化工学报 ›› 2020, Vol. 71 ›› Issue (2): 831-842.DOI: 10.11949/0438-1157.20190954
吴娜1,董依慧1,吉晓燕2,皇甫长安1,陆小华1
收稿日期:2019-08-21
修回日期:2019-11-13
出版日期:2020-02-05
发布日期:2020-02-05
通讯作者:
董依慧,陆小华
基金资助:Na WU1,Yihui DONG1,Xiaoyan JI2,Chang an HUANGFU1,Xiaohua LU1
Received:2019-08-21
Revised:2019-11-13
Online:2020-02-05
Published:2020-02-05
Contact:
Yihui DONG,Xiaohua LU
摘要:
采用电化学阳极氧化法,通过改变电解液氟离子浓度(0.4%、0.3%、0.2%(质量))和电压(15、25、35、45 V),制备一系列不同管径和粗糙度的TiO2纳米管阵列(TiO2 nanotube arrays, TNAs)。通过扫描电子显微镜以及原子力显微镜(atomic force microscopy, AFM)表征,结果表明随着电解液中氟离子浓度的降低,制备得到的TNAs表面平整度更好,壁厚增大,粗糙度降低。采用AFM力学表征研究了表面粗糙度以及管径对TNAs表面力学性质以及与细胞色素C(Cytochrome C, Cyt C)相互作用的影响,结果表明,黏附力与接触面积呈正比,随着TNAs管径增加,壁厚减小,TNAs与Cyt C的有效接触面积先增大后减小,两者之间作用力也先增加后减小;同时,同管径条件下粗糙度降低,TNAs有效面积增加,相互作用力也增加;由此可见,通过改变电解液氟离子浓度可以有效调控TNAs表面粗糙度及有效接触面积,进一步利于促进与蛋白分子之间相互作用。
中图分类号:
吴娜, 董依慧, 吉晓燕, 皇甫长安, 陆小华. TiO2纳米管阵列粗糙度调控及其与蛋白相互作用[J]. 化工学报, 2020, 71(2): 831-842.
Na WU, Yihui DONG, Xiaoyan JI, Chang an HUANGFU, Xiaohua LU. Interaction between proteins and roughness-regulated TiO2 nanotube arrays[J]. CIESC Journal, 2020, 71(2): 831-842.

图3 0.4%(质量)氟离子浓度下不同电压制备TNAs的AFM 2D以及高度图
Fig.3 AFM topographical and corresponding height images of TNAs prepared with different voltages at 0.4%(mass) F- concentration
| 电压/V | 粗糙度/nm | ||
|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 23.2±1.7 | 21.4±1.2 | 14.4±1.8 |
| 25 | 47.5±2.3 | 44.9±1.5 | 36.7±1.0 |
| 35 | 61.4±1.9 | 55.1±2.9 | 37.0±1.3 |
| 45 | 85.2±1.5 | 65.3±2.3 | 63.8±1.7 |
表1 不同外加电压以及氟离子浓度下TNAs粗糙度
Table 1 Roughness of TNAs under different applied voltages and F- concentrations
| 电压/V | 粗糙度/nm | ||
|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 23.2±1.7 | 21.4±1.2 | 14.4±1.8 |
| 25 | 47.5±2.3 | 44.9±1.5 | 36.7±1.0 |
| 35 | 61.4±1.9 | 55.1±2.9 | 37.0±1.3 |
| 45 | 85.2±1.5 | 65.3±2.3 | 63.8±1.7 |

图4 0.3%(质量)氟离子浓度下不同电压制备TNAs的AFM 2D以及高度图
Fig.4 AFM topographical and corresponding height images of TNAs prepared with different voltages at 0.3%(mass) F- concentration

图5 0.2%(质量)氟离子浓度下不同电压制备TNAs的AFM 2D以及高度图
Fig.5 AFM topographical and corresponding height images of TNAs prepared with different voltages at 0.2%(mass)F- concentration
| 电压/V | 壁厚/nm | 管径/nm | ||||
|---|---|---|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 9.3±0.2 | 9.4±0.4 | 9.4±0.4 | 24.7±1.9 | 23.1±2.8 | 21.5±0.8 |
| 25 | 8.0±0.4 | 9.0±0.9 | 9.2±0.5 | 51.1±2.3 | 49.9±1.5 | 48.7±1.9 |
| 35 | 7.3±0.4 | 8.4±0.6 | 8.8±0.3 | 65.7±2.9 | 65.0±1.6 | 62.3±1.3 |
| 45 | 6.0±0.5 | 7.3±0.4 | 8.0±0.3 | 91.6±2.5 | 87.2±1.9 | 78.8±2.1 |
表2 不同外加电压以及氟离子浓度条件下TNAs壁厚及管径
Table 2 Wall thickness and diameter of TNAs under different applied voltages and F- concentrations
| 电压/V | 壁厚/nm | 管径/nm | ||||
|---|---|---|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 9.3±0.2 | 9.4±0.4 | 9.4±0.4 | 24.7±1.9 | 23.1±2.8 | 21.5±0.8 |
| 25 | 8.0±0.4 | 9.0±0.9 | 9.2±0.5 | 51.1±2.3 | 49.9±1.5 | 48.7±1.9 |
| 35 | 7.3±0.4 | 8.4±0.6 | 8.8±0.3 | 65.7±2.9 | 65.0±1.6 | 62.3±1.3 |
| 45 | 6.0±0.5 | 7.3±0.4 | 8.0±0.3 | 91.6±2.5 | 87.2±1.9 | 78.8±2.1 |
| 电压/V | 管长/μm | ||
|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 1~1.3 | 1.0 | 0.8~1.2 |
| 25 | 5~6 | 5~5.4 | 4~5 |
| 35 | 5.7~6.5 | 5.5 | 5.5~6.5 |
| 45 | 10~11 | 10~11 | 10~10.5 |
表3 不同外加电压以及氟离子浓度下TNAs管长
Table 3 Tube length of TNAs under different applied voltages and F- concentrations
| 电压/V | 管长/μm | ||
|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 1~1.3 | 1.0 | 0.8~1.2 |
| 25 | 5~6 | 5~5.4 | 4~5 |
| 35 | 5.7~6.5 | 5.5 | 5.5~6.5 |
| 45 | 10~11 | 10~11 | 10~10.5 |
| 电压/V | 壁面积比率/% | 黏附力/nN | ||||
|---|---|---|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 36.1±0.5 | 38.0±0.6 | 41.4±0.7 | 2.6±0.3 | 4.0±0.4 | 4.3±0.5 |
| 25 | 41.0±.04 | 53.2±1.1 | 58.1±0.5 | 4.4±0.8 | 4.8±0.9 | 5.7±0.8 |
| 35 | 53.3±0.4 | 59.3±0.7 | 66.1±1.3 | 6.5±1.2 | 7.0±1.0 | 8.7±1.3 |
| 45 | 45.3±0.7 | 47.4±0.3 | 50.0±0.5 | 3.9±0.9 | 6.0±1.3 | 6.8±1.3 |
表4 不同外加电压以及氟离子浓度条件下TNAs壁面积比率以及表面黏附力
Table 4 Wall area ratio and adhesion force of TNAs under different applied voltages and F- concentrations
| 电压/V | 壁面积比率/% | 黏附力/nN | ||||
|---|---|---|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 36.1±0.5 | 38.0±0.6 | 41.4±0.7 | 2.6±0.3 | 4.0±0.4 | 4.3±0.5 |
| 25 | 41.0±.04 | 53.2±1.1 | 58.1±0.5 | 4.4±0.8 | 4.8±0.9 | 5.7±0.8 |
| 35 | 53.3±0.4 | 59.3±0.7 | 66.1±1.3 | 6.5±1.2 | 7.0±1.0 | 8.7±1.3 |
| 45 | 45.3±0.7 | 47.4±0.3 | 50.0±0.5 | 3.9±0.9 | 6.0±1.3 | 6.8±1.3 |
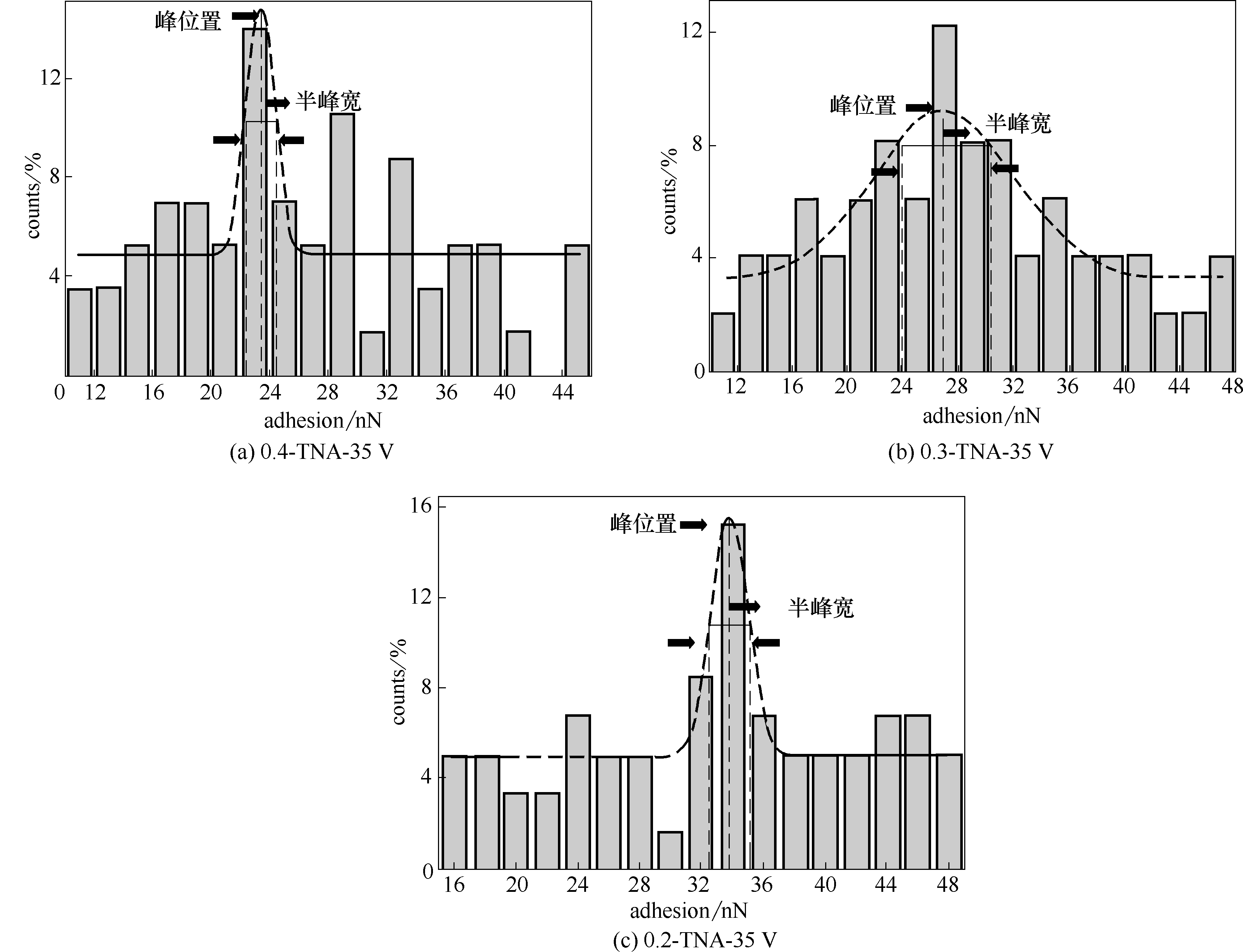
图10 Cyt C与不同氟离子浓度35 V下制备TNAs的表面黏附力分布
Fig.10 Histogram of adhesion forces measured for Cyt C with TNAs prepared with 35 V at different F- concentrations
| 电压/V | 黏附力/nN | ||
|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 13.4±1.5 | 14.9±1.2 | 18.0±4.5 |
| 25 | 14.9±2.6 | 17.5±3.5 | 25.2±1.6 |
| 35 | 23.4±1.2 | 27.0±3.2 | 33.8±1.3 |
| 45 | 19.5±2.0 | 25.1±2.1 | 30.6±1.4 |
表5 不同外加电压以及氟离子浓度条件下Cyt C-TNAs表面黏附力
Table 5 Adhesion force of Cyt C-TNAs under different applied voltages and F- concentrations
| 电压/V | 黏附力/nN | ||
|---|---|---|---|
| 0.4%(质量) F- | 0.3%(质量) F- | 0.2%(质量) F- | |
| 15 | 13.4±1.5 | 14.9±1.2 | 18.0±4.5 |
| 25 | 14.9±2.6 | 17.5±3.5 | 25.2±1.6 |
| 35 | 23.4±1.2 | 27.0±3.2 | 33.8±1.3 |
| 45 | 19.5±2.0 | 25.1±2.1 | 30.6±1.4 |
| 1 | Nel A E, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface[J]. Nat. Mater., 2009, 8(7): 543-557. |
| 2 | Leslie D C, Waterhouse A, Berthet J B, et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling[J]. Nat. Biotechnol., 2014, 32(11): 1134-1140. |
| 3 | Shao M F, Ning F Y, Zhao J W, et al. Preparation of Fe3O4@SiO2@layered double hydroxide core-shell microspheres for magnetic separation of proteins[J]. J. Am. Chem. Soc., 2012, 134(2): 1071-1077. |
| 4 | Zhen X, Wang X, Xie C, et al. Cellular uptake, antitumor response and tumor penetration of cisplatin-loaded milk protein nanoparticles[J]. Biomaterials, 2013, 34(4): 1372-1382. |
| 5 | Walkey C D, Chan W C W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment[J]. Chem. Soc. Rev., 2012, 41(7): 2780-2799. |
| 6 | Tang F Q, Li L L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery[J]. Adv. Mater., 2012, 24(12): 1504-1534. |
| 7 | Bayne L, Ulijn R V, Halling P J. Effect of pore size on the performance of immobilised enzymes[J]. Chem. Soc. Rev., 2013, 42(23): 9000-9010. |
| 8 | Huang Y, Zha G Y, Luo Q J, et al. The construction of hierarchical structure on Ti substrate with superior osteogenic activity and intrinsic antibacterial capability[J]. Sci. Rep., 2014: 6172. |
| 9 | Rajh T, Dimitrijevic N M, Bissonnette M, et al. Titanium dioxide in the service of the biomedical revolution[J]. Chem. Rev., 2014, 114(19): 10177-10216. |
| 10 | Chen W T, Chan A, Jovic V, et al. Effect of the TiO2 crystallite size, TiO2 polymorph and test conditions on the photo-oxidation rate of aqueous methylene blue[J]. Top Catal., 2015, 58(2/3): 85-102. |
| 11 | Si P, Ding S J, Yuan J, et al. Hierarchically structured one-dimensional TiO2 for protein immobilization, direct electrochemistry, and mediator-free glucose sensing[J]. ACS Nano, 2011, 5(9): 7617-7626. |
| 12 | Buettner K M, Valentine A M. Bioinorganic chemistry of titanium[J]. Chem. Rev., 2012, 112(3): 1863-1881. |
| 13 | Lee W, Kim D, Lee K, et al. Direct anodic growth of thick WO3 mesosponge layers and characterization of their photoelectrochemical response[J]. Electrochim. Acta, 2010, 56(2): 828-833. |
| 14 | Macak J M, Ghicov A, Hahn R, et al. Photoelectrochemical properties of N-doped self-organized titania nanotube layers with different thicknesses[J]. J. Mater. Res., 2006, 21(11): 2824-2828. |
| 15 | Feng C X, Xu G Q, Liu H P, et al. A flow-injection photoelectrochemical sensor based on TiO2 nanotube arrays for organic compound detection[J]. J. Electrochem. Soc., 2014, 161(3): H57-H61. |
| 16 | Xu X Q, Wang M, Wang L S. Electrochemical determination of hydrogen peroxide and glucose by titanium(Ⅳ) oxide nanotube arrays[J]. Anal. Lett., 2015, 48(11): 1698-1706. |
| 17 | Grigorescu S, Ungureanu C, Kirchgeorg R, et al. Various sized nanotubes on TiZr for antibacterial surfaces[J]. Appl. Surf. Sci., 2013, 270: 190-196. |
| 18 | Lee K, Mazare A, Schmuki P. One-dimensional titanium dioxide nanomaterials: nanotubes[J]. Chem. Rev., 2014, 114(19): 9385-9454. |
| 19 | Yuan Z Y, Su B L. Titanium oxide nanotubes, nanofibers and nanowires[J]. Colloid Surface A, 2004, 241(1/2/3): 173-183. |
| 20 | Grimes C A. Synthesis and application of highly ordered arrays of TiO2 nanotubes[J]. J. Mater. Chem., 2007, 17(15): 1451-1457. |
| 21 | Gundiah G, Mukhopadhyay S, Tumkurkar U G, et al. Hydrogel route to nanotubes of metal oxides and sulfates[J]. J. Mater. Chem., 2003, 13(9): 2118-2122. |
| 22 | Dong Y H, Ji X Y, Laaksonen A, et al. Determination of the small amount of proteins interacting with TiO2 nanotubes by AFM-measurement[J]. Biomaterials, 2019, 192: 368-376. |
| 23 | 饶超, 董依慧, 庄伟, 等. TiO2纳米管阵列孔径调控葡萄糖氧化酶生物传感器性能[J]. 化工学报, 2016, 67(10): 4324-4333. |
| Rao C, Dong Y H, Zhuang W, et al. Regulate properties of glucose oxidase biosensors through pore sizes of TiO2 nanotube arrays[J]. CIESC Journal, 2016, 67(10): 4324-4333. | |
| 24 | Roy P, Berger S, Schmuki P. TiO2 Nanotubes: synthesis and applications[J]. Angew. Chem. Int. Edit., 2011, 50(13): 2904-2939. |
| 25 | Moerz S T, Huber P. pH-dependent selective protein adsorption into mesoporous silica[J]. J. Phys. Chem. C, 2015, 119(48): 27072-27079. |
| 26 | Shao Q, Jiang S Y. Molecular understanding and design of zwitterionic materials[J]. Adv. Mater., 2015, 27(1): 15-26. |
| 27 | Zhou Z, Hartmann M. Progress in enzyme immobilization in ordered mesoporous materials and related applications[J]. Chem. Soc. Rev., 2013, 42(9): 3894-3912. |
| 28 | Wang F, Min Y, Geng X D. Fast separations of intact proteins by liquid chromatography[J]. J. Sep. Sci., 2012, 35(22): 3033-3045. |
| 29 | Su L A, Jia W Z, Hou C J, et al. Microbial biosensors: a review[J]. Biosensors & Bioelectronics, 2011, 26(5): 1788-1799. |
| 30 | Sun F, Ella-Menye J R, Galvan D D, et al. Stealth surface modification of surface-enhanced Raman scattering substrates for sensitive and accurate detection in protein solutions[J]. ACS Nano, 2015, 9(3): 2668-2676. |
| 31 | Fang T H, Chang W J, Weng C I. Surface analysis of nanomachined films using atomic force microscopy[J]. Mater. Chem. Phys., 2005, 92(2/3): 379-383. |
| 32 | Ye G, Lee J H, Perreault F, et al. Controlled architecture of dual-functional block copolymer brushes on thin-film composite membranes for integrated “defending” and “attacking” strategies against biofouling[J]. ACS Applied Materials & Interfaces, 2015, 7(41): 23069-23079. |
| 33 | Alsteens D, Gaub H E, Newton R, et al. Atomic force microscopy-based characterization and design of biointerfaces[J]. Nat. Rev. Mater., 2017, 2(5): 364-392. |
| 34 | An R, Zhuang W, Yang Z H, et al. Protein adsorptive behavior on mesoporous titanium dioxide determined by geometrical topography[J]. Chem. Eng. Sci., 2014, 117: 146-155. |
| 35 | Dong Y H, An R, Zhao S L, et al. Molecular interactions of protein with TiO2 by the AFM-measured adhesion force[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2017, 33(42): 11626-11634. |
| 36 | Dong Y H, Laaksonen A, Cao W, et al. AFM study of pH‐dependent adhesion of single protein to TiO2 surface[J]. Advanced Materials Interfaces, 2019, 6(14): 1900411. |
| 37 | Wang M S, Palmer L B, Schwartz J D, et al. Evaluating protein attraction and adhesion to biomaterials with the atomic force microscope[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2004, 20(18): 7753-7759. |
| 38 | Cai Q Y, Yang L X, Yu Y. Investigations on the self-organized growth of TiO2 nanotube arrays by anodic oxidization[J]. Thin Solid Films, 2006, 515(4): 1802-1806. |
| 39 | Peng C W, Liu J, Xie Y, et al. Molecular simulations of cytochrome C adsorption on positively charged surfaces: the influence of anion type and concentration[J]. Phys. Chem. Chem. Phys., 2016, 18(15): 9979-9989. |
| [1] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [2] | 王学良, 刘美红, 熊忠汾, 李鑫. 考虑表面粗糙度的柔性箔柱面气膜密封紊流特性分析[J]. 化工学报, 2022, 73(4): 1683-1694. |
| [3] | 熊桂龙, 谢静雯, 杨林军. 粗糙度对水汽在细颗粒表面异质核化影响的数值模拟[J]. 化工学报, 2021, 72(8): 4304-4313. |
| [4] | 周黄, 常禹, 范兴, 张楠楠, 陶长元. TiO2纳米管阵列电合成的扩散-反应耦合强化机制研究[J]. 化工学报, 2020, 71(10): 4663-4673. |
| [5] | 饶超, 董依慧, 庄伟, 邬新兵, 洪启亮, 刘畅, 陆小华. TiO2纳米管阵列孔径调控葡萄糖氧化酶生物传感器性能[J]. 化工学报, 2016, 67(10): 4324-4333. |
| [6] | 李洪懿, 翟丁, 周勇, 高从堦. 纳米聚苯胺改性聚哌嗪酰胺纳滤膜的制备[J]. 化工学报, 2015, 66(1): 142-148. |
| [7] | 许静, 彭旭东, 白少先, 李纪云, 王玉明. 端面微尺度效应和热黏效应对干气密封性能的影响[J]. 化工学报, 2013, 64(9): 3291-3300. |
| [8] | 李旦洋, 朱元正, 张良, 范利武, 徐旭, 俞自涛, 洪荣华, 胡亚才. 水基碳纳米管悬浮液的淬火沸腾特性[J]. 化工学报, 2013, 64(5): 1566-1572. |
| [9] | 李旦洋 朱元正 张良 范利武 徐旭 俞自涛 洪荣华 胡亚才. 水基碳纳米管悬浮液淬火沸腾特性的实验研究[J]. 化工学报, 2013, 64(5): 0-0. |
| [10] | 李旦洋 朱元正 张良 范利武 徐旭 俞自涛 洪荣华 胡亚才. 水基碳纳米管悬浮液淬火沸腾特性的实验研究[J]. 化工学报, 2013, 64(5): 0-0. |
| [11] | 邢卫红, 仲兆祥, 景文珩, 范益群. 基于膜表面与界面作用的膜污染控制方法[J]. 化工学报, 2013, 64(1): 173-181. |
| [12] | 刘海红1,李玉星1,王武昌1,陈鹏1,张庆东1,樊新斌2. 天然气水合物颗粒微观受力及聚集特性研究进展[J]. 化工进展, 2013, 32(08): 1796-1800. |
| [13] | 董如林,刘淑赟,陈智栋,金长春,王彩霞. TiO2/SiO2复合薄膜的制备及其自清洁性能[J]. 化工进展, 2013, 32(03): 645-651. |
| [14] | 鲁进利, 韩亚芳, 陈永平, 郝英立. 不锈钢微细圆管中去离子水流动特性[J]. 化工学报, 2012, 63(10): 3119-3124. |
| [15] | 阮艺平,张莉,徐宏. 铜表面物理化学有序度对蒸汽冷凝特性的影响[J]. 化工学报, 2012, 63(1): 90-95. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号