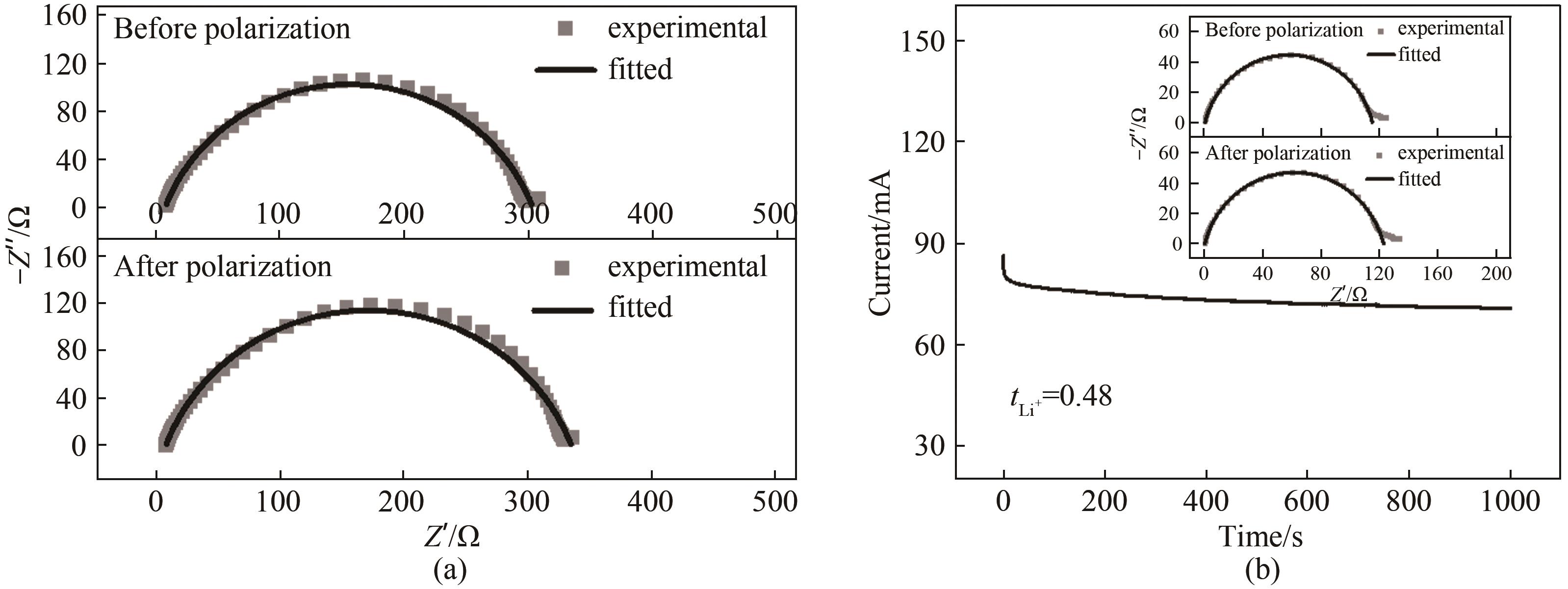
图A2 (a) Li|GPE|Li对称电池的阻抗拟合谱图; (b) Li|335C|Li对称电池的计时安培曲线(插图为对称电池极化前后的交流阻抗谱)
Fig.A2 (a) The impedance spectra of Li|GPE|Li symmetric cell before and after chronoamperometry. (b) The chronoamperometry profile of Li|335C|Li symmetric cell(The inset displays the impedance spectra before and after chronoamperometry)

图A3 对应图2(d)中的GPE和335C电解液在LSV曲线中被还原分解区域内[0 ~ 1.5 V(vs Li/Li+)]放大图
Fig.A3 The corresponding magnified views of two electrolytes decomposed at reduction reaction regions [0—1.5 V(vs Li/Li+)] in Fig. 2(d)

图A4 (a) 室温下Li|335C|Li对称电池在不同静置时间的交流阻抗谱图; (b)新鲜锂负极表面的SEM图; (c), (d) GPE所组装的锂负极经过20次循环后金属锂表面在不同尺度下的SEM图
Fig.A4 (a) AC impedance spectra of Li|335C|Li symmetrical cell for different time at room temperature; (b) Typical SEM images at the surface of fresh Li anode; (c),(d) Typical SEM images of the Li anode with GPE after 20th cycles under different scales
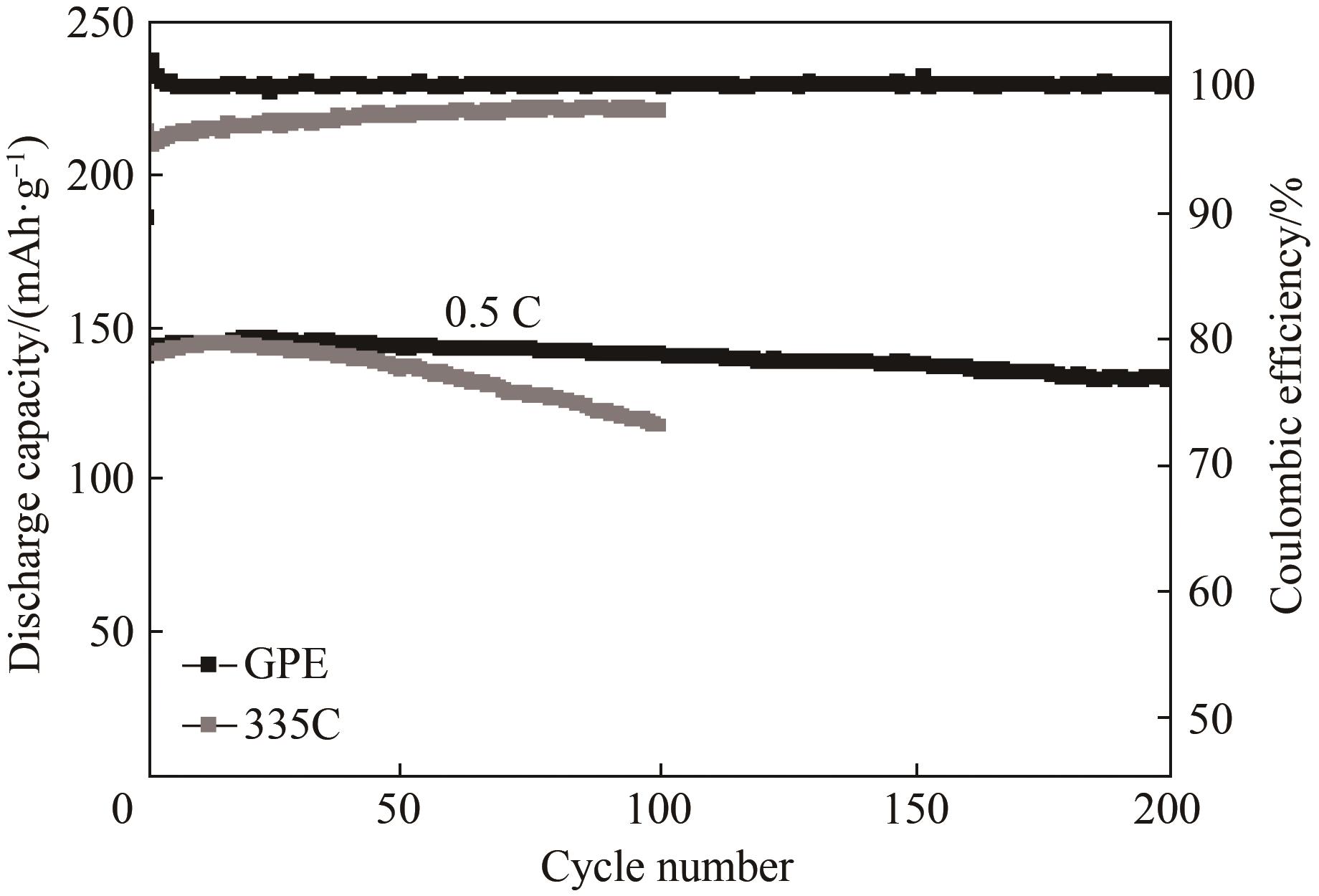
图A5 Li|LiFePO4电池在充放电电压为2.0 ~ 4.0 V以及电流密度为0.5 C条件下分别采用GPE和335C电解液的长期循环性能
Fig.A5 Long-term cycling performance of Li| LiFePO4 cells with GPE or 335C electrolyte at a current rate of 0.5 C and voltage range of 2.0—4.0 V
| 1 | Liu F Q, T, Li T, Yang Y J, et al. Investigation on the copolymer electrolyte of poly(1, 3-dioxolane-co-formaldehyde)[J]. Macromolecular Rapid Communications, 2020, 41(9): 2000047. |
| 2 | Bai M H, Xie K Y, Hong B, et al. An artificial Li3PO4 solid electrolyte interphase layer to achieve petal-shaped deposition of lithium[J]. Solid State Ionics, 2019, 333: 101-104. |
| 3 | Zhang Q K, Zhang X Q, Yuan H, et al. Thermally stable and nonflammable electrolytes for lithium metal batteries: progress and perspectives[J]. Small Science, 2021, 1(10): 2100058. |
| 4 | Jin D Q, Hu K, Hou R, et al. Vertical nanoarrays with lithiophilic sites suppress the growth of lithium dendrites for ultrastable lithium metal batteries[J]. Chemical Engineering Journal, 2021, 405: 126808. |
| 5 | Golozar M, Paolella A, Demers H, et al. Direct observation of lithium metal dendrites with ceramic solid electrolyte [J]. Scientific Reports, 2020, 10: 18410. |
| 6 | Ke X Y, Wang Y, Dai L M, et al. Cell failures of all-solid-state lithium metal batteries with inorganic solid electrolytes: lithium dendrites[J]. Energy Storage Materials, 2020, 33: 309-328. |
| 7 | Chi S S, Liu Y C, Zhao N, et al. Solid polymer electrolyte soft interface layer with 3D lithium anode for all-solid-state lithium batteries[J]. Energy Storage Materials, 2019, 17: 309-316. |
| 8 | Lu Y, Zhao C Z, Yuan H, et al. Critical current density in solid-state lithium metal batteries: mechanism, influences, and strategies[J]. Advanced Functional Materials, 2021, 31(18): 2009925. |
| 9 | Chen L, Li W X, Fan L Z, et al. Intercalated electrolyte with high transference number for dendrite-free solid-state lithium batteries[J]. Advanced Functional Materials, 2019, 29(28): 1901047. |
| 10 | Tabani Z, Maghsoudi H, Fathollahi Zonouz A. High electrochemical stability of polyvinylidene fluoride (PVDF) porous membranes using phase inversion methods for lithium-ion batteries[J]. Journal of Solid State Electrochemistry, 2021, 25(2): 651-657. |
| 11 | Tarascon J M, Gozdz A S, Schmutz C, et al. Performance of Bellcore’s plastic rechargeable Li-ion batteries[J]. Solid State Ionics, 1996, 86/87/88: 49-54. |
| 12 | Zhou J Q, Ji H Q, Liu J, et al. A new high ionic conductive gel polymer electrolyte enables highly stable quasi-solid-state lithium sulfur battery[J]. Energy Storage Materials, 2019, 22: 256-264. |
| 13 | Chen F, Yang D J, Zha W P, et al. Solid polymer electrolytes incorporating cubic Li7La3Zr2O12 for all-solid-state lithium rechargeable batteries[J]. Electrochimica Acta, 2017, 258: 1106-1114. |
| 14 | Wang Q Y, Xu X Q, Hong B, et al. Molecular engineering of a gel polymer electrolyte via in situ polymerization for high performance lithium metal batteries[J]. Chemical Engineering Journal, 2022, 428: 131331. |
| 15 | Deng B, Jing M X, Li L X, et al. Nano-zirconia boosting the ionic conductivity and lithium dendrite inhibition ability of a poly(1, 3-dioxolane) solid electrolyte for high-voltage solid-state lithium batteries[J]. Sustainable Energy & Fuels, 2021, 5(21): 5461-5470. |
| 16 | Zhang X L, Zhao S Y, Fan W, et al. Long cycling, thermal stable, dendrites free gel polymer electrolyte for flexible lithium metal batteries[J]. Electrochimica Acta, 2019, 301: 304-311. |
| 17 | Wu H, Tang B, Du X F, et al. LiDFOB initiated in situ polymerization of novel eutectic solution enables room-temperature solid lithium metal batteries[J]. Advanced Science, 2020, 7(23): 2003370. |
| 18 | Shen Y Q, Zhu W, Papadaki M, et al. Thermal decomposition of solid benzoyl peroxide using advanced reactive system screening tool: effect of concentration, confinement and selected acids and bases[J]. Journal of Loss Prevention in the Process Industries, 2019, 60: 28-34. |
| 19 | Zhong H, Wang C, Xu Z, et al. A novel quasi-solid state electrolyte with highly effective polysulfide diffusion inhibition for lithium-sulfur batteries[J]. Scientific Reports, 2016, 6: 25484. |
| 20 | Xie M, Wu Y, Liu Y, et al. Pathway of in situ polymerization of 1, 3-dioxolane in LiPF6 electrolyte on Li metal anode[J]. Materials Today Energy, 2021, 21: 100730. |
| 21 | Cui Y Y, Liang X M, Chai J C, et al. High performance solid polymer electrolytes for rechargeable batteries: a self-catalyzed strategy toward facile synthesis[J]. Advanced Science, 2017, 4(11): 1700174. |
| 22 | Ma Q, Yue J P, Fan M, et al. Formulating the electrolyte towards high-energy and safe rechargeable lithium-metal batteries[J]. Angewandte Chemie International Edition, 2021, 60(30): 16554-16560. |
| 23 | Tominaga Y, Kato S, Nishimura N. Preparation and electrochemical characterization of magnesium gel electrolytes based on crosslinked poly(tetrahydrofuran)[J]. Polymer, 2021, 224: 123743. |
| 24 | Li T, Zhang X Q, Shi P, et al. Fluorinated solid-electrolyte interphase in high-voltage lithium metal batteries[J]. Joule, 2019, 3(11): 2647-2661. |
| 25 | Yuan Y X, Wu F, Bai Y, et al. Regulating Li deposition by constructing LiF-rich host for dendrite-free lithium metal anode[J]. Energy Storage Materials, 2019, 16: 411-418. |
| 26 | Feng Y Y, Zhang C F, Li B, et al. Low-volume-change, dendrite-free lithium metal anodes enabled by lithophilic 3D matrix with LiF-enriched surface[J]. Journal of Materials Chemistry A, 2019, 7(11): 6090-6098. |
| 27 | Hoene R, Reichert K H W. Zur aufklärung des initiierungsschrittes der kationischen polymerisation von tetrahydrofuran mit PF5 [J]. Die Makromolekulare Chemie, 1976, 177(12): 3545-3570. |
| 28 | Zhang C J, Hu L F, Wu H L, et al. Dual organocatalysts for highly active and selective synthesis of linear poly(γ-butyrolactone)s with high molecular weights[J]. Macromolecules, 2018, 51(21): 8705-8711. |
| 29 | Huang S Q, Cui Z L, Qiao L X, et al. An in situ polymerized solid polymer electrolyte enables excellent interfacial compatibility in lithium batteries[J]. Electrochimica Acta, 2019, 299: 820-827. |
| 30 | O'Connor C J, Cleverly D R. Fourier-transform infrared assay of bile salt-stimulated lipase activity in reversed micelles[J]. Journal of Chemical Technology & Biotechnology, 1994, 61(3): 209-214. |
| 31 | 赵航, 魏闯, 康鑫, 等. 锂离子电池三元正极材料的研究进展[J]. 中国陶瓷, 2020, 56(5): 10-15. |
| Zhao H, Wei C, Kang X, et al. Research progress of ternary cathode materials for lithium ion battery[J]. China Ceramics, 2020, 56(5): 10-15. | |
| 32 | 蓝兹炜, 张建茹, 李园园, 等. 基于锂离子电池正极材料的一元/二元复合正极材料研究进展[J]. 储能科学与技术, 2021, 10(1): 27-39. |
| Lan Z W, Zhang J R, Li Y Y, et al. Research progress of mono/binary composite cathode materials based on lithium-ion battery cathode materials[J]. Energy Storage Science and Technology, 2021, 10(1): 27-39. | |
| 33 | Xu C, Sun B, Gustafsson T, et al. Interface layer formation in solid polymer electrolyte lithium batteries: an XPS study[J]. Journal of Materials Chemistry A, 2014, 2(20): 7256-7264. |
| 34 | Che H Y, Yang X R, Wang H, et al. Long cycle life of sodium-ion pouch cell achieved by using multiple electrolyte additives[J]. Journal of Power Sources, 2018, 407: 173-179. |
| 35 | Winkler V, Hanemann T, Bruns M. Comparative surface analysis study of the solid electrolyte interphase formation on graphite anodes in lithium-ion batteries depending on the electrolyte composition[J]. Surface and Interface Analysis, 2017, 49(5): 361-369. |
| 36 | Fu J L, Ji X, Chen J, et al. Lithium nitrate regulated sulfone electrolytes for lithium metal batteries[J]. Angewandte Chemie International Edition, 2020, 59(49): 22194-22201. |
| 37 | Qiao Y, Wu J W, Zhao J, et al. Synergistic effect of bifunctional catalytic sites and defect engineering for high-performance Li-CO2 batteries[J]. Energy Storage Materials, 2020, 27: 133-139. |
| 38 | Wang L N, Liu J Y, Yuan S Y, et al. To mitigate self-discharge of lithium–sulfur batteries by optimizing ionic liquid electrolytes[J]. Energy & Environmental Science, 2016, 9(1): 224-231. |
| 39 | Krotkov D, Schneier D, Menkin D S, et al. Operando terahertz spectroscopy of solid electrolyte interphase evolution on silicon anodes[J]. Batteries & Supercaps, 2022, 5(1): e202100183. |
| 40 | Hamidah N L, Wang F M, Nugroho G. The understanding of solid electrolyte interface (SEI) formation and mechanism as the effect of flouro-o-phenylenedimaleimaide (F-MI) additive on lithium-ion battery[J]. Surface and Interface Analysis, 2019, 51(3): 345-352. |
| [1] | 逯翠梅1,李 辉1,2,李昱琢1,谭先周2,李 贵2. 硅胶衍生尼古丁印迹整体柱的制备及其识别[J]. 化工进展, 2013, 32(07): 1613-1620. |
| [2] | 李杰, 刘红研, 汪树军, 游龙, 王肃凯. 密胺树脂硫磺微胶囊的制备及应用[J]. 化工学报, 2011, 62(6): 1716-1722. |
| [3] | 孙 凯,张步宁,晏凤梅,尹国强,崔英德. 石蜡微胶囊型相变储能材料制备及表征[J]. CIESC Journal, 2011, 30(12): 2676-. |
| [4] | 胡剑峰,夏正斌,司徒粤,陈焕钦. MF包封DCPD自修复微胶囊的合成 [J]. CIESC Journal, 2010, 61(11): 2978-2984. |
| [5] | 何秋星, 刘蕤, 涂伟萍. 铋掺杂氧化锡/聚氨酯的原位聚合动力学 [J]. 化工学报, 2008, 59(9): 2366-2370. |
| [6] | 方玉堂 匡胜严 张正国 高学农. 纳米胶囊相变材料的制备 [J]. CIESC Journal, 2007, 58(3): 771-775. |
| [7] | 庄秋虹,张正国,方晓明. 微/纳米胶囊相变材料的制备及应用进展 [J]. CIESC Journal, 2006, 25(4): 388-. |
| [8] | 张森;史鹏飞. 凝胶聚合物电解质的电化学性能 [J]. CIESC Journal, 2005, 56(2): 329-332. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号