化工学报 ›› 2023, Vol. 74 ›› Issue (4): 1598-1606.DOI: 10.11949/0438-1157.20230091
收稿日期:2023-02-20
修回日期:2023-03-20
出版日期:2023-04-05
发布日期:2023-06-02
通讯作者:
杨正金,徐铜文
作者简介:吕阳光(1996—),男,硕士研究生,yglv@mail.ustc.edu.cn
基金资助:
Yangguang LYU( ), Peipei ZUO, Zhengjin YANG(
), Peipei ZUO, Zhengjin YANG( ), Tongwen XU(
), Tongwen XU( )
)
Received:2023-02-20
Revised:2023-03-20
Online:2023-04-05
Published:2023-06-02
Contact:
Zhengjin YANG, Tongwen XU
摘要:
具备耐各种有机溶剂的微孔聚合物膜在有机纳滤领域逐渐受到重视。采用双氰基单体的超酸催化成环聚合反应,制备微孔框架聚合物薄膜(CTF-BP),该膜具备良好的力学性能,可耐受甲醇和正己烷等常见有机溶剂。CTF-BP膜内大量<1.0 nm的微孔通道使膜具备良好的筛分性能,其截留分子量为550。膜内含有的三嗪结构与羟基具有较强的亲和性,使甲醇的跨膜通量[1.10 L/(m2·h·bar)]显著高于黏度更低的正己烷通量[0.23 L/(m2·h·bar)]。采用纳滤操作将膜用于分离含低浓度甲醇的正己烷溶液[含5%(质量)甲醇的正己烷溶液],结果显示甲醇/正己烷分离因子最高可达到1485,渗透液的总流量超过3.21 kg/(m2·h)。证实CTF-BP膜有望实现高效甲醇/正己烷分离。
中图分类号:
吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606.
Yangguang LYU, Peipei ZUO, Zhengjin YANG, Tongwen XU. Triazine framework polymer membranes for methanol/n-hexane separation via organic solvent nanofiltration[J]. CIESC Journal, 2023, 74(4): 1598-1606.
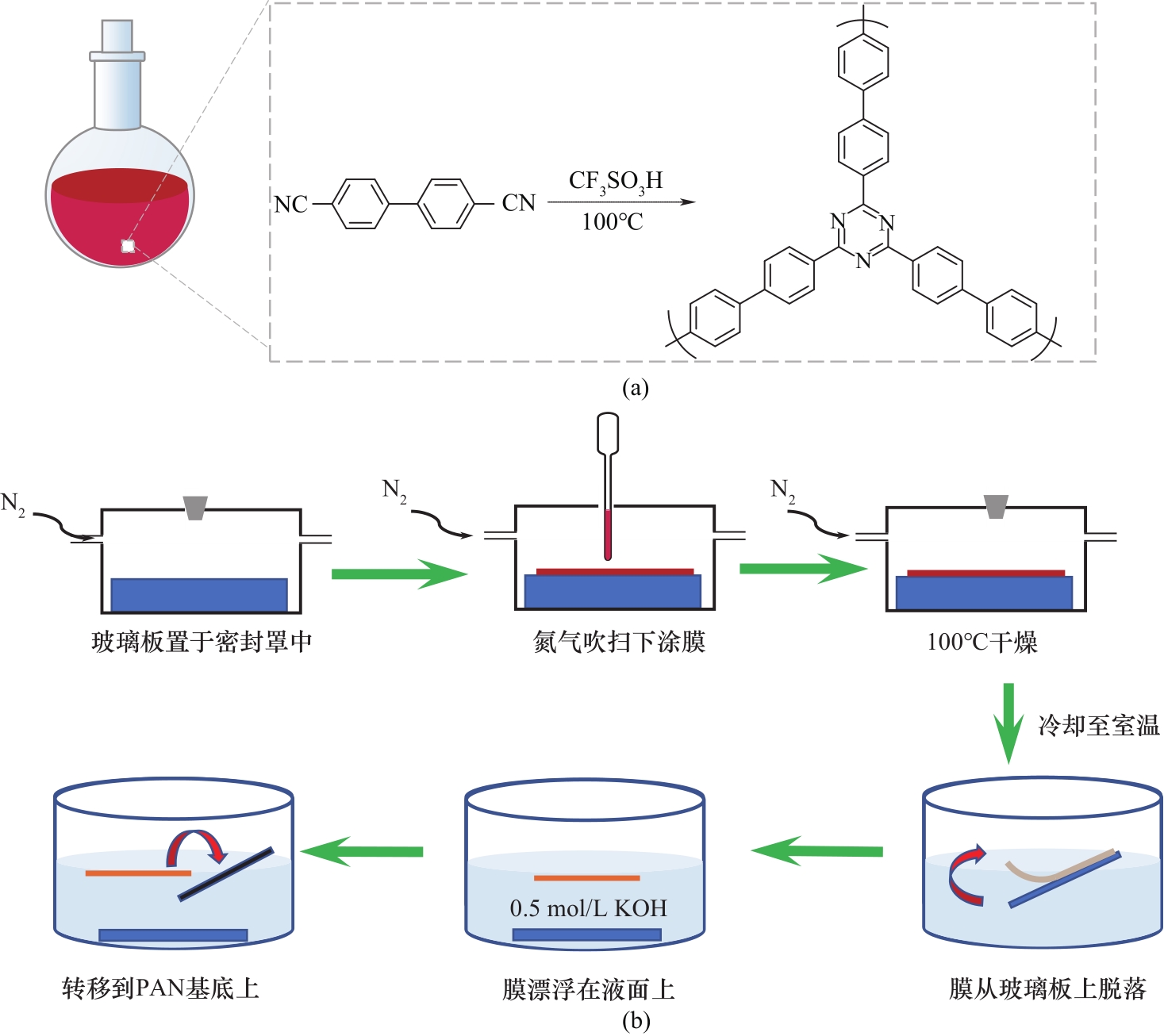
图1 4,4'-联苯甲腈的超酸催化聚合制备微孔三嗪框架聚合物(a)及膜制备过程(b)
Fig.1 Covalent triazine framework synthesized by the superacid catalytic polymerization of 4,4′-biphenyldicarbonitrile (a) and schematic diagram of the preparation of CTF-BP membranes (b)
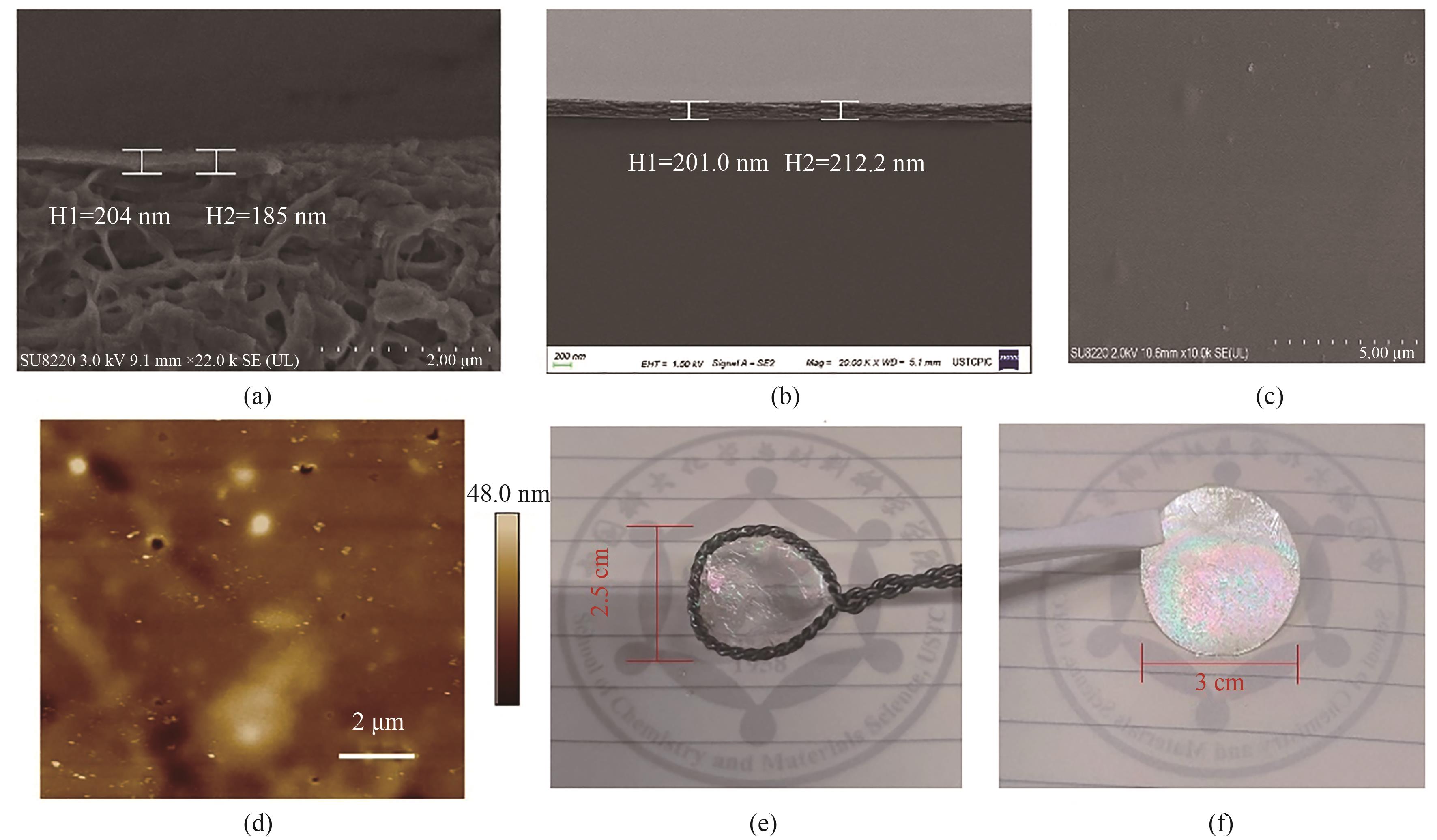
图4 以HPAN(a)和载玻片(b)为基底的CTF-BP膜断面SEM图;CTF-BP的表面SEM图(c);CTF-BP膜的表面AFM图(d);CTF-BP膜附着在铁圈上(e)和HPAN基底上(f)实物图
Fig.4 Cross-sectional SEM images of the CTF-BP membrane supported on porous HPAN (a) and glass plate (b), SEM (c) and AFM (d) images of the surface morphology of CTF-BP, photos of a free-standing CTF-BP membrane on an iron loop (e) and on an HPAN substrate (f)
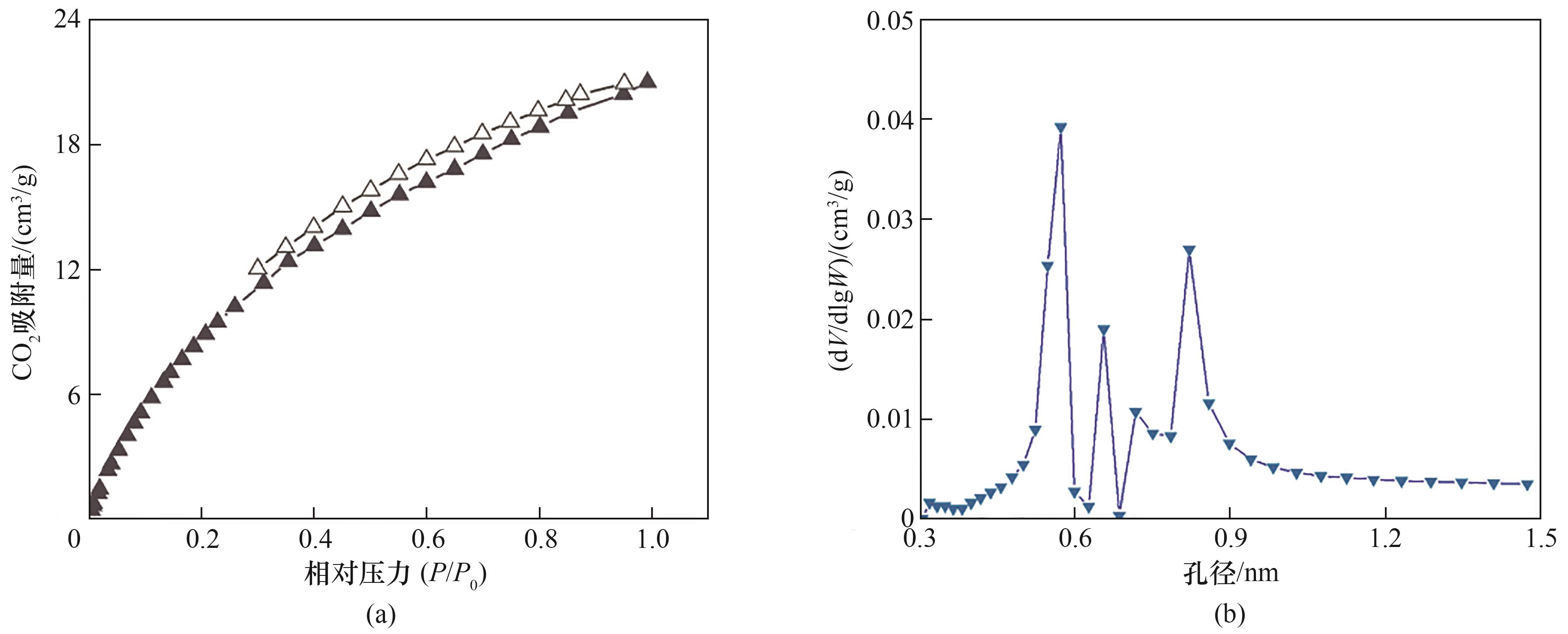
图5 CTF-BP等温CO2吸脱附曲线(273 K)(a);基于CO2等温吸附曲线,采用密度泛函理论模拟计算得到的CTF-BP的孔径分布(b)
Fig.5 CO2 sorption isotherms of CTF-BP membrane (273 K) (a), the pore size distribution of CTF-BP calculated according to density functional theory (DFT) based on the CO2 sorption isotherms (b)
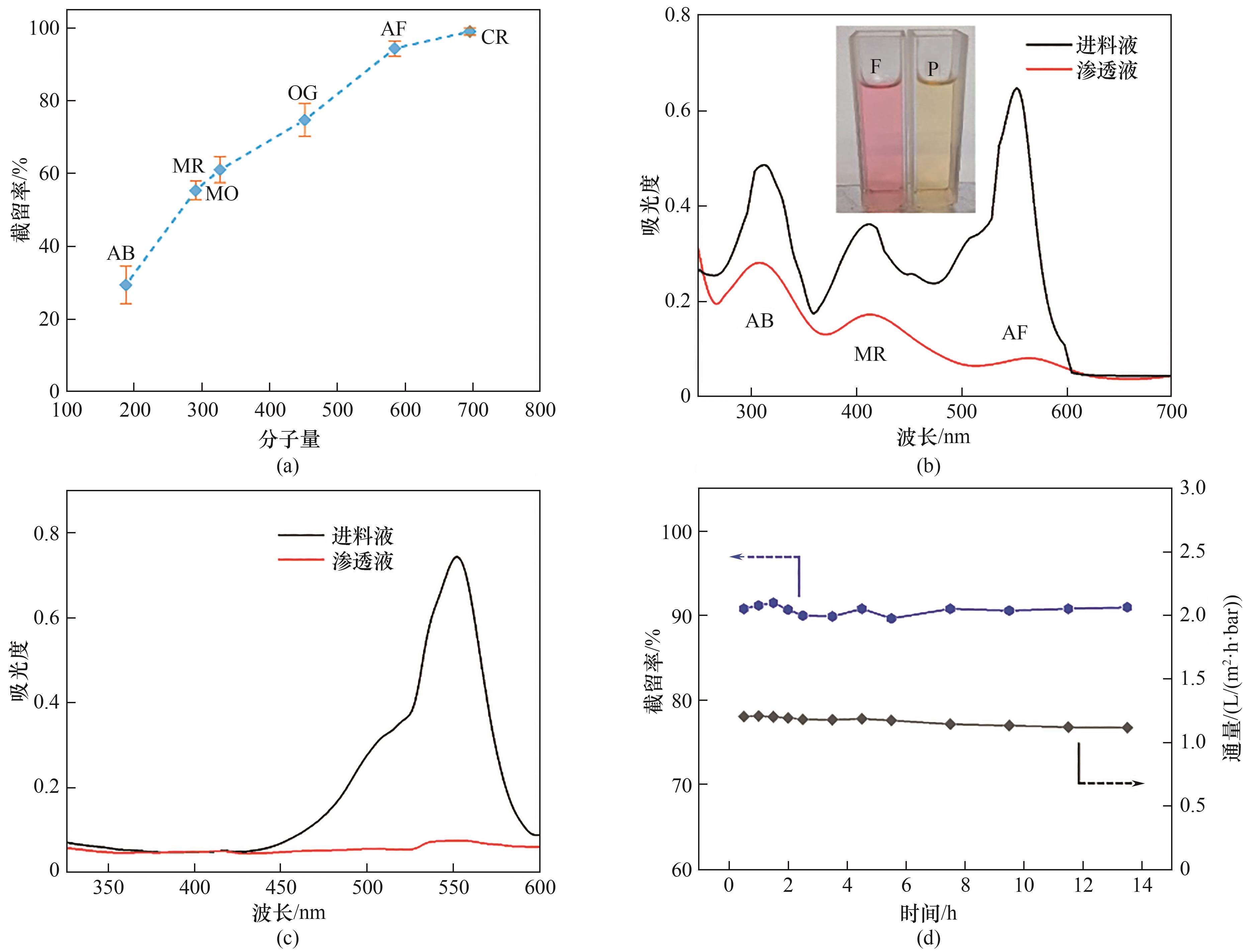
图6 CTF-BP膜对单个染料分子的截留率和对应的分子量(a);偶氮苯、甲基红、酸性品红混合物溶液跨膜渗透前后的紫外吸收波谱(b);酸性品红溶液跨膜渗透前后的紫外吸收波谱(c);长时间测试下酸性品红的截留率和渗透液通量(20 mg/L)(d)
Fig.6 Rejection rate as a function of the molecular weight of varied dyes (a), the UV-Vis spectra of the feed mixture consisting of AB, MR, AF and permeant (b), ultraviolet visible absorption spectra of AF before and after filtration through CTF-BP membranes (c), long-term OSN test of CTF-BP membrane in separating a solution mixture of acid fuchsine and methanol (20 mg/L) (d)
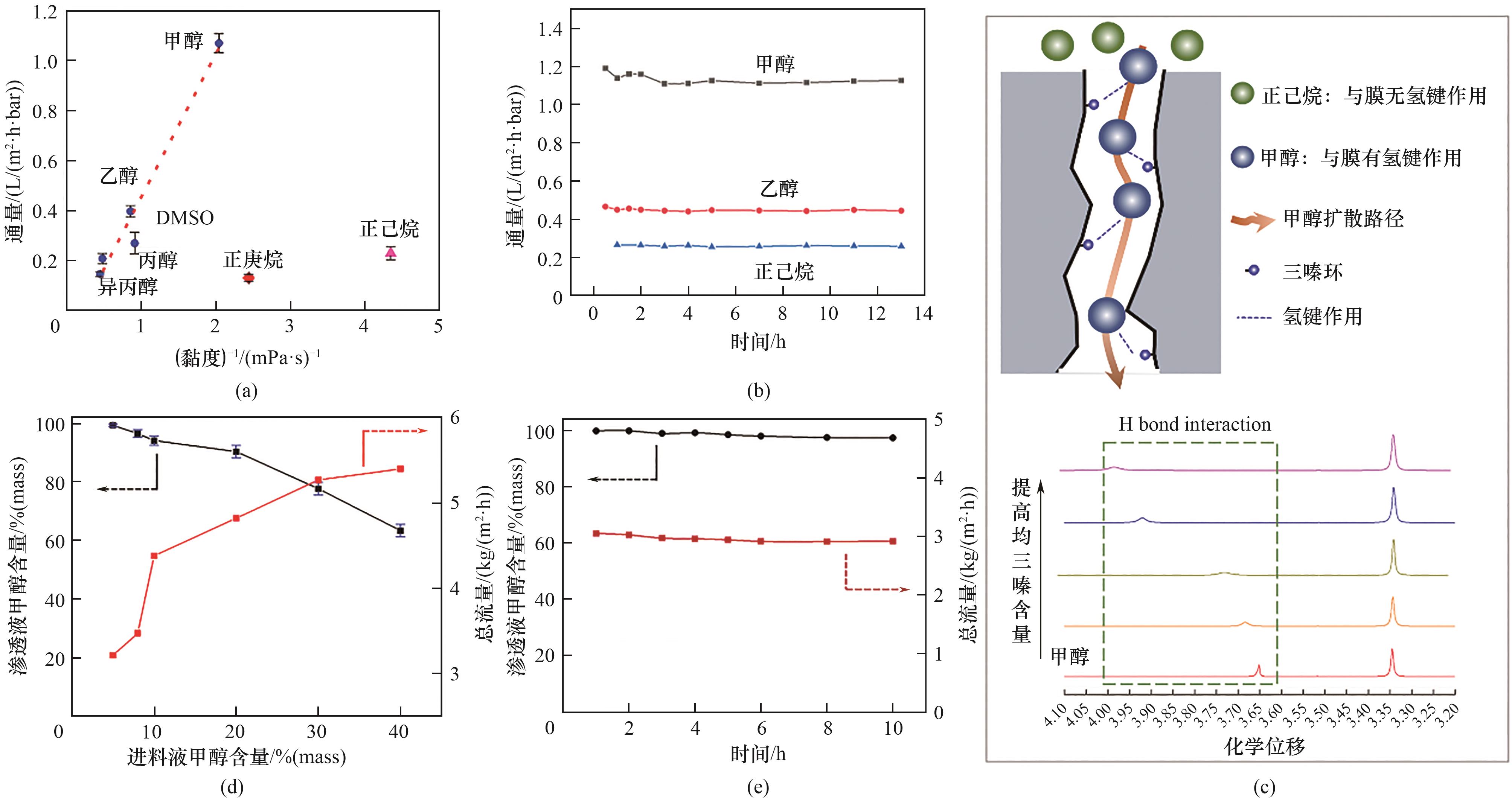
图7 溶剂通过CTF-BP膜的渗透通量和溶剂黏度关系(a);甲醇、乙醇、正己烷通过CTF-BP膜的渗透通量随时间的变化曲线(b);CTF-BP膜分离甲醇/正己烷混合溶液机理示意图及在100 μl甲醇中加入0.05、0.10、0.15、0.20 g 1,3,5-三嗪核磁滴定结果(c);进料液为不同浓度的甲醇/正己烷混合溶液时渗透液的总流量以及其中甲醇含量(d);进料液为8%(质量)甲醇/正己烷混合溶液时跨膜渗透液的总流量和其中甲醇含量随时间的变化(e)
Fig.7 Solvents permeance through the CTF-BP membrane versus the solvent viscosity for the membrane (a), plot of methanol, ethanol and n-hexane permeances with time for CTF-BP membranes (b), schematic diagram of the separation mechanism of methanol/n-hexane mixture (the upper panel) and 1H NMR spectra of 100 μl methanol with varied mass of 1,3,5-triazine from 0.05 g to 0.20 g (c), dependence of methanol concentration in permeate and total flux on methanol concentration in feed for membranes of methanol/n-hexane mixture (d), long time separation performance of methanol/n-hexane with the 8% (mass) methanol in feed solution (e)
| 1 | Paul M, Jons S D. Chemistry and fabrication of polymeric nanofiltration membranes: a review[J]. Polymer, 2016, 103: 417-456. |
| 2 | Bhosle B M, Subramanian R. New approaches in deacidification of edible oils—a review[J]. Journal of Food Engineering, 2005, 69(4): 481-494. |
| 3 | Abels C, Carstensen F, Wessling M. Membrane processes in biorefinery applications[J]. Journal of Membrane Science, 2013, 444: 285-317. |
| 4 | Gould R M, White L S, Wildemuth C R. Membrane separation in solvent lube dewaxing[J]. Environmental Progress, 2001, 20(1): 12-16. |
| 5 | Székely G, Bandarra J, Heggie W, et al. Organic solvent nanofiltration: a platform for removal of genotoxins from active pharmaceutical ingredients[J]. Journal of Membrane Science, 2011, 381(1/2): 21-33. |
| 6 | Siew W E, Livingston A G, Ates C, et al. Continuous solute fractionation with membrane cascades—a high productivity alternative to diafiltration[J]. Separation and Purification Technology, 2013, 102: 1-14. |
| 7 | Khan N A, Zhang R N, Wu H, et al. Solid-vapor interface engineered covalent organic framework membranes for molecular separation[J]. Journal of the American Chemical Society, 2020, 142(31): 13450-13458. |
| 8 | Cheng X Q, Wang Z X, Jiang X, et al. Towards sustainable ultrafast molecular-separation membranes: from conventional polymers to emerging materials[J]. Progress in Materials Science, 2018, 92: 258-283. |
| 9 | Vandezande P, Gevers L E M, Vankelecom I F J. Solvent resistant nanofiltration: separating on a molecular level[J]. Chemical Society Reviews, 2008, 37(2): 365-405. |
| 10 | Cheng C, Iyengar S A, Karnik R. Molecular size-dependent subcontinuum solvent permeation and ultrafast nanofiltration across nanoporous graphene membranes[J]. Nature Nanotechnology, 2021, 16(9): 989-995. |
| 11 | Jung B, Ma S C, Miang Khor C, et al. Impact of polarity reversal on inorganic scaling on carbon nanotube-based electrically-conducting nanofiltration membranes[J]. Chemical Engineering Journal, 2023, 452: 139216. |
| 12 | See Toh Y H, Lim F W, Livingston A G. Polymeric membranes for nanofiltration in polar aprotic solvents[J]. Journal of Membrane Science, 2007, 301(1/2): 3-10. |
| 13 | Chung T S. A critical review of polybenzimidazoles[J]. Polymer Reviews, 1997, 37(2): 277-301. |
| 14 | Sani N A A, Lau W J, Ismail A F. Polyphenylsulfone-based solvent resistant nanofiltration (SRNF) membrane incorporated with copper-1,3,5-benzenetricarboxylate (Cu-BTC) nanoparticles for methanol separation[J]. RSC Advances, 2015, 5(17): 13000-13010. |
| 15 | Zhu L F, Yu H W, Zhang H J, et al. Mixed matrix membranes containing MIL-53(Al) for potential application in organic solvent nanofiltration[J]. RSC Advances, 2015, 5(89): 73068-73076. |
| 16 | Lim S K, Goh K, Bae T H, et al. Polymer-based membranes for solvent-resistant nanofiltration: a review[J]. Chinese Journal of Chemical Engineering, 2017, 25(11): 1653-1675. |
| 17 | Geens J, van der Bruggen B, Vandecasteele C. Characterisation of the solvent stability of polymeric nanofiltration membranes by measurement of contact angles and swelling[J]. Chemical Engineering Science, 2004, 59(5): 1161-1164. |
| 18 | Yushkin A A, Anokhina T S, Bazhenov S D, et al. Sorption and nanofiltration characteristics of PIM-1 material in polar and non-polar solvents[J]. Petroleum Chemistry, 2018, 58(13): 1154-1158. |
| 19 | Wang C X, Li C X, Rutledge E R C, et al. Aromatic porous polymer network membranes for organic solvent nanofiltration under extreme conditions[J]. Journal of Materials Chemistry A, 2020, 8(31): 15891-15899. |
| 20 | Agarwal P, Hefner R E, Ge S R, et al. Nanofiltration membranes from crosslinked Troger’s base polymers of intrinsic microporosity (PIMs)[J]. Journal of Membrane Science, 2020, 595: 117501. |
| 21 | Abdulhamid M A, Szekely G. Organic solvent nanofiltration membranes based on polymers of intrinsic microporosity[J]. Current Opinion in Chemical Engineering, 2022, 36: 100804. |
| 22 | Zhou S Y, Zhao Y L, Zheng J F, et al. High-performance functionalized polymer of intrinsic microporosity (PIM) composite membranes with thin and stable interconnected layer for organic solvent nanofiltration[J]. Journal of Membrane Science, 2019, 591: 117347. |
| 23 | Dong G X, Lee Y M. Microporous polymeric membranes inspired by adsorbent for gas separation[J]. Journal of Materials Chemistry A, 2017, 5(26): 13294-13319. |
| 24 | Ma C H, Urban J J. Hydrogen-bonded polyimide/metal-organic framework hybrid membranes for ultrafast separations of multiple gas pairs[J]. Advanced Functional Materials, 2019, 29(32): 1903243. |
| 25 | Genduso G, Amelio A, Luis P, et al. Separation of methanol-tetrahydrofuran mixtures by heteroazeotropic distillation and pervaporation[J]. AIChE Journal, 2014, 60(7): 2584-2595. |
| 26 | Jiang L Y, Wang Y, Chung T S, et al. Polyimides membranes for pervaporation and biofuels separation[J]. Progress in Polymer Science, 2009, 34(11): 1135-1160. |
| 27 | Cabasso I. Organic liquid mixtures separation by permselective polymer membranes (Ⅰ): Selection and characteristics of dense isotropic membranes employed in the pervaporation process[J]. Industrial & Engineering Chemistry Product Research and Development, 1983, 22(2): 313-319. |
| 28 | Sheng F M, Hou L X, Wang X X, et al. Electro-nanofiltration membranes with positively charged polyamide layer for cations separation[J]. Journal of Membrane Science, 2020, 594: 117453. |
| 29 | He X, Sin H, Liang B, et al. Controlling the selectivity of conjugated microporous polymer membrane for efficient organic solvent nanofiltration[J]. Advanced Functional Materials, 2019, 29(32): 1900134. |
| 30 | Yasuda T, Shimizu T, Liu F, et al. Electro-functional octupolar π-conjugated columnar liquid crystals[J]. Journal of the American Chemical Society, 2011, 133(34): 13437-13444. |
| 31 | Zhu X, Tian C C, Mahurin S M, et al. A superacid-catalyzed synthesis of porous membranes based on triazine frameworks for CO2 separation[J]. Journal of the American Chemical Society, 2012, 134(25): 10478-10484. |
| 32 | Li J Q, Zhang M X, Feng W L, et al. PIM-1 pore-filled thin film composite membranes for tunable organic solvent nanofiltration[J]. Journal of Membrane Science, 2020, 601: 117951. |
| 33 | Cook M, Gaffney P R J, Peeva L G, et al. Roll-to-roll dip coating of three different PIMs for organic solvent nanofiltration[J]. Journal of Membrane Science, 2018, 558: 52-63. |
| 34 | Marchetti P, Jimenez Solomon M F, Szekely G, et al. Molecular separation with organic solvent nanofiltration: a critical review[J]. Chemical Reviews, 2014, 114(21): 10735-10806. |
| 35 | Huang T F, Moosa B A, Hoang P, et al. Molecularly-porous ultrathin membranes for highly selective organic solvent nanofiltration[J]. Nature Communications, 2020, 11(1): 1-10. |
| 36 | He P P, Zhao S, Mao C Y, et al. In-situ growth of double-layered polyaniline composite membrane for organic solvent nanofiltration[J]. Chemical Engineering Journal, 2021, 420: 129338. |
| 37 | Polotskaya G, Pulyalina A, Goikhman M, et al. Novel polyheteroarylene membranes for separation of methanol‒hexane mixture by pervaporation[J]. Scientific Reports, 2018, 8(1): 1-12. |
| [1] | 周国莉, 韩项珂, 武文佳, 王景涛, 张毛娃, 李凤丽. 异质结构g-C3N4@AM层状膜构筑及纳滤性能研究[J]. 化工学报, 2022, 73(2): 941-950. |
| [2] | 张后虎, 吴晓莉, 陈冲冲, 陈静静, 王景涛. CD-MOF二维层状膜制备及混合溶剂精准分离研究[J]. 化工学报, 2022, 73(10): 4539-4550. |
| [3] | 刘嘉玮, 郝雨峰, 苏延磊. 石墨烯量子点纳滤膜的仿生修饰及稳定性研究[J]. 化工学报, 2021, 72(6): 3390-3398. |
| [4] | 李燕, 王敏, 赵有璟, 王怀有, 杨红军, 祝增虎. 纳滤膜对高镁锂比盐湖卤水镁锂分离性能研究[J]. 化工学报, 2021, 72(6): 3130-3139. |
| [5] | 冉瑾,黄强,艾新宇,吴玉莹,张朋朋,窦焰. Zn-BTC/MoS2复合二维膜构筑及有机溶剂纳滤性能研究[J]. 化工学报, 2021, 72(4): 2148-2155. |
| [6] | 陆至彬, 谢星, 鲁思达, 何畅, 张冰剑, 陈清林. 基于代理模型的含盐废水多级纳滤系统的过程优化设计[J]. 化工学报, 2021, 72(3): 1400-1408. |
| [7] | 何鹏鹏, 赵颂, 毛晨岳, 王志, 王纪孝. 耐溶剂复合纳滤膜的研究进展[J]. 化工学报, 2021, 72(2): 727-747. |
| [8] | 杨丰瑞, 王志, 燕方正, 韩向磊, 王纪孝. 纳滤用于一价/二价无机盐溶液分离研究进展[J]. 化工学报, 2021, 72(2): 799-813. |
| [9] | 刘宁, 褚昌辉, 王乾, 孙世鹏. 用于混合一价盐分离的纳滤膜的制备及性能研究[J]. 化工学报, 2021, 72(1): 578-588. |
| [10] | 汪菊, 牛淑锋, 费莹, 漆虹. GO/Al2O3复合纳滤膜的制备及其稳定性能研究[J]. 化工学报, 2020, 71(6): 2795-2803. |
| [11] | 徐燕青, 李文飞, 吴梦瑶, 沈江南. 用于喷墨印花染料纯化的自组装GO/TiO2复合纳滤膜的制备[J]. 化工学报, 2020, 71(3): 1352-1361. |
| [12] | 刘丽雪, 张少峰, 赵长伟, 宝乐尔呼, 俞灵, 王军. β-环糊精为水相单体的复合纳滤膜制备及染料截留性能[J]. 化工学报, 2020, 71(2): 889-898. |
| [13] | 徐颜军, 徐泽海, 孟琴, 沈冲, 侯蕊, 张国亮. 新型还原氧化石墨烯/氮化碳复合纳滤膜制备及其性能[J]. 化工学报, 2019, 70(9): 3565-3572. |
| [14] | 唐元晖, 扈阳, 燕至琴, 李春玉. 高浓度含盐草甘膦溶液的纳滤分离实验研究[J]. 化工学报, 2019, 70(7): 2574-2583. |
| [15] | 许中煌, 雷萍萍, 洪昱斌, 丁马太, 何旭敏, 蓝伟光. 氧化石墨烯/碱式硫酸铝掺杂聚醚砜/聚酰胺复合纳滤膜的制备及其性能[J]. 化工学报, 2018, 69(9): 4066-4074. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号