化工学报 ›› 2024, Vol. 75 ›› Issue (6): 2283-2298.DOI: 10.11949/0438-1157.20240134
王岩1( ), 周佳文1, 孙培亮2, 陈勇1(
), 周佳文1, 孙培亮2, 陈勇1( ), 齐元红3, 彭冲2(
), 齐元红3, 彭冲2( )
)
收稿日期:2024-01-29
修回日期:2024-04-11
出版日期:2024-06-25
发布日期:2024-07-03
通讯作者:
陈勇,彭冲
作者简介:王岩(1998—),女,硕士研究生,wangyan040321@163.com
基金资助:
Yan WANG1( ), Jiawen ZHOU1, Peiliang SUN2, Yong CHEN1(
), Jiawen ZHOU1, Peiliang SUN2, Yong CHEN1( ), Yuanhong QI3, Chong PENG2(
), Yuanhong QI3, Chong PENG2( )
)
Received:2024-01-29
Revised:2024-04-11
Online:2024-06-25
Published:2024-07-03
Contact:
Yong CHEN, Chong PENG
摘要:
聚多巴胺(PDA)辅助负载聚氨基噻唑(PAT)法制备了磁性颗粒吸附剂Fe3O4@SiO2@PDA-PAT。对吸附剂进行了XRD、VSM、TG、SEM、XPS、EDS和Zeta电位等表征分析,考察了吸附剂对Hg2+的吸附性能。结果表明,Fe3O4@SiO2@PDA-PAT具有超顺磁性,在pH小于2时Zeta电位为正,pH大于2时Zeta电位为负。在303 K和Hg2+浓度为50 mg/L模拟废水中,当pH为1.3和5.0时,Hg2+的平衡吸附量分别为121.9 mg/g和153.1 mg/g。在强酸(如pH 1.3)和弱酸(如pH 5.0)环境下Fe3O4@SiO2@PDA-PAT吸附Hg2+的过程均是自发过程,且符合二级动力学模型和Langmuir等温吸附模型。强酸环境下(如pH 1.3)Fe3O4@SiO2@PDA-PAT吸附Hg2+是焓驱动的放热过程,弱酸(如pH 5.0)环境下是熵驱动的吸热过程。用2 mol/L混酸(盐酸和硝酸摩尔比为1∶1)作为解吸液可使Hg2+解吸率达91%以上。在303 K、pH 1.3、Hg2+浓度20 mg/L条件下,当Na+、K+、Mg2+、Ca2+、Cu2+、Zn2+、Ni2+质量浓度为Hg2+的20倍时,Fe3O4@SiO2@PDA-PAT的Hg2+平衡吸附量分别下降了33.2%、32.1%、20.6%、26.7%、21.2%、29.6%、17.8%。在模拟海水下,Hg2+吸附量下降40.9%。Fe3O4@SiO2@PDA-PAT具有较好的Hg2+选择性,具有净化海水脱除重金属的应用潜力。
中图分类号:
王岩, 周佳文, 孙培亮, 陈勇, 齐元红, 彭冲. 磁性聚氨基噻唑吸附剂脱除水体Hg2+性能[J]. 化工学报, 2024, 75(6): 2283-2298.
Yan WANG, Jiawen ZHOU, Peiliang SUN, Yong CHEN, Yuanhong QI, Chong PENG. Removal of Hg2+ from water by magnetic polyaminothiazole adsorbent[J]. CIESC Journal, 2024, 75(6): 2283-2298.
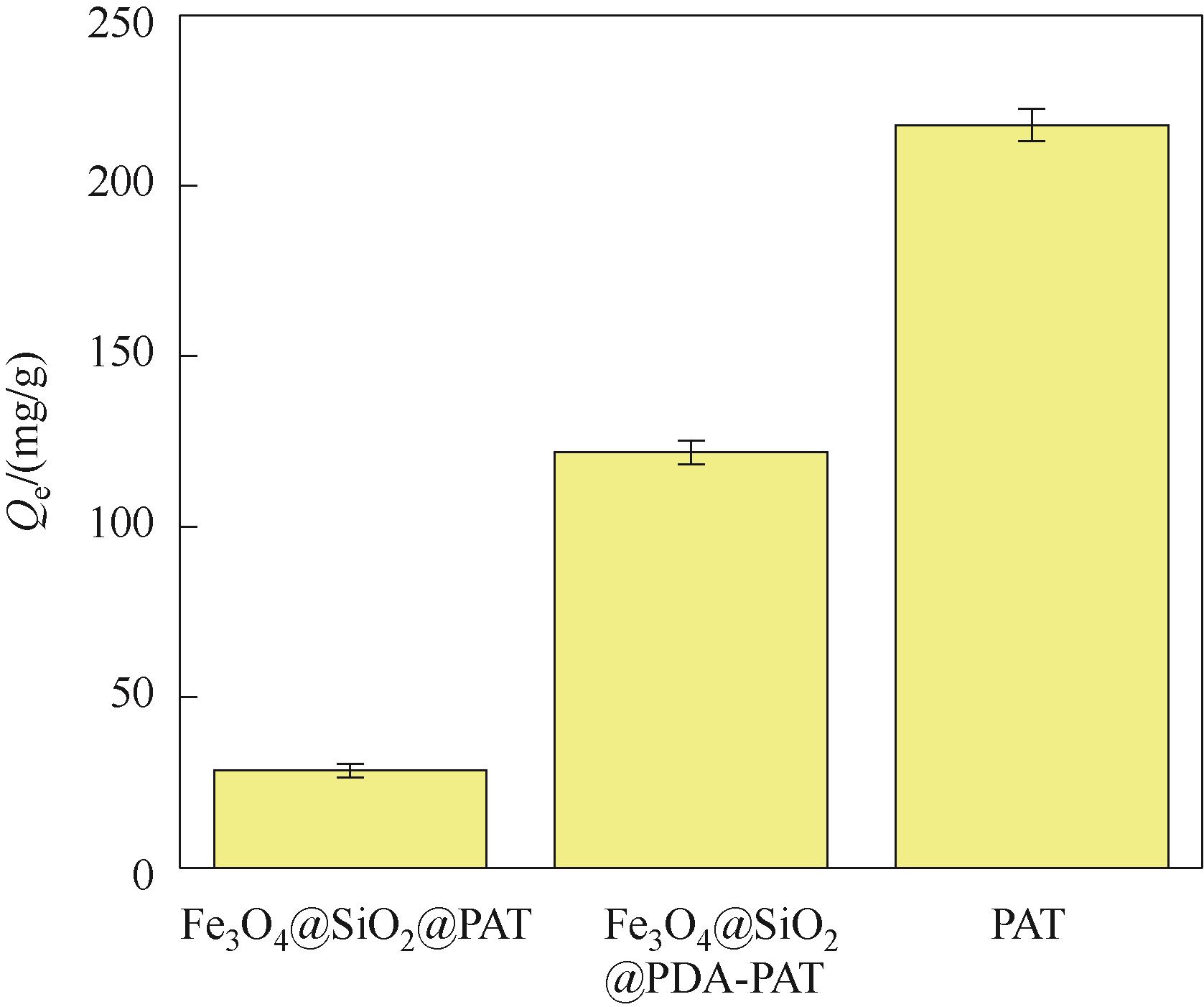
图2 Fe3O4@SiO2@PAT、Fe3O4@SiO2@PDA-PAT和PAT的Hg2+平衡吸附量(实验条件:pH 为1.3和Hg2+浓度为50 mg/L的废水100 ml,20 mg吸附剂在303 K下吸附24 h)
Fig.2 The Hg2+ equilibrium adsorption capacity of Fe3O4@SiO2 @PAT, Fe3O4@SiO2@PDA-PAT, PAT (experimental conditions: 100 ml of wastewater with pH of 1.3 and Hg2+ concentration of 50 mg/L was absorbed by 20 mg adsorbent at 303 K for 24 h)
| 元素 | 峰值/eV | 元素含量/% |
|---|---|---|
| Hg 4f | 103.18 | — |
| N 1s | 399.71 | 5.52 |
| S 2p | 163.91 | 1.25 |
| C 1s | 284.6 | 92.33 |
| Cu 2p | 932.73 | 0.53 |
表1 Fe3O4@SiO2@PDA-PAT的XPS元素含量
Table 1 Fe3O4@SiO2@PDA-PAT XPS elements content
| 元素 | 峰值/eV | 元素含量/% |
|---|---|---|
| Hg 4f | 103.18 | — |
| N 1s | 399.71 | 5.52 |
| S 2p | 163.91 | 1.25 |
| C 1s | 284.6 | 92.33 |
| Cu 2p | 932.73 | 0.53 |
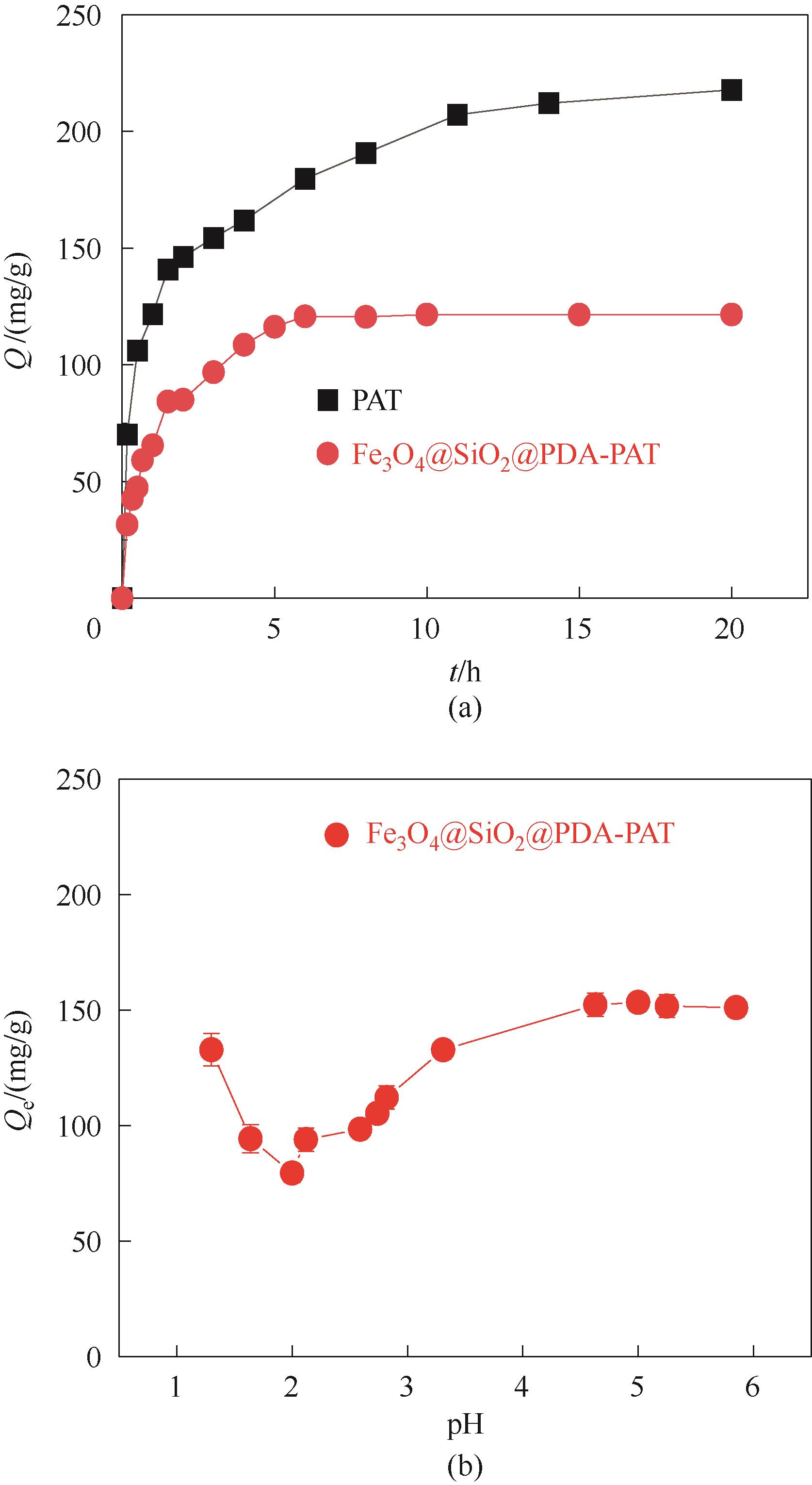
图8 吸附时间和pH对Fe3O4@SiO2@PDA-PAT吸附Hg2+的影响(实验条件:pH为1.3和Hg2+浓度为50 mg/L的废水100 ml,20 mg吸附剂在303 K下吸附24 h)
Fig.8 Effect of adsorption time and pH on Hg2+ adsorption by Fe3O4@SiO2@PDA-PAT (experimental conditions: 100 ml of wastewater with pH of 1.3 and Hg2+ concentration of 50 mg/L was absorbed by 20 mg adsorbent at 303 K for 24 h)
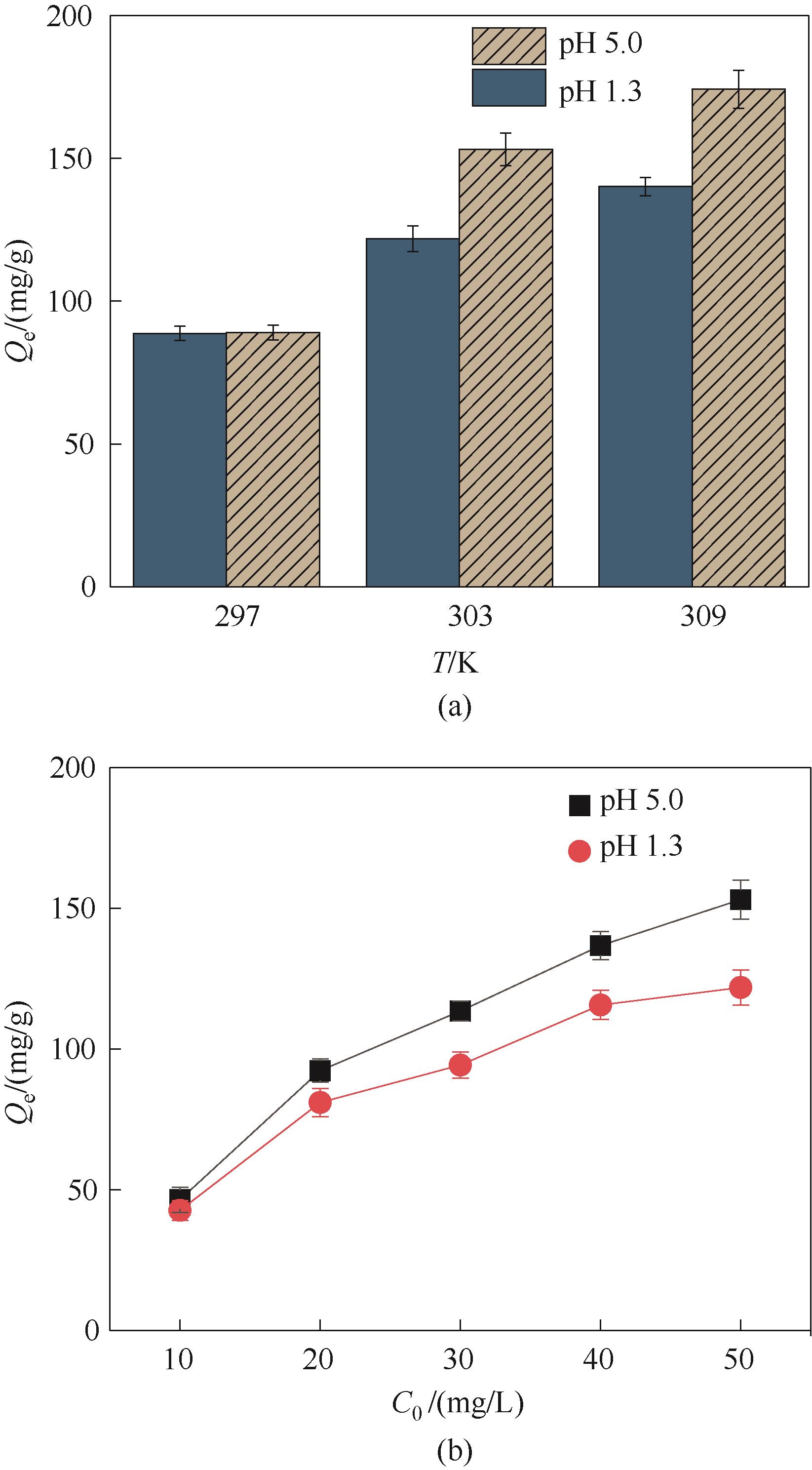
图9 温度和Hg2+浓度对Fe3O4@SiO2@PDA-PAT吸附性能的影响(实验条件:pH为1.3或pH为5.0和Hg2+浓度为50 mg/L的废水100 ml,20 mg吸附剂在303 K下吸附24 h)
Fig.9 Effect of temperature and Hg2+ concentration on the adsorption properties of Fe3O4@SiO2@PDA-PAT (experimental conditions: 100 ml wastewater with pH of 1.3 or pH of 5.0 and Hg2+ concentration of 50 mg/L was adsorbed by 20 mg adsorbent at 303 K for 24 h)
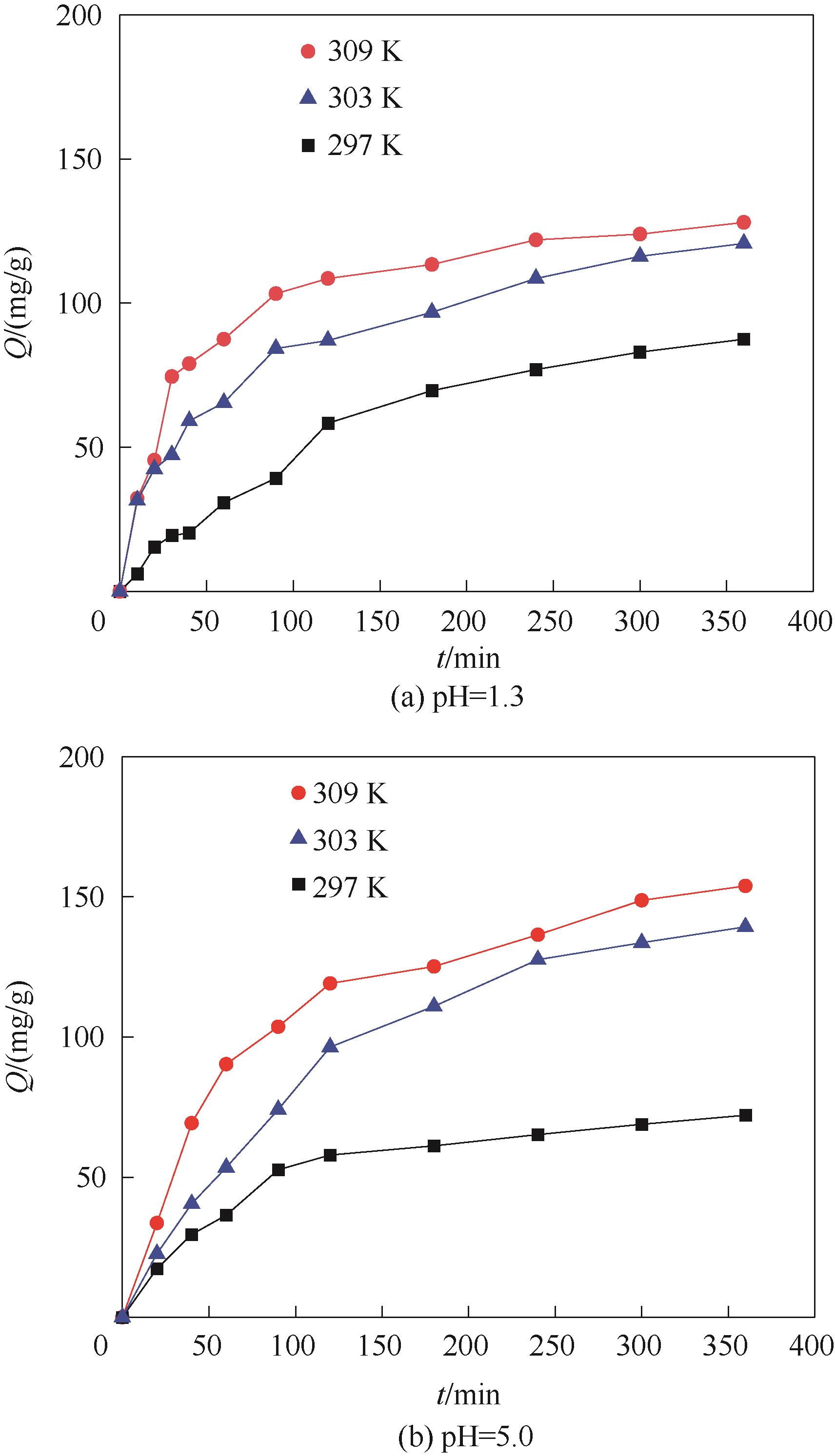
图10 不同pH时Fe3O4@SiO2@PDA-PAT的Hg2+吸附时间与吸附量的关系(实验条件:pH为1.3或5.0和Hg2+浓度为50 mg/L的废水100 ml,20 mg吸附剂)
Fig.10 Relationship between mercury adsorption time and adsorption capacity of Fe3O4@SiO2@PDA-PAT at different pH (experimental conditions: 100 ml of wastewater with pH 1.3 or pH 5.0 and Hg2+ concentration of 50 mg/L, 20 mg of adsorbent)
| pH | T/K | 准一级吸附动力学 | 准二级吸附动力学 | 内扩散吸附动力学 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe/(mg/g) | k1/min-1 | R2 | Qe/(mg/g) | k2/(g/(mg·min)) | R2 | kin | C | R2 | ||
| 1.3 | 297 | 98.99 | 0.0105 | 0.9602 | 119.8 | 6.26×10-5 | 0.9893 | 5.469 | -10.03 | 0.9789 |
| 303 | 84.95 | 0.0089 | 0.9513 | 135.9 | 1.29×10-4 | 0.9845 | 5.632 | 20.21 | 0.9744 | |
| 309 | 109.2 | 0.0051 | 0.9686 | 138.5 | 2.17×10-4 | 0.9987 | 7.253 | 24.20 | 0.9052 | |
| 5.0 | 297 | 56.42 | 0.0060 | 0.9553 | 86.43 | 1.59×10-4 | 0.9948 | 3.591 | 9.681 | 0.9007 |
| 303 | 134.8 | 0.0065 | 0.9539 | 179.5 | 7.97×10-5 | 0.9975 | 8.383 | -8.289 | 0.9684 | |
| 309 | 122.6 | 0.0048 | 0.9502 | 204.5 | 3.12×10-5 | 0.9929 | 7.253 | 24.20 | 0.9052 | |
表2 Fe3O4@SiO2@PDA-PAT吸附Hg2+的动力学参数
Table 2 Kinetic parameters of adsorption of Hg2+ by Fe3O4@SiO2@PDA-PAT
| pH | T/K | 准一级吸附动力学 | 准二级吸附动力学 | 内扩散吸附动力学 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe/(mg/g) | k1/min-1 | R2 | Qe/(mg/g) | k2/(g/(mg·min)) | R2 | kin | C | R2 | ||
| 1.3 | 297 | 98.99 | 0.0105 | 0.9602 | 119.8 | 6.26×10-5 | 0.9893 | 5.469 | -10.03 | 0.9789 |
| 303 | 84.95 | 0.0089 | 0.9513 | 135.9 | 1.29×10-4 | 0.9845 | 5.632 | 20.21 | 0.9744 | |
| 309 | 109.2 | 0.0051 | 0.9686 | 138.5 | 2.17×10-4 | 0.9987 | 7.253 | 24.20 | 0.9052 | |
| 5.0 | 297 | 56.42 | 0.0060 | 0.9553 | 86.43 | 1.59×10-4 | 0.9948 | 3.591 | 9.681 | 0.9007 |
| 303 | 134.8 | 0.0065 | 0.9539 | 179.5 | 7.97×10-5 | 0.9975 | 8.383 | -8.289 | 0.9684 | |
| 309 | 122.6 | 0.0048 | 0.9502 | 204.5 | 3.12×10-5 | 0.9929 | 7.253 | 24.20 | 0.9052 | |
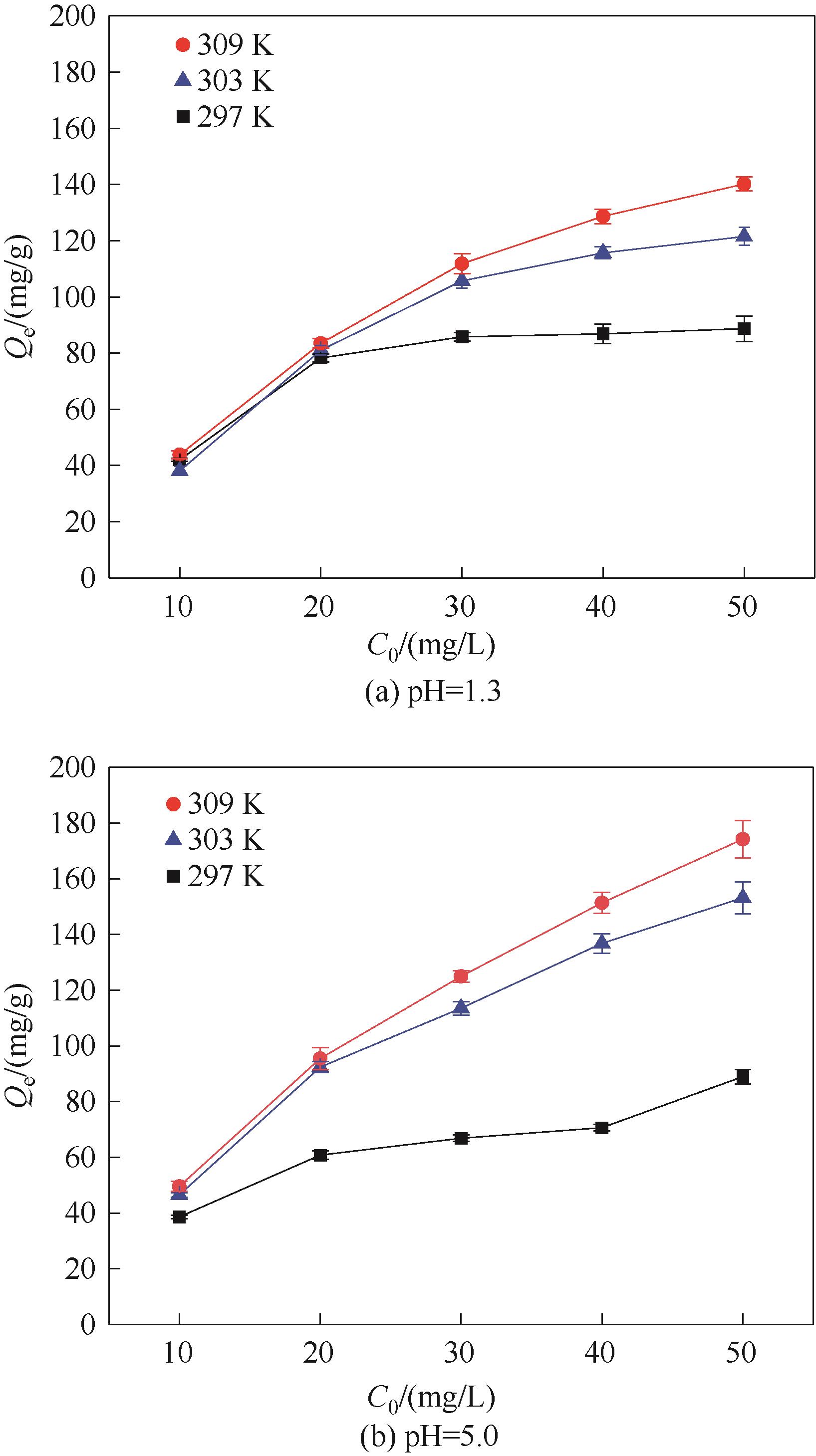
图12 不同pH时Fe3O4@SiO2@PDA-PAT的Hg2+初始浓度与平衡吸附量的关系(实验条件:pH为1.3或5.0的废水100 ml,20 mg吸附剂吸附24 h)
Fig.12 The relationship between the initial Hg2+ concentration of Fe3O4@SiO2@PDA-PAT and the equilibrium adsorption capacity at pH 1.3 and 5.0 (experimental conditions: 100 ml of wastewater with pH 1.3 or pH 5.0 was absorbed by 20 mg adsorbent for 24 h)
| pH | T/K | Langmuir等温吸附模型 | Freundlich等温吸附模型 | ||||
|---|---|---|---|---|---|---|---|
| Qm/(mg/g) | KL/(L/mg) | R2 | 1/n | KF/(mg/g) | R2 | ||
| 1.3 | 297 | 92.42 | 0.7723 | 0.9989 | 0.2233 | 44.95 | 0.7599 |
| 303 | 131.9 | 0.4446 | 0.9999 | 0.4246 | 35.22 | 0.7760 | |
| 309 | 159.7 | 0.3110 | 0.9996 | 0.3951 | 45.54 | 0.9426 | |
| 5.0 | 297 | 83.26 | 0.3099 | 0.9825 | 0.2487 | 33.01 | 0.9528 |
| 303 | 163.1 | 0.5104 | 0.9926 | 0.3120 | 62.69 | 0.8851 | |
| 309 | 176.7 | 1.018 | 0.9884 | 0.2899 | 73.89 | 0.9658 | |
表3 Fe3O4@SiO2@PDA-PAT吸附Hg2+的等温吸附模型
Table 3 Fe3O4@SiO2@PDA-PAT isotherm adsorption model for Hg2+ adsorption
| pH | T/K | Langmuir等温吸附模型 | Freundlich等温吸附模型 | ||||
|---|---|---|---|---|---|---|---|
| Qm/(mg/g) | KL/(L/mg) | R2 | 1/n | KF/(mg/g) | R2 | ||
| 1.3 | 297 | 92.42 | 0.7723 | 0.9989 | 0.2233 | 44.95 | 0.7599 |
| 303 | 131.9 | 0.4446 | 0.9999 | 0.4246 | 35.22 | 0.7760 | |
| 309 | 159.7 | 0.3110 | 0.9996 | 0.3951 | 45.54 | 0.9426 | |
| 5.0 | 297 | 83.26 | 0.3099 | 0.9825 | 0.2487 | 33.01 | 0.9528 |
| 303 | 163.1 | 0.5104 | 0.9926 | 0.3120 | 62.69 | 0.8851 | |
| 309 | 176.7 | 1.018 | 0.9884 | 0.2899 | 73.89 | 0.9658 | |
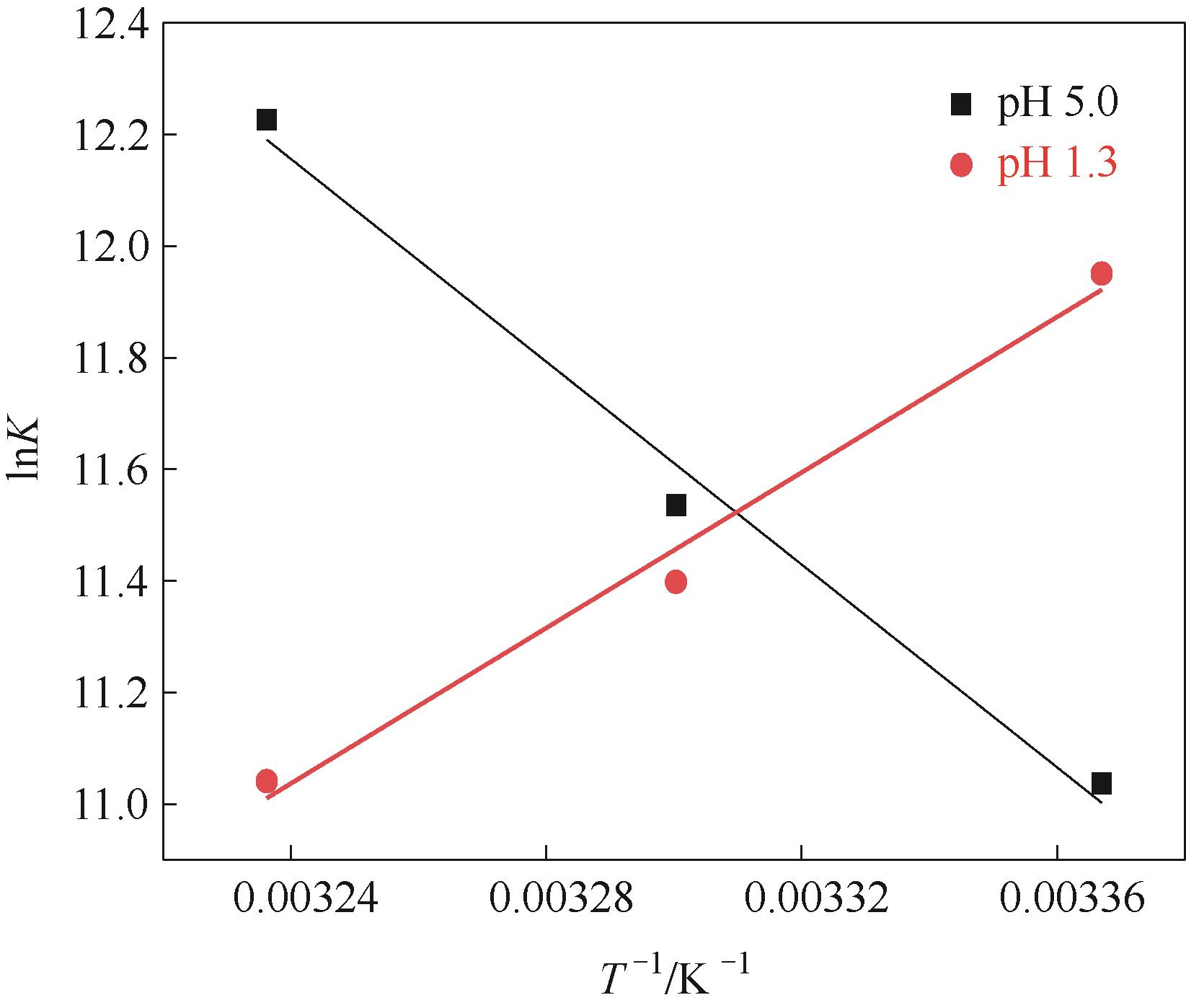
图14 Fe3O4@SiO2@PDA-PAT吸附Hg2+的吸附热力学参数lnK与1/T的关系图
Fig.14 Plot of lnKvs 1/T for estimation of thermodynamic parameters for the adsorption of mercury onto Fe3O4@SiO2@PDA-PAT
| pH | T/K | R2 | ΔH/(kJ/mol) | ΔS/(J/(mol·K)) | ΔG/(kJ/mol) |
|---|---|---|---|---|---|
| 1.3 | 297 | 0.9876 | -57.91 | -95.89 | -29.51 |
| 303 | -28.71 | ||||
| 309 | -28.36 | ||||
| 5.0 | 297 | 0.9892 | 75.52 | 345.8 | -27.25 |
| 303 | -29.06 | ||||
| 309 | -31.41 |
表4 Fe3O4@SiO2@PDA-PAT吸附Hg2+的热力学参数
Table 4 Thermodynamic parameters of adsorption of Hg2+ by Fe3O4@SiO2@PDA-PAT
| pH | T/K | R2 | ΔH/(kJ/mol) | ΔS/(J/(mol·K)) | ΔG/(kJ/mol) |
|---|---|---|---|---|---|
| 1.3 | 297 | 0.9876 | -57.91 | -95.89 | -29.51 |
| 303 | -28.71 | ||||
| 309 | -28.36 | ||||
| 5.0 | 297 | 0.9892 | 75.52 | 345.8 | -27.25 |
| 303 | -29.06 | ||||
| 309 | -31.41 |
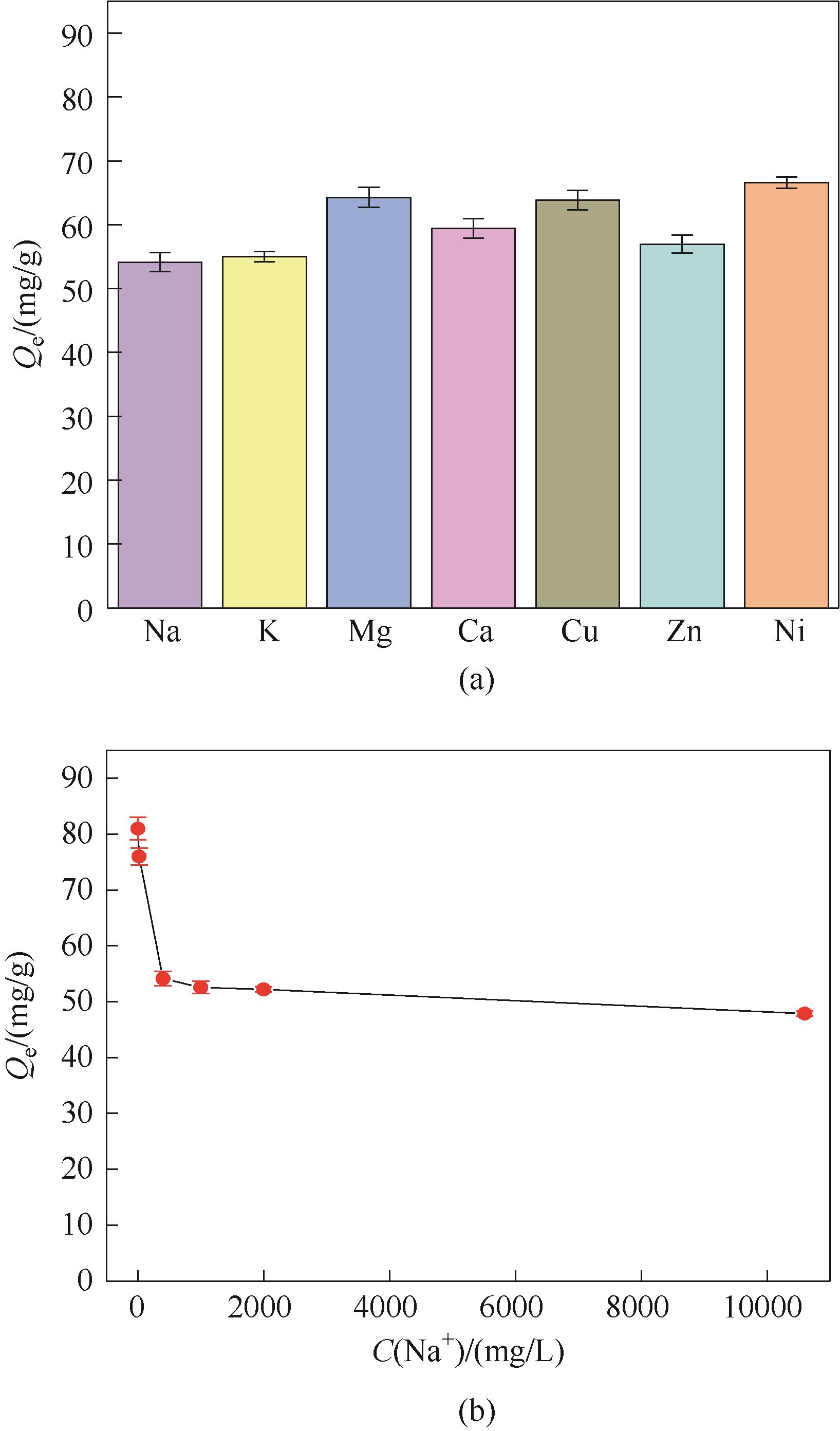
图16 常见金属阳离子(a)以及Na+浓度(b)对Fe3O4@SiO2 @PDA-PAT吸附Hg2+的影响(实验条件:pH 1.3、Hg2+为20 mg/L和400 mg/L的模拟废水100 ml,20 mg吸附剂在303 K下吸附24 h)
Fig.16 Effect of common metal cations (a) and Na+ (b) on the adsorption of mercury ions by Fe3O4@SiO2@PDA-PAT(experimental conditions: 100 ml of simulated wastewater with pH 1.3, Hg2+ at 20 mg/L and 400 mg/L, 20 mg adsorbent at 303 K for 24 h)
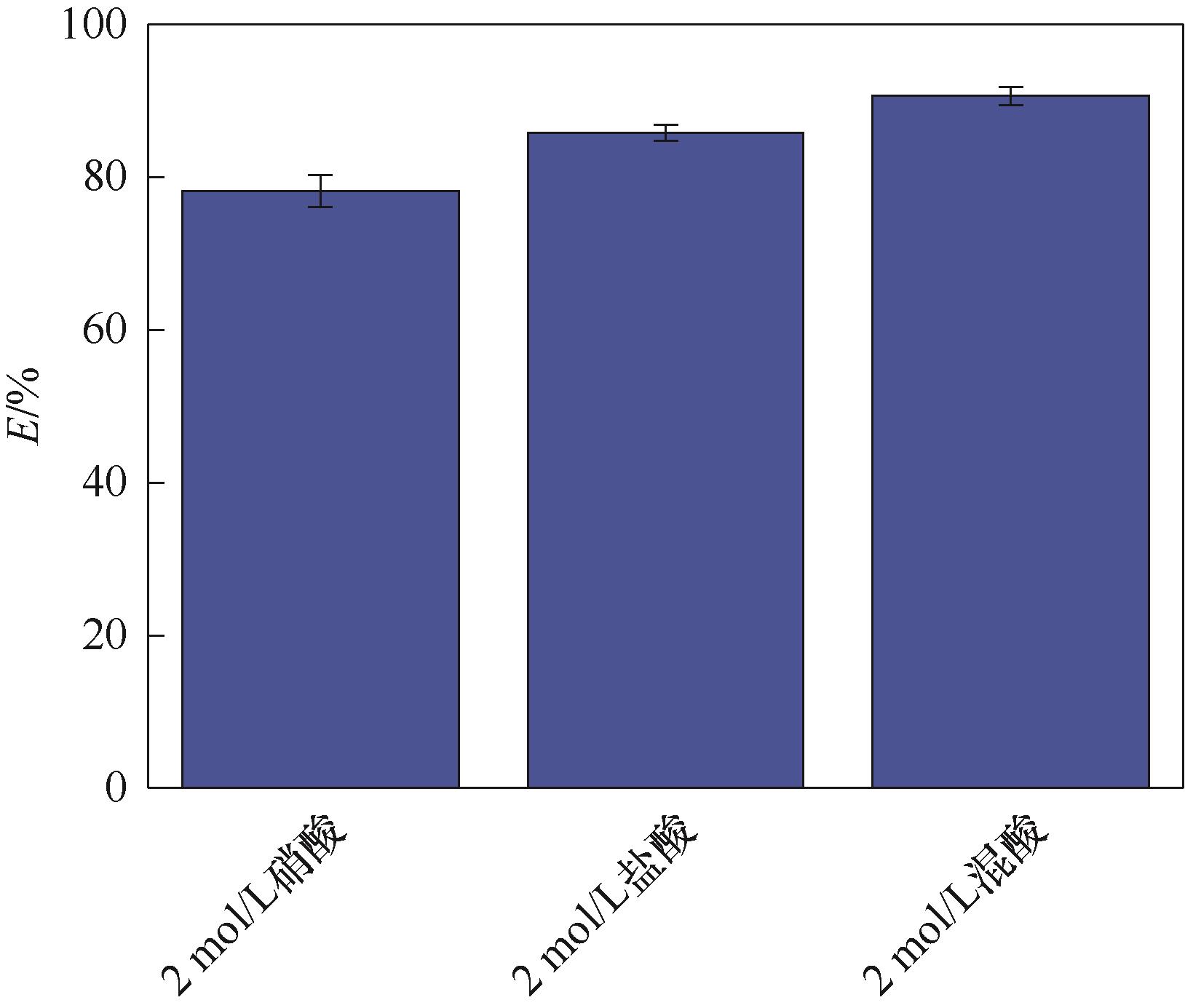
图17 Fe3O4@SiO2@PDA-PAT的解吸效率(实验条件:20 mg饱和吸附后的吸附剂加入50 ml解吸液中,在303 K下解吸24 h)
Fig.17 Desorption efficiency of Fe3O4@SiO2@PDA-PAT(experimental conditions: the adsorbent after 20 mg saturation adsorption was added into 50 ml desorption solution and desorbed at 303 K for 24 h)
| 1 | 谭哲亚, 刘胜男. 水污染中重金属的吸附净化处理研究[J]. 环境科学与管理, 2021, 46(6): 100-103. |
| Tan Z Y, Liu S N. Study on adsorption and purification of heavy metals in water pollution[J]. Environmental Science and Management, 2021, 46(6): 100-103. | |
| 2 | Engstler R, Reipert J, Karimi S, et al. A reverse osmosis process to recover and recycle trivalent chromium from electroplating wastewater[J]. Membranes, 2022, 12(9): 853. |
| 3 | Rakesh S, Sagar B, Sijan D, et al. Technological trends in heavy metals removal from industrial wastewater: a review[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105688. |
| 4 | Wang Z H, Qin Y Z, Xu X P, et al. Laminated graphene oxide membrane for recovery of mercury-containing wastewater by pervaporation[J]. Applied Water Science, 2021, 11(7): 129. |
| 5 | Zhang Y L, Zhang L J, Liang X J, et al. Competitive exchange between divalent metal ions[Cu(Ⅱ), Zn(Ⅱ), Ca(Ⅱ)] and Hg(Ⅱ) bound to thiols and natural organic matter[J]. Journal of Hazardous Materials, 2022, 424: 127388. |
| 6 | 陈立国, 白萍萍, 仇志峰. 基于电渗析法去除聚驱采油废水中阳离子影响因素研究[J]. 数字印刷, 2022(6): 109-116. |
| Chen L G, Bai P P, Qiu Z F. Study on the influence factors of removing cations from polymer flooding wastewater based on electrodialysis[J]. Digital Printing, 2022(6): 109-116. | |
| 7 | 苗时雨, 毛振钢, 刘锐平, 等. 实验室废液降危减量化处理工艺与工程案例[J]. 环境工程学报, 2020, 14(7): 1944-1949. |
| Miao S Y, Mao Z G, Liu R P, et al. Treatment process and engineering cases for the risk and quantity reduction of laboratory liquid waste[J]. Chinese Journal of Environmental Engineering, 2020, 14(7): 1944-1949. | |
| 8 | Chao C, Jing K, Jimin S, et al. Selective and efficient removal of Hg(Ⅱ) from aqueous media by a low-cost dendrimer-grafted polyacrylonitrile fiber: performance and mechanism[J]. Chemosphere, 2021, 1(262): 127836. |
| 9 | Xia M D, Chen Z X, Li Y, et al. Removal of Hg(Ⅱ) in aqueous solutions through physical and chemical adsorption principles[J]. RSC Advances, 2019, 9(36): 20941-20953. |
| 10 | Arief B M, Gusti W Y, Surya R B, et al. Mercury removal using modified activated carbon of peat soil and coal in simulated landfill leachate[J]. Environmental Technology & Innovation, 2021, 24: 102022. |
| 11 | Dmitry B, Sanya E, Nadezhda K, et al. Applicability of the modified diatomite for treatment of wastewater containing heavy metals[J]. E3S Web of Conferences, 2021, 4(5): 01052. |
| 12 | Beutel M W, Dent S R, Newcombe R L, et al. Mercury removal from municipal secondary effluent with hydrous ferric oxide reactive filtration[J]. Water Environment Research, 2019, 91(2): 132-143. |
| 13 | Heidarinejad Z, Dehghani M H, Heidari M, et al. Methods for preparation and activation of activated carbon: a review[J]. Environmental Chemistry Letters, 2020, 18(2): 393-415. |
| 14 | Lanas S G, Valiente M, Tolazzi M, et al. Thermodynamics of Hg2+ and Ag+ adsorption by 3-mercaptopropionic acid-functionalized superparamagnetic iron oxide nanoparticles[J]. Journal of Thermal Analysis and Calorimetry, 2019, 136(3): 1153-1162. |
| 15 | Yuhei K, Fumihiko O, Chalermpong S, et al. Adsorption/desorption capability of potassium-type zeolite prepared from coal fly ash for removing of Hg2+ [J]. Sustainability, 2021, 13(8): 4269. |
| 16 | Shi Z N, Xu C, Lu P, et al. Preparation and the adsorption ability of thiolated magnetic core-shell Fe3O4@SiO2@C-SH for removing Hg2+ in water solution[J]. Materials Letters, 2018, 225: 130-133. |
| 17 | Zhao J J, Luan L P, Li Z W, et al. The adsorption property and mechanism for Hg(Ⅱ) and Ag(Ⅰ) by Schiff base functionalized magnetic Fe3O4 from aqueous solution[J]. Journal of Alloys and Compounds, 2020, 825: 154051. |
| 18 | Wang X, Wang L, Zou H, et al. Simple synthesis of conducting poly(2-aminothiazole) with high molecular weight[J]. Colloid and Polymer Science, 2015, 293(7): 2027-2034. |
| 19 | Gao M D, Yang L Y, Yang S J, et al. Simple aminated modified zeolite 4A synthesized using fly ash and its remediation of mercury contamination: characteristics and mechanism[J]. Sustainability, 2022, 14(23): 15924. |
| 20 | Gomaa H, Sayed A, Mahross M H, et al. A hybrid spongy-like porous carbon-based on azopyrazole-benzenesulfonamide derivative for highly selective Fe3+-adsorption from real water samples[J]. Microporous and Mesoporous Materials, 2022, 330: 111578. |
| 21 | Zou H, Lv P F, Wang X, et al. Electrospun poly(2-aminothiazole)/cellulose acetate fiber membrane for removing Hg(Ⅱ) from water[J]. Journal of Applied Polymer Science, 2017, 134(21): e44879. |
| 22 | Wang X, Lv P F, Zou H, et al. Synthesis of poly(2-aminothiazole) for selective removal of Hg(Ⅱ) in aqueous solutions[J]. Industrial & Engineering Chemistry Research, 2016, 55(17): 4911-4918. |
| 23 | Zou H, Wu D. Revisiting the synthesis of poly(2-aminothiazole) for removal of Hg(Ⅱ) in aqueous solution[J]. European Polymer Journal, 2020, 125: 109514. |
| 24 | 张春晓, 张腾, 秦泗超, 等. Fe3O4@SiO2@EDTA磁性复合微球的制备及吸附性能研究[J]. 化工新型材料, 2020, 48(9): 196-201. |
| Zhang C X, Zhang T, Qin S C, et al. Preparation and adsorption property of Fe3O4@SiO2@EDTA magnetic composite microsphere[J]. New Chemical Materials, 2020, 48(9): 196-201. | |
| 25 | 徐要辉, 邓迟, 肖志刚, 等. 氨基功能化Fe3O4@SiO2的合成及对Pb2+、Hg2+、Cd2+和Cr3+的吸附研究[J]. 乐山师范学院学报, 2019, 34(4): 22-27. |
| Xu Y H, Deng C, Xiao Z G, et al. Synthesis of amino-functionalized Fe3O4@SiO2 nanoparticles for removal of Pb2+, Hg2+, Cd2+ and Cr3+ [J]. Journal of Leshan Normal University, 2019, 34(4): 22-27. | |
| 26 | Jahanshahi P, Mamaghani M. Efficient and straightforward access to diverse and densely functionalized chromenes by 3-amino-1,2,4-triazole supported on hydroxyapatite-encapsulated-γ-Fe2O3(γ- Fe2O3@HAp@CPTMS@AT) as a new magnetic basic nanocatalyst[J]. Reaction Kinetics, Mechanisms and Catalysis, 2020, 130(2): 955-977. |
| 27 | Zou H, Wu D, Sun H, et al. Poly(2-aminothiazole)-silica nanocomposite particles: synthesis and morphology control[J]. Applied Surface Science, 2018, 436: 1083-1092. |
| 28 | Li Q, Lin G Y, Zhang S, et al. Conducting and stretchable emulsion styrene butadiene rubber composites using SiO2@Ag core-shell particles and polydopamine coated carbon nanotubes[J]. Polymer Testing, 2022, 115: 107722. |
| 29 | 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 文献著录: 第5部分 非书资料: [S]. 北京: 中国标准出版社, 2014. |
| General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of China. Bibliography(part 5): Non-book materials: [S]. Beijing: Standards Press of China, 2014. | |
| 30 | Sio J E L, Escobar E C, Kim H, et al. Hydroxypicolinic acid tethered on magnetite core-silica shell (HPCA@SiO2@Fe3O4) as an effective and reusable adsorbent for practical Co(Ⅱ) recovery[J]. Chemosphere, 2022, 298: 134301. |
| 31 | 冯晴. 简易热重分析仪测定胆矾中结晶水含量[J]. 化学教学, 2016(10): 58-60. |
| Feng Q. Determination of crystal water content in gallstone by simple thermogravimetric analyzer[J]. Education in Chemistry, 2016(10): 58-60. | |
| 32 | 周庆莹, 刘四华, 厍景国, 等. 曝气辅助聚多巴胺共沉积膜的制备与表征[J]. 膜科学与技术, 2022, 42(5): 17-23, 32. |
| Zhou Q Y, Liu S H, She J G, et al. Preparation and characterization of aeration-assisted polydopamine co-deposition membranes[J]. Membrane Science and Technology, 2022, 42(5): 17-23, 32. | |
| 33 | 付亚康, 李锡忠, 夏晓, 等. 羟基磷灰石Zeta电位对其蛋白吸附性能的影响[J]. 现代生物医学进展, 2016, 16(19): 3610-3613. |
| Fu Y K, Li X Z, Xia X, et al. Effect of Zeta potential of hydroxyapatite on protein adsorption[J]. Progress in Modern Biomedicine, 2016, 16(19): 3610-3613. | |
| 34 | 龚建康, 李国滔, 叶坪, 等. 磁性Zr基MOFs材料的合成及吸附水中磷的性能研究[J]. 功能材料, 2023, 54(4): 4176-4188. |
| Gong J K, Li G T, Ye P, et al. Synthesis of magnetic Zr-based MOFs materials and performance of phosphorus removal from water[J]. Journal of Functional Materials, 2023, 54(4): 4176-4188. | |
| 35 | 殷俞, 张壮壮, 徐丹, 等. 多孔材料基π络合吸附材料的合成及其应用[J]. 化学进展, 2017, 29(6): 628-636. |
| Yin Y, Zhang Z Z, Xu D, et al. π complexation adsorbents based on porous materials: preparation and application[J]. Progress in Chemistry, 2017, 29(6): 628-636. | |
| 36 | 许砚铭, 张书园, 曹红杰, 等. L-半胱氨酸和聚吡咯功能化磁性高岭土对水中Hg2+的吸附[J]. 工业水处理, 2023, 43(1): 95-101. |
| Xu Y M, Zhang S Y, Cao H J, et al. Adsorption of Hg2+ with L-cysteine and polypyrrole functionalized magnetic Kaolin[J]. Industrial Water Treatment, 2023, 43(1): 95-101. | |
| 37 | 陈金垒, 王嘉豪, 黄雪君, 等. 纳米矿晶对氧氟沙星的吸附性能研究[J]. 山东化工, 2022, 51(11): 210-212. |
| Chen J L, Wang J H, Huang X J, et al. Study on the adsorption performance of ofloxacin by nano-crystalline mineral[J]. Shandong Chemical Industry, 2022, 51(11): 210-212. | |
| 38 | 那立艳, 张丽影, 张凤杰, 等. 固液界面吸附热力学参数的计算[J]. 材料导报, 2020, 34(22): 22030-22035. |
| Na L Y, Zhang L Y, Zhang F J, et al. Calculation of adsorption thermodynamic parameters at solid-liquid interfaces[J]. Materials Reports, 2020, 34(22): 22030-22035. | |
| 39 | 叶竞华, 蓝桥发, 柯兆华. CL-P507树脂吸附Nd3+过程热力学及动力学研究[J]. 冶金管理, 2022(1): 25-27. |
| Ye J H, Lan Q F, Ke Z H. Study on thermodynamics and kinetics of adsorption of Nd3+ by CL-P507 resin[J]. China Steel Focus, 2022(1): 25-27. | |
| 40 | Vicente-Martínez Y, Caravaca M, Soto-Meca A. Simultaneous adsorption of mercury species from aquatic environments using magnetic nanoparticles coated with nanomeric silver functionalized with L-cysteine[J]. Chemosphere, 2021, 282: 131128. |
| 41 | 唐艳妮, 吕利. 改性成都粘土预处理垃圾渗滤液的吸附热力学和动力学研究[J]. 离子交换与吸附, 2019, 35(2): 181-192. |
| Tang Y N, Lv L. Study on adsorption thermodynamics and kinetics of modified Chengdu clay pretreatment of landfill leachate[J]. Ion Exchange and Adsorption, 2019, 35(2): 181-192. | |
| 42 | Pearson R G. Hard and soft acids and bases, HSAB(part Ⅱ): Underlying theories[J]. Journal of Chemical Education, 1968, 45(10): 643. |
| 43 | 罗德纹, 陈思莉, 谢磊, 等. 镉在红壤上的吸附行为和机理[J]. 水处理技术, 2021, 47(7): 54-59, 64. |
| Luo D W, Chen S L, Xie L, et al. Adsorption behavior and mechanism of cadmium on red soil[J]. Technology of Water Treatment, 2021, 47(7): 54-59, 64. | |
| 44 | Naini N, Sid Kalal H, Almasian M R, et al. Phosphine-functionalized Fe3O4/SiO2/composites as efficient magnetic nanoadsorbents for the removal of palladium ions from aqueous solution: kinetic, thermodynamic and isotherm studies[J]. Materials Chemistry and Physics, 2022, 287: 126242. |
| 45 | 马博文. 咪唑型聚离子液体对Cr(Ⅵ)、Mo(Ⅵ)、W(Ⅵ)同多酸根负离子和Hg(Ⅱ)氯配阴离子的吸附性能研究[D]. 兰州: 兰州大学, 2020. |
| Ma B W. Adsorption performance of imidazolium poly(ionic liquid)s for Cr(Ⅵ), Mo(Ⅵ), W(Ⅵ) isopolyacid anions and chloro-mercury complex anions[D]. Lanzhou: Lanzhou University, 2020. |
| [1] | 尹飞, 王翠, 童少平. rGO-Fe3O4活化过硫酸盐处理酸性红73[J]. 化工学报, 2019, 70(1): 207-213. |
| [2] | 贾尚宁, 常娟娟, 李宁波, 乔洁. 核壳结构磁性纳米复合物的合成及载药性能[J]. 化工学报, 2018, 69(S1): 170-175. |
| [3] | 马千里,董相廷,王进贤,刘桂霞,于文生. 纳米四氧化三铁的化学制备方法研究进展[J]. 化工进展, 2012, 31(03): 562-573. |
| [4] | 宋艳艳1,2,孔维宝1,宋 昊1,华绍烽1,夏春谷1. 磁性壳聚糖微球的研究进展[J]. 化工进展, 2012, 31(02 ): 345-354. |
| [5] | 竺柏康,王东光,任益枰,邹志斌,楼银龙,卢林平. 新型磁性纳米锂离子筛的合成 [J]. CIESC Journal, 2011, 62(7): 2067-2074. |
| [6] | 朱春山,宋 佳,邱 莉,张 强. 油酸低温水洗改性磁性四氧化三铁纳米粒子 [J]. CIESC Journal, 2011, 30(7): 1552-. |
| [7] | 吴兰峰, 吴德峰, 张明. 聚苯硫醚/四氧化三铁复合材料的力学和磁性能 [J]. 化工学报, 2008, 59(11): 2941-2945. |
| [8] | 宋丽贤,卢忠远,刘德春,崔绍波,肖相齐. 分解沉淀法制备磁性纳米Fe3O4的研究及表征 [J]. CIESC Journal, 2006, 25(1): 54-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号