化工学报 ›› 2025, Vol. 76 ›› Issue (12): 6398-6409.DOI: 10.11949/0438-1157.20250514
夏铭1( ), 黄帅2, 石慧3, 牛丛丛4, 李德宝5, 乔旭1,6
), 黄帅2, 石慧3, 牛丛丛4, 李德宝5, 乔旭1,6
收稿日期:2025-05-09
修回日期:2025-06-13
出版日期:2025-12-31
发布日期:2026-01-23
通讯作者:
夏铭
作者简介:夏铭(1987—),男,博士,副研究员,Sciengart@163.com, mxia@njtech.edu.cn
基金资助:
Ming XIA1( ), Shuai HUANG2, Hui SHI3, Congcong NIU4, Debao LI5, Xu QIAO1,6
), Shuai HUANG2, Hui SHI3, Congcong NIU4, Debao LI5, Xu QIAO1,6
Received:2025-05-09
Revised:2025-06-13
Online:2025-12-31
Published:2026-01-23
Contact:
Ming XIA
摘要:
采用集总动力学思想与链增长概率模型,结合工业单管试验数据,建立了钴基费托合成包含合成气消耗速率和集总组分选择性模型的复合集总宏观动力学模型。研究结果表明,建立的宏观集总动力学模型计算的单管CO单程转化率与试验值吻合良好,计算的集总组分CH4、C3H8、C12H26和C29H60的时空收率与对应工况的试验值亦符合良好,大多数相对偏差在15%以内;将该复合集总宏观动力学模型应用于工业单管装置的全流程建模与模拟,结果表明,该模型较好地计算不同工况下蜡油、水的流率以及CO的转化率。本文提出的钴基费托合成复合集总宏观动力学模型及其全流程模型方法,具有待定参数数量适中,能够同时模拟合成气转化率和集总组分生成速率的优点,表现出较好的应用与学术价值,有望拓展应用至其他类似的反应体系。
中图分类号:
夏铭, 黄帅, 石慧, 牛丛丛, 李德宝, 乔旭. 钴基费托合成复合集总宏观动力学模型及工业单管模拟[J]. 化工学报, 2025, 76(12): 6398-6409.
Ming XIA, Shuai HUANG, Hui SHI, Congcong NIU, Debao LI, Xu QIAO. The hybrid lumping macroscopic kinetic model for cobalt-based fischer-tropsch synthesis and its application in industrial single-tube simulation[J]. CIESC Journal, 2025, 76(12): 6398-6409.
| 时间 | M0 | 反应条件 | 放料间隔/h | 产物生成质量/g | CO 总转化率/ % | CO单程转化率/% | 进料中各组分与 CO的摩尔比 | 单程转化的 CO物质的量/mol | 系数 | 实验数据计算的平均 分子量/(g/mol) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/K | P/MPa | U | V | V0/L | t | CH4 | C3H8 | C12H26 | C29H60 | H2O | x | xexp | xcalc | R1 | R2 | R3 | R4 | mCO | A | B | C | D | E | |||||||
| 2019.06.02 | 3.914 | 464.05 | 6.07 | 2.121 | 141.346 | 141346 | 8 | 1771.64 | 601.57 | 2948.62 | 2964.91 | 9924 | 79.18 | 31.378 | 30.97 | 2.414 | 0 | 0.295 | 0.205 | 1276.53 | 0.087 | 3.821 | 4.761 | 1.707 | 140.862 | 16 | 40.22 | 158.24 | 443.8 | 18 |
| 2019.06.05 | 4.087 | 467.85 | 6.08 | 2.009 | 137.583 | 137583 | 8 | 2026.62 | 773.86 | 3573.76 | 2482.79 | 10898 | 75.69 | 36.038 | 36.46 | 2.431 | 0 | 0.444 | 0.212 | 1137.498 | 0.111 | 4.616 | 5.335 | 1.443 | 148.139 | 16 | 41.02 | 163.9 | 420.89 | 18 |
| 2019.06.09 | 3.534 | 473.35 | 6.07 | 1.994 | 136.235 | 136235 | 8 | 1871.86 | 684.61 | 4503.84 | 2803.50 | 11687 | 77.48 | 34.439 | 33.94 | 1.921 | 0 | 0.421 | 0.192 | 1333.259 | 0.088 | 4.799 | 7.632 | 1.963 | 183.702 | 16 | 40.36 | 166.96 | 404.16 | 18 |
| 2019.06.10 | 3.303 | 473.45 | 6.06 | 2.102 | 139.371 | 139371 | 8 | 1735.6 | 658.86 | 3798.21 | 3561.24 | 11469 | 74.36 | 30.922 | 30.48 | 1.804 | 0 | 0.312 | 0.187 | 1400.732 | 0.077 | 4.977 | 7.082 | 2.418 | 192.905 | 16 | 40.08 | 162.37 | 445.87 | 18 |
| 2019.06.12 | 3.150 | 473.65 | 6.08 | 1.971 | 139.147 | 139147 | 8 | 1241.75 | 545.73 | 3890.46 | 3719.36 | 11437 | 74.07 | 29.439 | 29.98 | 1.675 | 0 | 0.244 | 0.231 | 1460.689 | 0.053 | 4.422 | 7.346 | 2.580 | 201.711 | 16 | 39.18 | 168.13 | 457.73 | 18 |
| 2019.06.13 | 3.001 | 473.65 | 6.08 | 1.996 | 142.245 | 142245 | 8 | 1273.47 | 561.31 | 3882.04 | 3647.42 | 11252 | 73.37 | 28.011 | 27.96 | 1.598 | 0 | 0.207 | 0.196 | 1552.535 | 0.051 | 4.893 | 7.859 | 2.824 | 208.301 | 16 | 38.23 | 164.6 | 430.31 | 18 |
| 2019.06.21 | 3.085 | 484.05 | 6.07 | 2.076 | 140.504 | 140504 | 8 | 1226.43 | 503.67 | 4221.75 | 4793.27 | 12939 | 83.38 | 30.848 | 32.24 | 1.520 | 0 | 0.307 | 0.258 | 1695.303 | 0.045 | 4.044 | 7.531 | 3.497 | 233.009 | 16 | 40.37 | 181.72 | 444.36 | 18 |
| 2019.06.24 | 3.280 | 488.95 | 6.07 | 2.005 | 132.947 | 132947 | 8 | 1314.00 | 374.07 | 5383.6 | 4094.02 | 13514 | 88.25 | 33.178 | 33.63 | 1.380 | 0 | 0.503 | 0.397 | 1596.877 | 0.051 | 2.873 | 9.407 | 3.000 | 228.896 | 16 | 39.69 | 174.48 | 415.99 | 18 |
| 2019.07.01 | 3.009 | 493.55 | 6.04 | 2.063 | 139.369 | 139369 | 8 | 1167.82 | 620.68 | 5413.03 | 5152.76 | 15192 | 87.66 | 35.540 | 33.23 | 1.310 | 0 | 0.35 | 0.349 | 1812.581 | 0.040 | 5.290 | 10.832 | 3.837 | 280.492 | 16 | 38.99 | 166.08 | 446.34 | 18 |
| 2019.07.08 | 3.062 | 493.85 | 6.05 | 1.97 | 145.156 | 145156 | 8 | 1479.35 | 708.24 | 4875.58 | 5282.83 | 15253 | 85.89 | 35.684 | 37.09 | 1.440 | 0 | 0.372 | 0.25 | 1817.709 | 0.051 | 6.122 | 9.904 | 3.915 | 276.744 | 16 | 37.78 | 160.77 | 440.72 | 18 |
| 2019.07.12 | 3.049 | 498.95 | 6.05 | 2.044 | 148.188 | 148188 | 8 | 1626.57 | 724.48 | 5405.9 | 6390.77 | 17146 | 86.10 | 37.749 | 37.66 | 1.400 | 0 | 0.362 | 0.287 | 1868.146 | 0.054 | 6.196 | 11.422 | 4.466 | 312.416 | 16 | 38.35 | 155.22 | 469.36 | 18 |
表1 工业单管试验数据及其向集总动力学数据的换算
Table 1 Industrial single-tube test data and their conversion to lumped kinetics data
| 时间 | M0 | 反应条件 | 放料间隔/h | 产物生成质量/g | CO 总转化率/ % | CO单程转化率/% | 进料中各组分与 CO的摩尔比 | 单程转化的 CO物质的量/mol | 系数 | 实验数据计算的平均 分子量/(g/mol) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/K | P/MPa | U | V | V0/L | t | CH4 | C3H8 | C12H26 | C29H60 | H2O | x | xexp | xcalc | R1 | R2 | R3 | R4 | mCO | A | B | C | D | E | |||||||
| 2019.06.02 | 3.914 | 464.05 | 6.07 | 2.121 | 141.346 | 141346 | 8 | 1771.64 | 601.57 | 2948.62 | 2964.91 | 9924 | 79.18 | 31.378 | 30.97 | 2.414 | 0 | 0.295 | 0.205 | 1276.53 | 0.087 | 3.821 | 4.761 | 1.707 | 140.862 | 16 | 40.22 | 158.24 | 443.8 | 18 |
| 2019.06.05 | 4.087 | 467.85 | 6.08 | 2.009 | 137.583 | 137583 | 8 | 2026.62 | 773.86 | 3573.76 | 2482.79 | 10898 | 75.69 | 36.038 | 36.46 | 2.431 | 0 | 0.444 | 0.212 | 1137.498 | 0.111 | 4.616 | 5.335 | 1.443 | 148.139 | 16 | 41.02 | 163.9 | 420.89 | 18 |
| 2019.06.09 | 3.534 | 473.35 | 6.07 | 1.994 | 136.235 | 136235 | 8 | 1871.86 | 684.61 | 4503.84 | 2803.50 | 11687 | 77.48 | 34.439 | 33.94 | 1.921 | 0 | 0.421 | 0.192 | 1333.259 | 0.088 | 4.799 | 7.632 | 1.963 | 183.702 | 16 | 40.36 | 166.96 | 404.16 | 18 |
| 2019.06.10 | 3.303 | 473.45 | 6.06 | 2.102 | 139.371 | 139371 | 8 | 1735.6 | 658.86 | 3798.21 | 3561.24 | 11469 | 74.36 | 30.922 | 30.48 | 1.804 | 0 | 0.312 | 0.187 | 1400.732 | 0.077 | 4.977 | 7.082 | 2.418 | 192.905 | 16 | 40.08 | 162.37 | 445.87 | 18 |
| 2019.06.12 | 3.150 | 473.65 | 6.08 | 1.971 | 139.147 | 139147 | 8 | 1241.75 | 545.73 | 3890.46 | 3719.36 | 11437 | 74.07 | 29.439 | 29.98 | 1.675 | 0 | 0.244 | 0.231 | 1460.689 | 0.053 | 4.422 | 7.346 | 2.580 | 201.711 | 16 | 39.18 | 168.13 | 457.73 | 18 |
| 2019.06.13 | 3.001 | 473.65 | 6.08 | 1.996 | 142.245 | 142245 | 8 | 1273.47 | 561.31 | 3882.04 | 3647.42 | 11252 | 73.37 | 28.011 | 27.96 | 1.598 | 0 | 0.207 | 0.196 | 1552.535 | 0.051 | 4.893 | 7.859 | 2.824 | 208.301 | 16 | 38.23 | 164.6 | 430.31 | 18 |
| 2019.06.21 | 3.085 | 484.05 | 6.07 | 2.076 | 140.504 | 140504 | 8 | 1226.43 | 503.67 | 4221.75 | 4793.27 | 12939 | 83.38 | 30.848 | 32.24 | 1.520 | 0 | 0.307 | 0.258 | 1695.303 | 0.045 | 4.044 | 7.531 | 3.497 | 233.009 | 16 | 40.37 | 181.72 | 444.36 | 18 |
| 2019.06.24 | 3.280 | 488.95 | 6.07 | 2.005 | 132.947 | 132947 | 8 | 1314.00 | 374.07 | 5383.6 | 4094.02 | 13514 | 88.25 | 33.178 | 33.63 | 1.380 | 0 | 0.503 | 0.397 | 1596.877 | 0.051 | 2.873 | 9.407 | 3.000 | 228.896 | 16 | 39.69 | 174.48 | 415.99 | 18 |
| 2019.07.01 | 3.009 | 493.55 | 6.04 | 2.063 | 139.369 | 139369 | 8 | 1167.82 | 620.68 | 5413.03 | 5152.76 | 15192 | 87.66 | 35.540 | 33.23 | 1.310 | 0 | 0.35 | 0.349 | 1812.581 | 0.040 | 5.290 | 10.832 | 3.837 | 280.492 | 16 | 38.99 | 166.08 | 446.34 | 18 |
| 2019.07.08 | 3.062 | 493.85 | 6.05 | 1.97 | 145.156 | 145156 | 8 | 1479.35 | 708.24 | 4875.58 | 5282.83 | 15253 | 85.89 | 35.684 | 37.09 | 1.440 | 0 | 0.372 | 0.25 | 1817.709 | 0.051 | 6.122 | 9.904 | 3.915 | 276.744 | 16 | 37.78 | 160.77 | 440.72 | 18 |
| 2019.07.12 | 3.049 | 498.95 | 6.05 | 2.044 | 148.188 | 148188 | 8 | 1626.57 | 724.48 | 5405.9 | 6390.77 | 17146 | 86.10 | 37.749 | 37.66 | 1.400 | 0 | 0.362 | 0.287 | 1868.146 | 0.054 | 6.196 | 11.422 | 4.466 | 312.416 | 16 | 38.35 | 155.22 | 469.36 | 18 |
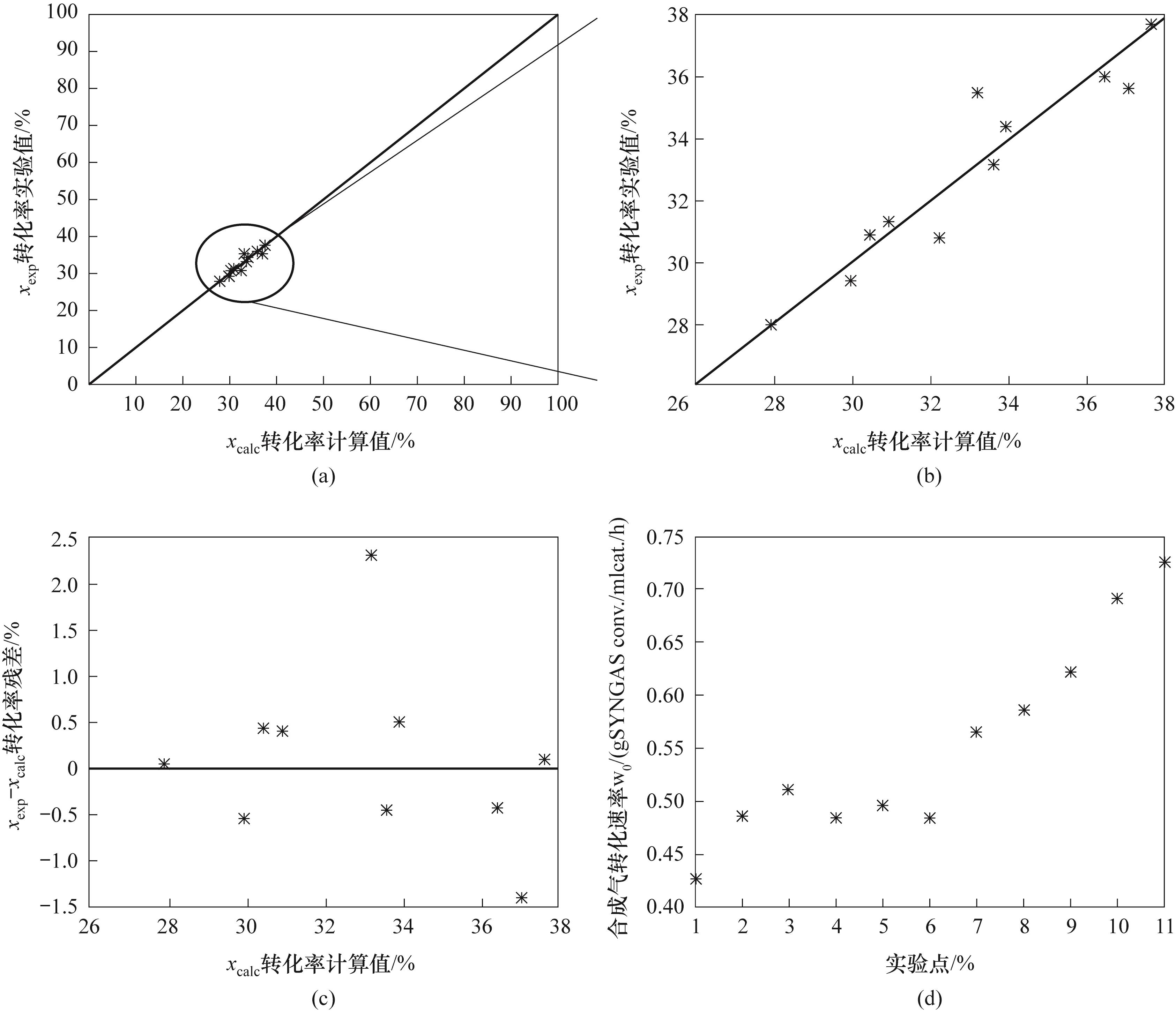
图2 (a)、(b)不同范围转化率计算值与转化率实验值的对比;(c)转化率残差;(d)合成气转化速率
Fig.2 (a),(b) Comparison of calculated conversion and experimental conversion within different ranges; (c) Conversion residual plot (d) Syngas consumption rate
| MP | Mi | F | ρ2 | F0.05 |
|---|---|---|---|---|
| 3 | 11 | 341.306 | 0.992 | 34.406 |
表2 动力学模型统计检验
Table 2 Statistical test results of the kinetics model
| MP | Mi | F | ρ2 | F0.05 |
|---|---|---|---|---|
| 3 | 11 | 341.306 | 0.992 | 34.406 |
| T/K | P/MPa | GHSV/h-1 | R1 | α1, exp | α1, calc | W1, exp | W1, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.5419 | 0.5262 | 0.2138 | 0.2245 | -5.01 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.5269 | 0.5260 | 0.2288 | 0.2246 | 1.82 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.5604 | 0.5803 | 0.1898 | 0.1762 | 7.17 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.5737 | 0.5954 | 0.1779 | 0.1637 | 7.99 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.6309 | 0.6153 | 0.1321 | 0.1480 | -12.02 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.6260 | 0.6271 | 0.1360 | 0.1391 | -2.25 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.6574 | 0.6395 | 0.1141 | 0.1300 | -13.88 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.6525 | 0.6613 | 0.1177 | 0.1147 | 2.54 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.6885 | 0.6772 | 0.0945 | 0.1042 | -10.26 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.6495 | 0.6513 | 0.1198 | 0.1216 | -1.46 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.6563 | 0.6579 | 0.1150 | 0.1170 | -1.78 |
表3 甲烷生成的碳链增长概率因子α1计算值及其与实验值对比
Table 3 Calculated values of the carbon chain growth probability factor α1 for methane formation in comparison with the experimental values
| T/K | P/MPa | GHSV/h-1 | R1 | α1, exp | α1, calc | W1, exp | W1, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.5419 | 0.5262 | 0.2138 | 0.2245 | -5.01 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.5269 | 0.5260 | 0.2288 | 0.2246 | 1.82 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.5604 | 0.5803 | 0.1898 | 0.1762 | 7.17 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.5737 | 0.5954 | 0.1779 | 0.1637 | 7.99 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.6309 | 0.6153 | 0.1321 | 0.1480 | -12.02 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.6260 | 0.6271 | 0.1360 | 0.1391 | -2.25 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.6574 | 0.6395 | 0.1141 | 0.1300 | -13.88 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.6525 | 0.6613 | 0.1177 | 0.1147 | 2.54 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.6885 | 0.6772 | 0.0945 | 0.1042 | -10.26 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.6495 | 0.6513 | 0.1198 | 0.1216 | -1.46 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.6563 | 0.6579 | 0.1150 | 0.1170 | -1.78 |
| T/K | P/MPa | GHSV/h-1 | R1 | α3, exp | α3, calc | W3, exp | W3, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.8120 | 0.8052 | 0.0726 | 0.0738 | -1.65 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.7868 | 0.7931 | 0.0874 | 0.0808 | 7.56 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.8120 | 0.8103 | 0.0694 | 0.0709 | -2.13 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.8120 | 0.8141 | 0.0675 | 0.0687 | -1.72 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.8281 | 0.8274 | 0.0581 | 0.0612 | -5.38 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.8259 | 0.8366 | 0.0599 | 0.0561 | 6.48 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.8492 | 0.8309 | 0.0469 | 0.0592 | -26.35 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.8754 | 0.8745 | 0.0335 | 0.0361 | -7.80 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.8436 | 0.8439 | 0.0502 | 0.0521 | -3.63 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.8289 | 0.8349 | 0.0574 | 0.0570 | 0.64 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.8400 | 0.8417 | 0.0512 | 0.0533 | -4.02 |
表4 C2~5烃(集总为C3)生成的碳链增长概率因子α3计算值及其与实验值对比
Table 4 Calculated values of the carbon chain growth probability factor α3 generated by C2-5 hydrocarbons (with a total set of C3) in comparison with the experimental values
| T/K | P/MPa | GHSV/h-1 | R1 | α3, exp | α3, calc | W3, exp | W3, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.8120 | 0.8052 | 0.0726 | 0.0738 | -1.65 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.7868 | 0.7931 | 0.0874 | 0.0808 | 7.56 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.8120 | 0.8103 | 0.0694 | 0.0709 | -2.13 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.8120 | 0.8141 | 0.0675 | 0.0687 | -1.72 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.8281 | 0.8274 | 0.0581 | 0.0612 | -5.38 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.8259 | 0.8366 | 0.0599 | 0.0561 | 6.48 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.8492 | 0.8309 | 0.0469 | 0.0592 | -26.35 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.8754 | 0.8745 | 0.0335 | 0.0361 | -7.80 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.8436 | 0.8439 | 0.0502 | 0.0521 | -3.63 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.8289 | 0.8349 | 0.0574 | 0.0570 | 0.64 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.8400 | 0.8417 | 0.0512 | 0.0533 | -4.02 |
| T/K | P/MPa | GHSV/h-1 | R1 | α12, exp | α12, calc | W12, exp | W12, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.9143 | 0.9155 | 0.3558 | 0.3840 | -7.91 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.9182 | 0.9177 | 0.4035 | 0.3840 | 4.84 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.9232 | 0.9187 | 0.4566 | 0.3839 | 15.93 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.9171 | 0.9170 | 0.3894 | 0.3840 | 1.39 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.9232 | 0.9236 | 0.4140 | 0.3825 | 7.61 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.9232 | 0.9244 | 0.4146 | 0.3821 | 7.84 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.9174 | 0.9223 | 0.3929 | 0.3830 | 2.52 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.9236 | 0.9248 | 0.4822 | 0.3819 | 20.80 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.9232 | 0.9168 | 0.4381 | 0.3840 | 12.36 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.9175 | 0.9179 | 0.3949 | 0.3839 | 2.78 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.9165 | 0.9185 | 0.3821 | 0.3839 | -0.47 |
表5 C6~19烃(集总为C12)生成的碳链增长概率因子α12计算值及其与实验值对比
Table 5 Calculated values of the carbon chain growth probability factor α12 generated by C6-19 hydrocarbons (aggregated as C12) in comparison with the experimental values
| T/K | P/MPa | GHSV/h-1 | R1 | α12, exp | α12, calc | W12, exp | W12, calc | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.9143 | 0.9155 | 0.3558 | 0.3840 | -7.91 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.9182 | 0.9177 | 0.4035 | 0.3840 | 4.84 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.9232 | 0.9187 | 0.4566 | 0.3839 | 15.93 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.9171 | 0.9170 | 0.3894 | 0.3840 | 1.39 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.9232 | 0.9236 | 0.4140 | 0.3825 | 7.61 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.9232 | 0.9244 | 0.4146 | 0.3821 | 7.84 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.9174 | 0.9223 | 0.3929 | 0.3830 | 2.52 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.9236 | 0.9248 | 0.4822 | 0.3819 | 20.80 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.9232 | 0.9168 | 0.4381 | 0.3840 | 12.36 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.9175 | 0.9179 | 0.3949 | 0.3839 | 2.78 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.9165 | 0.9185 | 0.3821 | 0.3839 | -0.47 |
| T/K | P/MPa | GHSV/h-1 | R1 | α29, exp | α29, calc | W29, exp | W29, exp | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.9698 | 0.9650 | 0.3578 | 0.3743 | -4.62 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.9676 | 0.9678 | 0.2803 | 0.3734 | -33.22 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.9601 | 0.9681 | 0.2842 | 0.3732 | -31.30 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.9649 | 0.9673 | 0.3651 | 0.3738 | -2.39 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.9707 | 0.9687 | 0.3958 | 0.3726 | 5.86 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.9705 | 0.9683 | 0.3895 | 0.3730 | 4.23 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.9716 | 0.9703 | 0.4461 | 0.3704 | 16.98 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.9649 | 0.9684 | 0.3667 | 0.3729 | -1.71 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.9711 | 0.9691 | 0.4171 | 0.3721 | 10.79 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.9713 | 0.9703 | 0.4279 | 0.3703 | 13.46 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.9717 | 0.9709 | 0.4517 | 0.3690 | 18.31 |
表6 C20+烃(集总为C29)生成的碳链增长概率因子α29计算值及其与实验值对比
Table 6 Calculated values of the carbon chain growth probability factor α29 generated by C20+ hydrocarbons (aggregated as C29) in comparison with the experimental values
| T/K | P/MPa | GHSV/h-1 | R1 | α29, exp | α29, calc | W29, exp | W29, exp | RD/% |
|---|---|---|---|---|---|---|---|---|
| 464.05 | 6.07 | 3715.27 | 2.414 | 0.9698 | 0.9650 | 0.3578 | 0.3743 | -4.62 |
| 467.85 | 6.08 | 3728.25 | 2.431 | 0.9676 | 0.9678 | 0.2803 | 0.3734 | -33.22 |
| 473.35 | 6.07 | 3789.95 | 1.921 | 0.9601 | 0.9681 | 0.2842 | 0.3732 | -31.30 |
| 473.45 | 6.06 | 3786.64 | 1.804 | 0.9649 | 0.9673 | 0.3651 | 0.3738 | -2.39 |
| 473.65 | 6.08 | 3764.47 | 1.675 | 0.9707 | 0.9687 | 0.3958 | 0.3726 | 5.86 |
| 473.65 | 6.08 | 3774.52 | 1.598 | 0.9705 | 0.9683 | 0.3895 | 0.3730 | 4.23 |
| 484.05 | 6.07 | 3961.53 | 1.52 | 0.9716 | 0.9703 | 0.4461 | 0.3704 | 16.98 |
| 488.95 | 6.07 | 4209.79 | 1.38 | 0.9649 | 0.9684 | 0.3667 | 0.3729 | -1.71 |
| 493.55 | 6.04 | 4193.09 | 1.31 | 0.9711 | 0.9691 | 0.4171 | 0.3721 | 10.79 |
| 493.85 | 6.05 | 4204.55 | 1.44 | 0.9713 | 0.9703 | 0.4279 | 0.3703 | 13.46 |
| 498.95 | 6.05 | 4336.51 | 1.4 | 0.9717 | 0.9709 | 0.4517 | 0.3690 | 18.31 |
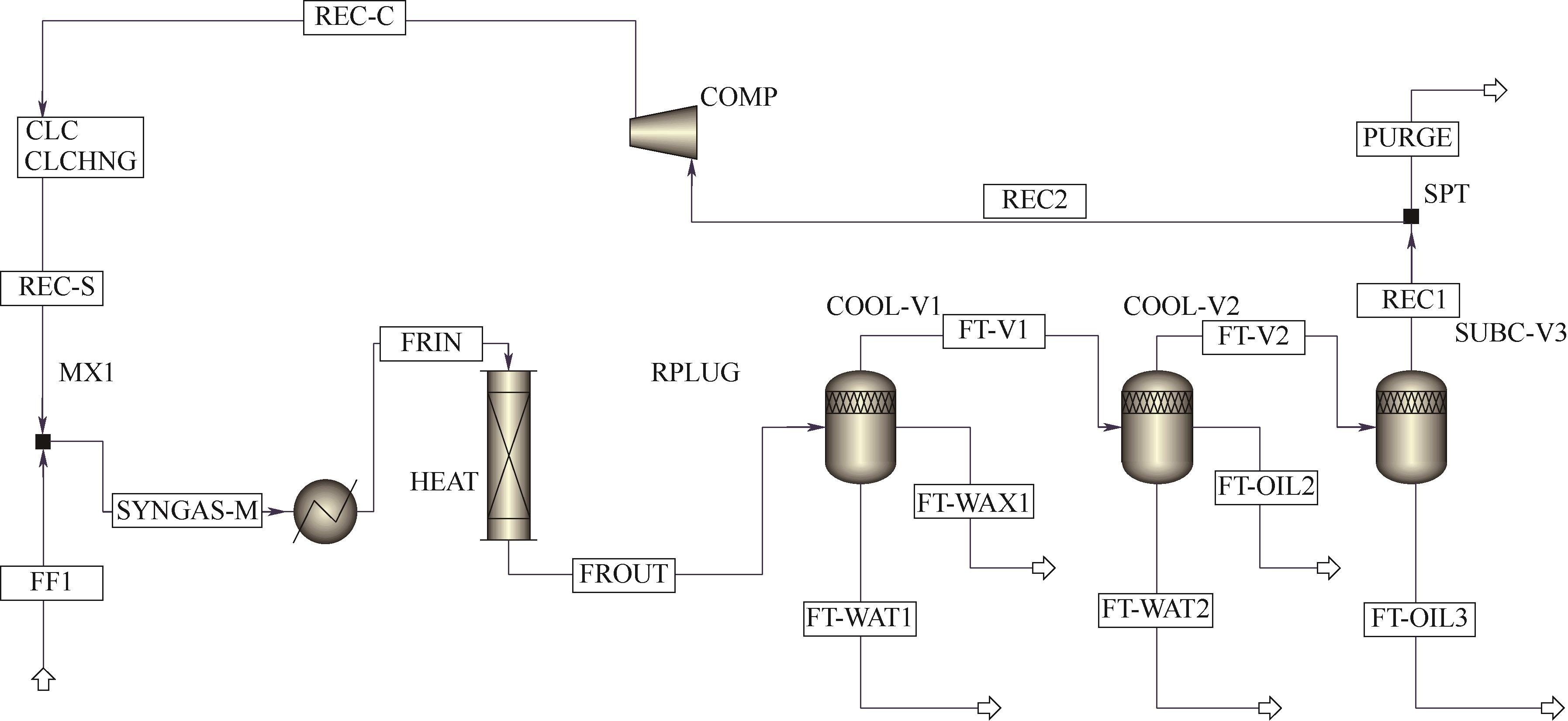
图3 采用Aspen Plus进行钴基费托合成工业单管装置全流程模拟示意图HEAT—进料预热器;RPLUG—固定床反应器;COOL-V1—常冷器;COOL-V2—水冷器;CUB-V3—深冷器;COMP—循环压缩机;FF1—新鲜气进料;REC-S—循环气物料;SYNGAS-M—合成气混合气;FRIN—反应器进气;FROUT—反应器出料;FT-WAT1—常冷器费托合成水;FT-WAX1—常冷器费托合成蜡油;FT-V1—常冷器汽相物料;FT-WAT2—水冷器费托合成水;FT-WAX2—水冷器费托合成蜡油;FT-OIL3—深冷器费托合成油;REC1—去循环和弛放气
Fig.3 A simplified diagram of the full-process simulation of the industrial single-tube device for cobalt-based Fischer-Tropsch synthesis using Aspen PlusHEAT—feed preheater; RPLUG—fixed-bed reactor; COOL-V1—normal-temperature cooler; COOL-V2—water cooler; CUB-V3—subcooler; COMP—recycle compressor;FF1—fresh feed; REC-S—recycle stream; SYNGAS-M—synthesis gas mixed; FRIN—inlet stream of the reactor; FROUT—outlet stream of the reactor; FT-WAT1—FT synthesized water; FT-WAX1—FT synthesized wax1; FT-V1—vapor stream from the COOL-V1; FT-WAT2—FT synthesized water2; FT-OIL2—FT synthesized oil2; FT-OIL3—FT synthesized oil3; REC1—stream to recycle and purge
| 对比组 | 流股 | 新鲜气 (FF1) | 循环气 (REC-S) | 入器气 (FRIN) | 费托蜡1 (FT-WAX1) | 费托油2 (FT-OIL2) | 费托油3 (FT-OIL3) | 费托水1 (FT-WAT1) | 费托水2 (FT-WAT2) | 弛放气 (PURGE) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 模拟值 | 流量/(kg/h) | 4.052 | 5.507 | 9.558 | 0.763 | 0.067 | 0.003 | 1.077 | 0.273 | 1.868 | |
| SUM = 0.833 | SUM=1.350 | ||||||||||
| 组分 | 摩尔分数 | 摩尔分数 | 摩尔分数 | 质量分数 | 质量分数 | 质量分数 | 质量分数 | 质量分数 | 摩尔分数 | ||
| H2 | 0.6703 | 0.5636 | 0.6138 | 0.0005 | 0.0004 | 0.0004 | 0 | 0 | 0.5636 | ||
| CO | 0.2886 | 0.2245 | 0.2547 | 0.0044 | 0.0050 | 0.0061 | 0 | 0 | 0.2245 | ||
| N2 | 0.0281 | 0.0723 | 0.0515 | 0.0086 | 0.0214 | 0.0418 | 0.0010 | 0.0006 | 0.0723 | ||
| CH4 | 0.0129 | 0.1269 | 0.0732 | 0.0026 | 0.0039 | 0.0055 | 0 | 0 | 0.1269 | ||
| C3H8 | 0 | 0.0114 | 0.0060 | 0.0038 | 0.0131 | 0.0308 | 0 | 0 | 0.0114 | ||
| C12H26 | 0 | 0 | 0 | 0.4719 | 0.9530 | 0.9047 | 0 | 0 | 0 | ||
| C29H60 | 0 | 0 | 0 | 0.4991 | 0.0001 | 0 | 0 | 0 | 0 | ||
| H2O | 0 | 0.0014 | 0.0007 | 0.0092 | 0.0031 | 0.0108 | 0.9989 | 0.9993 | 0.0014 | ||
| 试验值 | 流量/(kg/h) | 3.814 | — | — | SUM = 0.713, 各组分已全分析,不便列出 | SUM = 1.241, 不便列出 | 1.822 | ||||
| 组分 | 摩尔分数 | 摩尔分数 | 摩尔分数 | — | — | — | — | — | 摩尔分数 | ||
| H2 | 0.6728 | 0.5875 | 0.6114 | — | — | — | — | — | 0.5875 | ||
| CO | 0.2990 | 0.2334 | 0.2533 | — | — | — | — | — | 0.2334 | ||
| N2 | 0.018 | 0.0460 | 0.0518 | — | — | — | — | — | 0.0460 | ||
| CH4 | 0.0023 | 0.1060 | 0.0747 | — | — | — | — | — | 0.1060 | ||
| C2H6+ | — | — | — | — | — | — | — | — | — | ||
| H2O | — | — | — | — | — | — | — | — | — | ||
相对 误差/% | (模拟值-试验值)/试验值×100 | 6.24 | — | — | 16.8 | — | — | 8.87 | — | 2.52 | |
表7 钴基费托合成工业单管装置全流程关键物料数据的模拟值与试验值对比
Table 7 Comparison of simulated and experimental values of key material data for the entire process of cobalt-based Fischer-Tropsch synthesis industrial single-tube equipment
| 对比组 | 流股 | 新鲜气 (FF1) | 循环气 (REC-S) | 入器气 (FRIN) | 费托蜡1 (FT-WAX1) | 费托油2 (FT-OIL2) | 费托油3 (FT-OIL3) | 费托水1 (FT-WAT1) | 费托水2 (FT-WAT2) | 弛放气 (PURGE) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 模拟值 | 流量/(kg/h) | 4.052 | 5.507 | 9.558 | 0.763 | 0.067 | 0.003 | 1.077 | 0.273 | 1.868 | |
| SUM = 0.833 | SUM=1.350 | ||||||||||
| 组分 | 摩尔分数 | 摩尔分数 | 摩尔分数 | 质量分数 | 质量分数 | 质量分数 | 质量分数 | 质量分数 | 摩尔分数 | ||
| H2 | 0.6703 | 0.5636 | 0.6138 | 0.0005 | 0.0004 | 0.0004 | 0 | 0 | 0.5636 | ||
| CO | 0.2886 | 0.2245 | 0.2547 | 0.0044 | 0.0050 | 0.0061 | 0 | 0 | 0.2245 | ||
| N2 | 0.0281 | 0.0723 | 0.0515 | 0.0086 | 0.0214 | 0.0418 | 0.0010 | 0.0006 | 0.0723 | ||
| CH4 | 0.0129 | 0.1269 | 0.0732 | 0.0026 | 0.0039 | 0.0055 | 0 | 0 | 0.1269 | ||
| C3H8 | 0 | 0.0114 | 0.0060 | 0.0038 | 0.0131 | 0.0308 | 0 | 0 | 0.0114 | ||
| C12H26 | 0 | 0 | 0 | 0.4719 | 0.9530 | 0.9047 | 0 | 0 | 0 | ||
| C29H60 | 0 | 0 | 0 | 0.4991 | 0.0001 | 0 | 0 | 0 | 0 | ||
| H2O | 0 | 0.0014 | 0.0007 | 0.0092 | 0.0031 | 0.0108 | 0.9989 | 0.9993 | 0.0014 | ||
| 试验值 | 流量/(kg/h) | 3.814 | — | — | SUM = 0.713, 各组分已全分析,不便列出 | SUM = 1.241, 不便列出 | 1.822 | ||||
| 组分 | 摩尔分数 | 摩尔分数 | 摩尔分数 | — | — | — | — | — | 摩尔分数 | ||
| H2 | 0.6728 | 0.5875 | 0.6114 | — | — | — | — | — | 0.5875 | ||
| CO | 0.2990 | 0.2334 | 0.2533 | — | — | — | — | — | 0.2334 | ||
| N2 | 0.018 | 0.0460 | 0.0518 | — | — | — | — | — | 0.0460 | ||
| CH4 | 0.0023 | 0.1060 | 0.0747 | — | — | — | — | — | 0.1060 | ||
| C2H6+ | — | — | — | — | — | — | — | — | — | ||
| H2O | — | — | — | — | — | — | — | — | — | ||
相对 误差/% | (模拟值-试验值)/试验值×100 | 6.24 | — | — | 16.8 | — | — | 8.87 | — | 2.52 | |
| [1] | Yates I C, Satterfield C N. Intrinsic kinetics of the Fischer-Tropsch synthesis on a cobalt catalyst[J]. Energy and Fuels, 1991, 5(1): 168-173. |
| [2] | Zennaro R, Tagliabue M, Bartholomew C H. Kinetics of Fischer–Tropsch synthesis on titania-supported cobalt[J]. Catalysis Today, 2000, 58(4): 309-319. |
| [3] | Atashi H, Mansouri M, Hosseini S H, et al. Intrinsic kinetics of the Fischer-Tropsch synthesis over an impregnated cobalt-potassium catalyst[J]. Korean Journal of Chemical Engineering, 2012, 29(3): 304-309. |
| [4] | Ma W P, Jacobs G, Sparks D E, et al. Fischer–Tropsch synthesis: support and cobalt cluster size effects on kinetics over Co/Al2O3 and Co/SiO2 catalysts[J]. Fuel, 2011, 90(2): 756-765. |
| [5] | Visconti C G, Tronconi E, Lietti L, et al. Detailed kinetics of the Fischer-Tropsch synthesis on cobalt catalysts based on H-assisted CO activation[J]. Topics in Catalysis, 2011, 54(13): 786. |
| [6] | Kaiser P, Pöhlmann F, Jess A. Intrinsic and effective kinetics of cobalt-catalyzed Fischer-Tropsch synthesis in view of a power-to-liquid process based on renewable energy[J]. Chemical Engineering & Technology, 2014, 37(6): 964-972. |
| [7] | Visconti C G, Lietti L, Tronconi E, et al. Kinetics of low-temperature Fischer-Tropsch synthesis on cobalt catalysts: are both slurry autoclave and tubular packed-bed reactors adequate to collect relevant data at lab-scale?[J]. The Canadian Journal of Chemical Engineering, 2016, 94(4): 685-695. |
| [8] | Ostadi M, Rytter E, Hillestad M. Evaluation of kinetic models for Fischer-Tropsch cobalt catalysts in a plug flow reactor[J]. Chemical Engineering Research and Design, 2016, 114: 236-246. |
| [9] | Keyvanloo K, Lanham S J, Hecker W C. Kinetics of Fischer-Tropsch synthesis on supported cobalt: effect of temperature on CO and H2 partial pressure dependencies[J]. Catalysis Today, 2016, 270: 9-18. |
| [10] | Moazami N, Wyszynski M L, Rahbar K, et al. A comprehensive study of kinetics mechanism of Fischer-Tropsch synthesis over cobalt-based catalyst[J]. Chemical Engineering Science, 2017, 171: 32-60. |
| [11] | Golestan S, Mirzaei A A, Atashi H. Kinetic and mechanistic studies of Fischer-Tropsch synthesis over the nano-structured iron-cobalt-manganese catalyst prepared by hydrothermal procedure[J]. Fuel, 2017, 200: 407-418. |
| [12] | Asiaee A, Benjamin K M. A density functional theory based elementary reaction mechanism for early steps of Fischer-Tropsch synthesis over cobalt catalyst. 1. Reaction kinetics[J]. Molecular Catalysis, 2017, 436: 218-227. |
| [13] | Mousavi S, Zamaniyan A, Irani M, et al. Statistical investigation of macro kinetics for iron and cobalt based Fischer-Tropsch synthesis: Mechanistic and kinetic implications[J]. Journal of Natural Gas Science and Engineering, 2016, 34: 1333-1346. |
| [14] | Parnian M J, Taheri Najafabadi A, Mortazavi Y, et al. Ru promoted cobalt catalyst on γ-Al2O3: influence of different catalyst preparation method and Ru loadings on Fischer–Tropsch reaction and kinetics[J]. Applied Surface Science, 2014, 313: 183-195. |
| [15] | Botes F G. Influences of water and syngas partial pressure on the kinetics of a commercial alumina-supported cobalt Fischer-Tropsch catalyst[J]. Industrial and Engineering Chemistry Research, 2009, 48(4): 1859-1865. |
| [16] | Keyser M J, Everson R C, Espinoza R L. Fischer-Tropsch studies with cobalt-manganese oxide catalysts: synthesis performance in a fixed bed reactor[J]. Applied Catalysis A: General, 1998, 171(1): 99-107. |
| [17] | Das T K, Conner W A, Li J L, et al. Fischer–Tropsch synthesis: kinetics and effect of water for a Co/SiO2Catalyst[J]. Energy & Fuels, 2005, 19(4): 1430-1439. |
| [18] | Yang C-H, Massoth F E, Oblad A G. Kinetics of CO + H2 Reaction over Co-Cu-Al2O3 Catalyst. In Hydrocarbon Synthesis from Carbon Monoxide and Hydrogen[M]. American Chemical Society: Washington, 1979: 35-46. |
| [19] | Schulz H. Short history and present trends of Fischer-Tropsch synthesis[J]. Applied Catalysis A: General, 1999, 186(1/2): 3-12. |
| [20] | Vervloet D, Kapteijn F, Nijenhuis J, et al. Fischer-Tropsch reaction-diffusion in a cobalt catalyst particle: aspects of activity and selectivity for a variable chain growth probability[J]. Catalysis Science & Technology, 2012, 2(6): 1221-1233. |
| [21] | 鲁丰乐. 费托合成催化剂反应动力学研究与反应器数学模拟[D]. 上海: 华东理工大学, 2010. |
| Lu F L. Study on reaction kinetics of Fischer-Tropsch synthesis catalyst and mathematical simulation of reactor[D]. Shanghai: East China University of Science and Technology, 2010. | |
| [22] | 鲁丰乐, 张海涛, 马向东, 等. 钴基催化剂费托合成动力学模型[J]. 中国科技论文在线, 2009, 4(9): 632-637. |
| Lu F L, Zhang H T, Ma X D, et al. Kinetics models of Fischer-Tropsch synthesis on the cobalt-based catalyst[J]. Sciencepaper Online, 2009, 4(9): 632-637. | |
| [23] | Lox E S, Froment G F. Kinetics of the Fischer-Tropsch reaction on a precipitated promoted iron catalyst. 1. Experimental procedure and results[J]. Industrial & Engineering Chemistry Research, 1993, 32(1): 61-70. |
| [24] | Lox E S, Froment G F. Kinetics of the Fischer-Tropsch reaction on a precipitated promoted iron catalyst. 2. Kinetic modeling[J]. Industrial & Engineering Chemistry Research, 1993, 32(1): 71-82. |
| [25] | 吉媛媛, 杨继礼, 相宏伟, 等. Fe-Mn工业催化剂F-T合成详细机理动力学研究 I.反应性能及初步反应机理[J]. 燃料化学学报, 1999, 27(S1): 130-137. |
| Ji Y Y, Yang J L, Xiang H W, et al. Study of F-T synthesis detailed mechanism kinetics over Fe-Mn industrial catalyst I. Catalyst Performance and Preliminary Mechanism[J]. Journal of Fuel Chemistry and Technology, 1999, 27(S1): 130-137. | |
| [26] | 马文平, 李永旺, 赵玉龙, 等. 工业Fe-Cu-K催化剂上费托合成反应动力学 ( Ⅱ ) : 模型筛选与参数估值[J]. 化工学报, 1999, 50(2): 167-173. |
| Ma W P, Li Y W, Zhao Y L, et al. Kinetics of Fischer - Tropsch synthesis over Fe - Cu - K catalyst ( Ⅱ ) model discrimination and parameter estimation[J]. Journal of Chemical Industry and Engineering (China), 1999, 50(2): 167-173. | |
| [27] | 马文平, 李永旺, 赵玉龙, 等. 工业Fe-Cu-K催化剂上费托合成反应动力学(Ⅰ): 基于机理的动力学模型[J]. 化工学报, 1999, 50(2): 159-166. |
| Ma W P, Li Y W, Zhao Y L, et al. Kinetics of Fischer - Tropsch synthesis over Fe - Cu -K catalyst ( Ⅰ ) kinetic model on the basis of mechanism[J]. Journal of Chemical Industry and Engineering (China), 1999, 50(2): 159-166. | |
| [28] | Wang Y N, Ma W P, Lu Y J, et al. Kinetics modelling of Fischer-Tropsch synthesis over an industrial Fe-Cu-K catalyst[J]. Fuel, 2003, 82(2): 195-213. |
| [29] | Yang J, Liu Y, Chang J, et al. Detailed kinetics of Fischer-Tropsch synthesis on an industrial Fe-Mn catalyst[J]. Industrial & Engineering Chemistry Research, 2003, 42(21): 5066-5090. |
| [30] | Niu C C, Guo S P, Xia M, et al. A hybrid kinetics integrating feed-consumption rate and product selectivity models for Fischer-Tropsch synthesis over an industrial cobalt-based catalyst[J]. Chemical Engineering Journal, 2023, 455: 140817. |
| [1] | 刘鑫, 潘阳, 刘公平, 方静, 李春利, 李浩. 渗透汽化-隔壁塔精馏耦合初步分离费托合成水的过程研究[J]. 化工学报, 2022, 73(5): 2020-2030. |
| [2] | 金科, 王晨光, 马隆龙, 张琦. 核壳纳米材料制备及其在CO/CO2热催化加氢中的应用[J]. 化工学报, 2022, 73(3): 990-1007. |
| [3] | 王吴玉, 史玉竹, 严龙, 张兴华, 马隆龙, 张琦. 负载型Co基双功能催化剂上戊酸酯生物燃料的制备[J]. 化工学报, 2022, 73(2): 689-698. |
| [4] | 陈康伟, 熊文婷, 符继乐, 陈秉辉. 合成气费托合成制重质烃Ru-Co/SiC催化剂的制备及性能[J]. 化工学报, 2021, 72(7): 3648-3657. |
| [5] | 应景涛, 李涛. 费托合成蛋壳型催化剂活性组分厚度的模拟计算[J]. 化工学报, 2019, 70(9): 3404-3411. |
| [6] | 宋楠, 潘敏建, 陈炳旭, 钱刚, 段学志, 周兴贵. 费托催化剂η-Fe2C (011)上CH4形成及C-C耦合机理研究[J]. 化工学报, 2019, 70(7): 2540-2547. |
| [7] | 刘意, 刘勇, 陈建峰, 张燚. 不同氧化锰载体对费托钴基催化剂合成低碳烯烃的影响[J]. 化工学报, 2015, 66(9): 3413-3420. |
| [8] | 王燕1,葛喜慧2,张敏卿1,朱怀工3,张子建2,王明1. 费托合成高温油相产品中正构烃的分离[J]. 化工进展, 2014, 33(11): 2894-2898. |
| [9] | 孙启文,吴建民,张宗森,庞利峰. 煤间接液化技术及其研究进展[J]. 化工进展, 2013, 32(01): 1-12. |
| [10] | 管国锋,王 磊,王锋娜. 氧化物助剂对费托合成钴基催化剂的促进作用[J]. 化工进展, 2012, 31(12): 2595-2602. |
| [11] | 张海娟,李江红,张舒冬,王振宇,张喜文. 丙烷催化脱氢反应宏观动力学模型[J]. 化工进展, 2012, 31(07): 1464-1467. |
| [12] | 赵钟兴1,廖丹葵1,孙建华1,黄科林2,孙果宋2,金维维1,谢美萱1,吴志洪2,童张法1. 碱性蛋白酶水解蚕蛹蛋白集总动力学 [J]. CIESC Journal, 2011, 62(9): 2588-2594. |
| [13] | 岳晨, 史翊翔, 蔡宁生. 煤气化费托合成/电联产系统建模及热力学分析 [J]. 化工学报, 2011, 62(4): 1070-1076. |
| [14] | 侯朝鹏,夏国富,李明丰,聂 红,李大东. FT合成反应器的研究进展 [J]. CIESC Journal, 2011, 30(2): 251-. |
| [15] | 王 祥 云. 反应循环气中二氧化碳脱除技术的进展 [J]. CIESC Journal, 2011, 30(1): 52-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号