化工学报 ›› 2024, Vol. 75 ›› Issue (S1): 251-258.DOI: 10.11949/0438-1157.20240534
收稿日期:2024-05-20
修回日期:2024-06-11
出版日期:2024-12-25
发布日期:2024-12-17
通讯作者:
郑仁朝
作者简介:宋世萍(1999—),女,硕士研究生,3206757276@qq.com
基金资助:
Shiping SONG( ), Xiaoling TANG, Renchao ZHENG(
), Xiaoling TANG, Renchao ZHENG( )
)
Received:2024-05-20
Revised:2024-06-11
Online:2024-12-25
Published:2024-12-17
Contact:
Renchao ZHENG
摘要:
谷胱甘肽(GSH)是细胞内丰富的非蛋白质硫醇,其巯基作为活性基团,参与生物体内氧化还原反应,具有多种生理功能,在食品、医药及化妆品等行业得到广泛应用。谷胱甘肽双功能合成酶是合成GSH的关键酶,其催化效率的提升对GSH高效合成具有重要意义。本研究以Streptococcus thermophilus来源谷胱甘肽双功能合成酶(St-GshF)为研究对象,构建了一种基于谷胱甘肽和邻苯二甲醛反应产生荧光显色效应的高通量筛选方法,通过半理性设计对St-GshF进行分子改造,筛选获得有益突变体St-GshF(S27Q/G510P),其酶活为野生型的1.75倍。在此基础上,偶联多聚磷酸激酶,构建高效ATP循环系统并进行反应体系优化,最终谷胱甘肽的产量为17.36 g/L,产率达94.22%,为谷胱甘肽的规模化生产奠定了重要基础。
中图分类号:
宋世萍, 汤晓玲, 郑仁朝. 谷胱甘肽双功能合成酶分子改造及应用[J]. 化工学报, 2024, 75(S1): 251-258.
Shiping SONG, Xiaoling TANG, Renchao ZHENG. Molecular modification of glutathione bifunctional synthase and its application[J]. CIESC Journal, 2024, 75(S1): 251-258.
| 位点 | 引物序列 |
|---|---|
| S27MNN | 5´-ACGCAGMNNTTCACGTTCGATACCGAAGTTAGC-3´ |
| S27NNK | 5´-CGTGAANNKCTGCGTGTTGACCGTCAGGGTCAG-3´ |
| P99MNN | 5´-AGACAGMNNCCACAGAACTTCGTCGGTAGCGAT-3´ |
| P99NNK | 5´-CTGTGGNNKCTGTCTATGCCGCCGCGTCTGAAA-3´ |
| L136MNN | 5´-AGCCTGMNNTTTGGTACCGTATTTTTCAGCCAG-3´ |
| L136NNK | 5´-ACCAAANNKCAGGCTATCTCTGGTATCCACTAC-3´ |
| T505MNN | 5´-CAGAGAMNNGAATTCGTCACCAGACGGAACCG-3´ |
| T505NNK | 5´-GAATTCNNKTCTCTGGAAGAAGGTCTGGCTTAC-3´ |
| G510MNN | 5´-GAAGAAMNNCTGGCTTACTACCCGCTGATCAAA-3´ |
| G510NNK | 5´-AGCCAGNNKTTCTTCCAGAGAGGTGAATTCGTC-3´ |
表1 饱和突变引物
Table 1 Primers for saturation mutation
| 位点 | 引物序列 |
|---|---|
| S27MNN | 5´-ACGCAGMNNTTCACGTTCGATACCGAAGTTAGC-3´ |
| S27NNK | 5´-CGTGAANNKCTGCGTGTTGACCGTCAGGGTCAG-3´ |
| P99MNN | 5´-AGACAGMNNCCACAGAACTTCGTCGGTAGCGAT-3´ |
| P99NNK | 5´-CTGTGGNNKCTGTCTATGCCGCCGCGTCTGAAA-3´ |
| L136MNN | 5´-AGCCTGMNNTTTGGTACCGTATTTTTCAGCCAG-3´ |
| L136NNK | 5´-ACCAAANNKCAGGCTATCTCTGGTATCCACTAC-3´ |
| T505MNN | 5´-CAGAGAMNNGAATTCGTCACCAGACGGAACCG-3´ |
| T505NNK | 5´-GAATTCNNKTCTCTGGAAGAAGGTCTGGCTTAC-3´ |
| G510MNN | 5´-GAAGAAMNNCTGGCTTACTACCCGCTGATCAAA-3´ |
| G510NNK | 5´-AGCCAGNNKTTCTTCCAGAGAGGTGAATTCGTC-3´ |
| 突变体 | 酶活/(U/g wcw) | 相对酶活/% |
|---|---|---|
| St-GshF | 45.0 | 100 |
| St-GshF(S27Q) | 72.0 | 160.0 |
| St-GShF(G510P) | 76.2 | 169.3 |
| St-GshF(S27Q/G510P) | 78.75 | 175.1 |
表2 St-GshF及其突变体的相对酶活
Table 2 The relative enzymatic activity of St-GshF and its variants
| 突变体 | 酶活/(U/g wcw) | 相对酶活/% |
|---|---|---|
| St-GshF | 45.0 | 100 |
| St-GshF(S27Q) | 72.0 | 160.0 |
| St-GShF(G510P) | 76.2 | 169.3 |
| St-GshF(S27Q/G510P) | 78.75 | 175.1 |
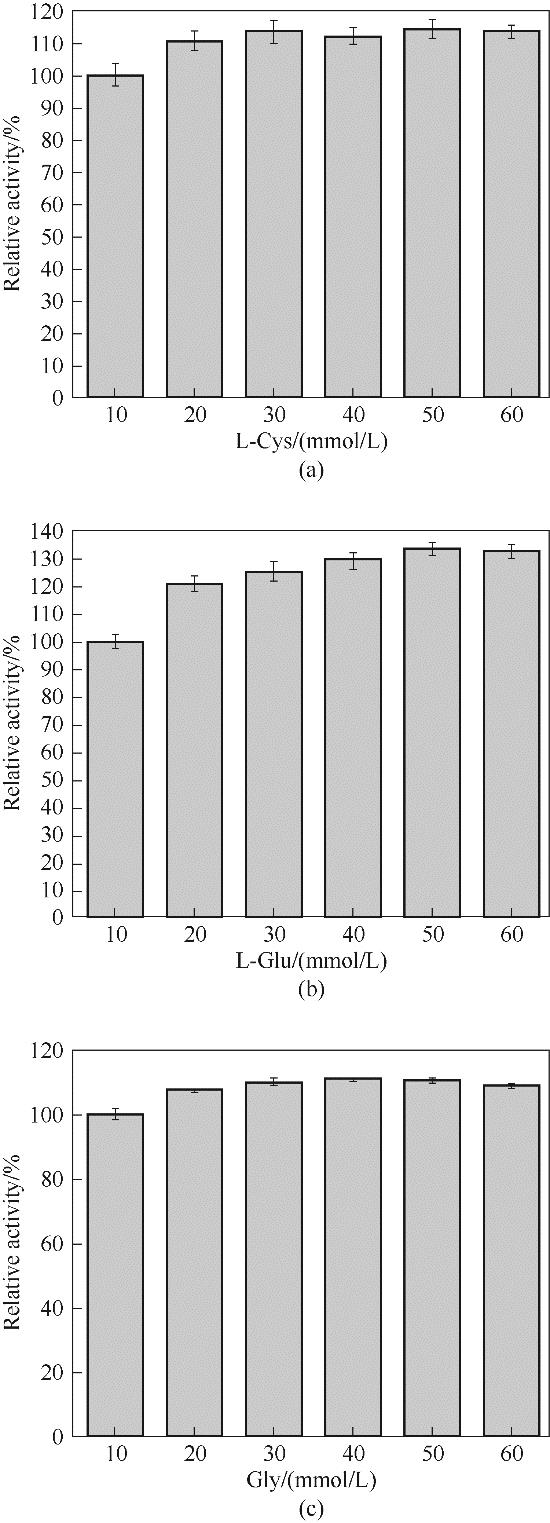
图10 不同浓度底物(L-Cys, L-Glu, Gly)对St-GshF(S27Q/G510P)催化效率的影响
Fig.10 The effect of substrate concentrations (L-Cys, L-Glu, Gly) on the catalytic efficiency of St-GshF (S27Q/G510P)
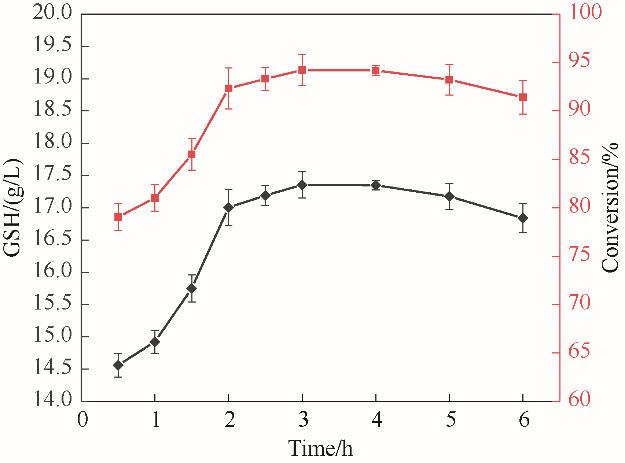
图11 谷胱甘肽双功能合成酶和多聚磷酸激酶偶联体系催化合成GSH的反应进程
Fig.11 The catalytic synthesis process of GSH by the coupling system of glutathione bifunctional synthase and polyphosphate kinase
| 1 | Lushchak V I. Glutathione homeostasis and functions: potential targets for medical interventions[J]. Journal of Amino Acids, 2012, 2012: 736837. |
| 2 | Kretzschmar M. Regulation of hepatic glutathione metabolism and its role in hepatotoxicity[J]. Experimental and Toxicologic Pathology, 1996, 48(5): 439-446. |
| 3 | Ishkaeva RA, Nizamov IS, Blokhin DS, et al. Dithiophosphate-induced redox conversions of reduced and oxidized glutathione[J]. Molecules, 2021, 26(10): 2973. |
| 4 | Jeong E M, Yoon J H, Lim J, et al. Real-time monitoring of glutathione in living cells reveals that high glutathione levels are required to maintain stem cell function[J]. Stem Cell Reports, 2018, 10(2): 600-614. |
| 5 | Raj Rai S, Bhattacharyya C, Sarkar A, et al. Glutathione: role in oxidative/nitrosative stress, antioxidant defense, and treatments[J]. ChemistrySelect, 2021, 6(18): 4566-4590. |
| 6 | Bachhawat A K, Yadav S. The glutathione cycle: glutathione metabolism beyond the γ‐glutamyl cycle[J]. IUBMB Life, 2018, 70(7): 585-592. |
| 7 | Witoo D, Teerapon D, Piyameth D. The clinical effect of glutathione on skin color and other related skin conditions: a systematic review[J]. Journal of Cosmetic Dermatology, 2019, 18(3): 728-737. |
| 8 | Zhang P P, Li S S, Guo Z F, et al. Nitric oxide regulates glutathione synthesis and cold tolerance in forage legumes[J]. Environmental and Experimental Botany, 2019, 167: 103851. |
| 9 | Morellato A E, Umansky C, Pontel L B. The toxic side of one-carbon metabolism and epigenetics[J]. Redox Biology, 2021, 40: 101850. |
| 10 | Wang H L, Zhang J, Li Y P, et al. Potential use of glutathione as a treatment for P a r k i n s o n ' s disease[J]. Experimental and Therapeutic Medicine, 2021, 21(2): 125. |
| 11 | Gogoi K, Manna P, Dey T, et al. Circulatory heavy metals (cadmium, lead, mercury, and chromium) inversely correlate with plasma GST activity and GSH level in COPD patients and impair NOX4/Nrf2/GCLC/GST signaling pathway in cultured monocytes[J]. Toxicology in Vitro, 2019, 54: 269-279. |
| 12 | 徐菁苒, 毛健, 刘双平, 等. 高产GSH果酒酵母的筛选及对苹果酒褐变的抑制[J]. 食品与生物技术学报, 2018, 37(5): 455-462. |
| Xu J R, Mao J, Liu S P, et al. Study on the screening of high GSH -yielding wine yeast and its effect on wine browning[J]. Journal of Food Science and Biotechnology, 2018, 37(5): 455-462. | |
| 13 | 任赐, 张方方, 李莹, 等. 富含谷胱甘肽酵母制品在葡萄酒酿造中的应用进展[J]. 食品与发酵工业, 2023: 1-9. |
| Ren C, Zhang F F, Li Y, et al. Advances in the application of glutathione-enriched inactive dry yeast derivatives during winemaking[J]. Food and Fermentation Industries, 2023: 1-9. | |
| 14 | Gotti R, Andrisano V, Cavrini V, et al. Determination of glutathione in pharmaceuticals and cosmetics by HPLC with UV and fluorescence detection[J]. Chromatographia, 1994, 39(1): 23-28. |
| 15 | Cui X W, Wan J X, Zhang X, et al. Efficient glutathione production in metabolically engineered Escherichia coli strains using constitutive promoters[J]. Journal of Biotechnology, 2019, 289: 39-45. |
| 16 | Huang C S, Chang L S, Anderson M E, et al. Catalytic and regulatory properties of the heavy subunit of rat kidney gamma-glutamylcysteine synthetase[J]. Journal of Biological Chemistry, 1993, 268(26): 19675-19680. |
| 17 | Huang C, Yin Z. Highly efficient synthesis of glutathione via a genetic engineering enzymatic method coupled with yeast ATP generation[J]. Catalysts, 2019, 10(1): 33. |
| 18 | Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes[J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2013, 1830(5): 3217-3266. |
| 19 | Richman PG, Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione[J]. Journal of Biological Chemistry, 1975, 250(4):1422-1426. |
| 20 | Griffith O W, Mulcahy R T. The Enzymes of Glutathione Synthesis: γ-Glutamylcysteine Synthetase[M]. Advances in Enzymology and Related Areas of Molecular Biology, 1999: 209-267. |
| 21 | Janowiak B E, Griffith O W. Glutathione synthesis in Streptococcus agalactiae: one protein accounts for γ-glutamylcysteine synthetase and glutathione synthetase activities[J]. Journal of Biological Chemistry, 2005, 280(12): 11829-11839. |
| 22 | Yang J, Li W, Wang D, et al. Characterization of bifunctional L-glutathione synthetases from Actinobacillus pleuropneumoniae and Actinobacillus succinogenes for efficient glutathione biosynthesis[J]. Applied microbiology and biotechnology, 2016, 100(14):6279-6289. |
| 23 | Andexer J N, Richter M. Emerging enzymes for ATP regeneration in biocatalytic processes[J]. Chembiochem: a European Journal of Chemical Biology, 2015, 16(3): 380-386. |
| 24 | Wang D Z, Wang C, Wu H, et al. Glutathione production by recombinant Escherichia coli expressing bifunctional glutathione synthetase[J]. Journal of Microbiology and Biotechnology, 2016, 43(1): 45-53. |
| 25 | Huang X, Li Y M, Du C, et al. Expression of polyphosphate kinase from Sphingobacterium siyangensis and its application in ATP regeneration system[J]. Chinese Journal of Biotechnology, 2022, 38(12): 4669-4680. |
| 26 | Cao H, Li C, Zhao J, et al. Enzymatic production of glutathione coupling with an ATP regeneration system based on polyphosphate kinase[J]. Applied Biochemistry and Biotechnology, 2018, 185(2): 385-395. |
| 27 | Coates T L, Young N, Jarrett A J, et al. Current computational methods for enzyme design[J]. Modern Physics Letters B, 2021, 35(9): 2150155. |
| 28 | Eberhardt J, Santos-Martins D, Tillack AF, et al. AutoDock Vina 1.2.0: new docking methods, expanded force field, and python bindings[J]. Journal of Chemical Information and Modeling, 2021, 61(8): 3891-3898. |
| 29 | Chen R F, Scott C, Trepman E. Fluorescence properties of o-phthaldialdehyde derivatives of amino acids[J]. Biochimica et Biophysica Acta (BBA) - Protein Structure, 1979, 576(2): 440-455. |
| 30 | Bae W, Yoon T Y, Jeong C. Direct evaluation of self-quenching behavior of fluorophores at high concentrations using an evanescent field[J]. PLoS One, 2021, 16(2): e0247326. |
| [1] | 吴哲明, 张碧云, 郑仁朝. 腈水解酶立体选择性改造及其合成布瓦西坦[J]. 化工学报, 2024, 75(7): 2633-2643. |
| [2] | 安绍杰, 许洪峰, 李思, 许远航, 李佳锡. 利用分子机器的组装与分解构建pH敏感性谷胱甘肽过氧化物人工酶[J]. 化工学报, 2022, 73(8): 3669-3678. |
| [3] | 冯旭东, 吕波, 李春. 酶分子稳定性改造研究进展[J]. 化工学报, 2016, 67(1): 277-284. |
| [4] | 王玉磊,卫功元,邵娜,聂敏. 基于能量代谢分析的S-腺苷蛋氨酸和谷胱甘肽联合高产方法[J]. 化工学报, 2012, 63(1): 223-229. |
| [5] | 董颖颖,卫功元,张君丽,陈学东. 谷胱甘肽生物合成过程中酸胁迫的作用及其机制[J]. CIESC Journal, 2011, 62(11): 3228-3235. |
| [6] | 卫功元, 王大慧, 陈坚. 不同溶氧控制方式下的谷胱甘肽分批发酵过程分析 [J]. 化工学报, 2007, 58(9): 2329-2335. |
| [7] | 卫功元;李寅;堵国成;陈坚 . 产朊假丝酵母分批发酵生产谷胱甘肽的代谢通量分析 [J]. CIESC Journal, 2006, 57(6): 1410-1417. |
| [8] | 聂薇, 卫功元, 李寅, 堵国成, 陈坚. 利用低pH胁迫作用促进产朊假丝酵母生产谷胱甘肽 [J]. 化工学报, 2005, 56(9): 1750-1756. |
| [9] | 梅乐和,林东强,朱自强. 双水相分配结合温度诱导相分离从酵母中提取谷胱甘肽 [J]. CIESC Journal, 1998, 49(4): 470-475. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号