化工学报 ›› 2024, Vol. 75 ›› Issue (5): 1890-1902.DOI: 10.11949/0438-1157.20240155
收稿日期:2024-02-01
修回日期:2024-03-29
出版日期:2024-05-25
发布日期:2024-06-25
通讯作者:
安霞
作者简介:徐欣欣(2000—),女,硕士研究生,2408795631@qq.com
基金资助:
Xinxin XU( ), Yunli JI, Xianfeng WU, Xia AN(
), Yunli JI, Xianfeng WU, Xia AN( ), Xu WU
), Xu WU
Received:2024-02-01
Revised:2024-03-29
Online:2024-05-25
Published:2024-06-25
Contact:
Xia AN
摘要:
NH3-SCR催化剂同时去除NO x 和挥发性有机物(VOCs)引起了人们的广泛关注,然而,VOCs的存在会对脱硝反应产生负面影响,尤其在低温条件下。本研究选定水滑石衍生复合氧化物(Cu)MgFe-LDO催化剂探索协同脱除NO x 和甲醇性能,着重考察Cu的引入以及CuO x 和FeO x 相互作用对协同反应的影响,并对所制备的催化剂进行表征测试。结果表明,含Cu催化剂的脱硝活性均高于MgFe-LDO催化剂,最佳催化剂Cu0.5MgFe-LDO在230~300℃温窗内具有较好的脱硝活性和甲醇氧化性能。适量引入Cu加强了Cu、Fe物种间的相互作用,有利于氧化还原循环,从而产生更多的氧缺陷及活性氧,过量Cu掺杂会破坏催化剂结构,降低表面酸性,引入Cu可以减缓甲醇对SCR反应的抑制作用。这些结果可为实际应用SCR催化剂协同去除VOCs提供指导。
中图分类号:
徐欣欣, 冀芸丽, 武鲜凤, 安霞, 吴旭. 水滑石衍生CuMgFe-LDO催化剂协同净化氮氧化物和甲醇[J]. 化工学报, 2024, 75(5): 1890-1902.
Xinxin XU, Yunli JI, Xianfeng WU, Xia AN, Xu WU. Hydrotalcite-derived CuMgFe-LDO catalyst for simultaneous abatement of nitrogen oxides and methanol[J]. CIESC Journal, 2024, 75(5): 1890-1902.
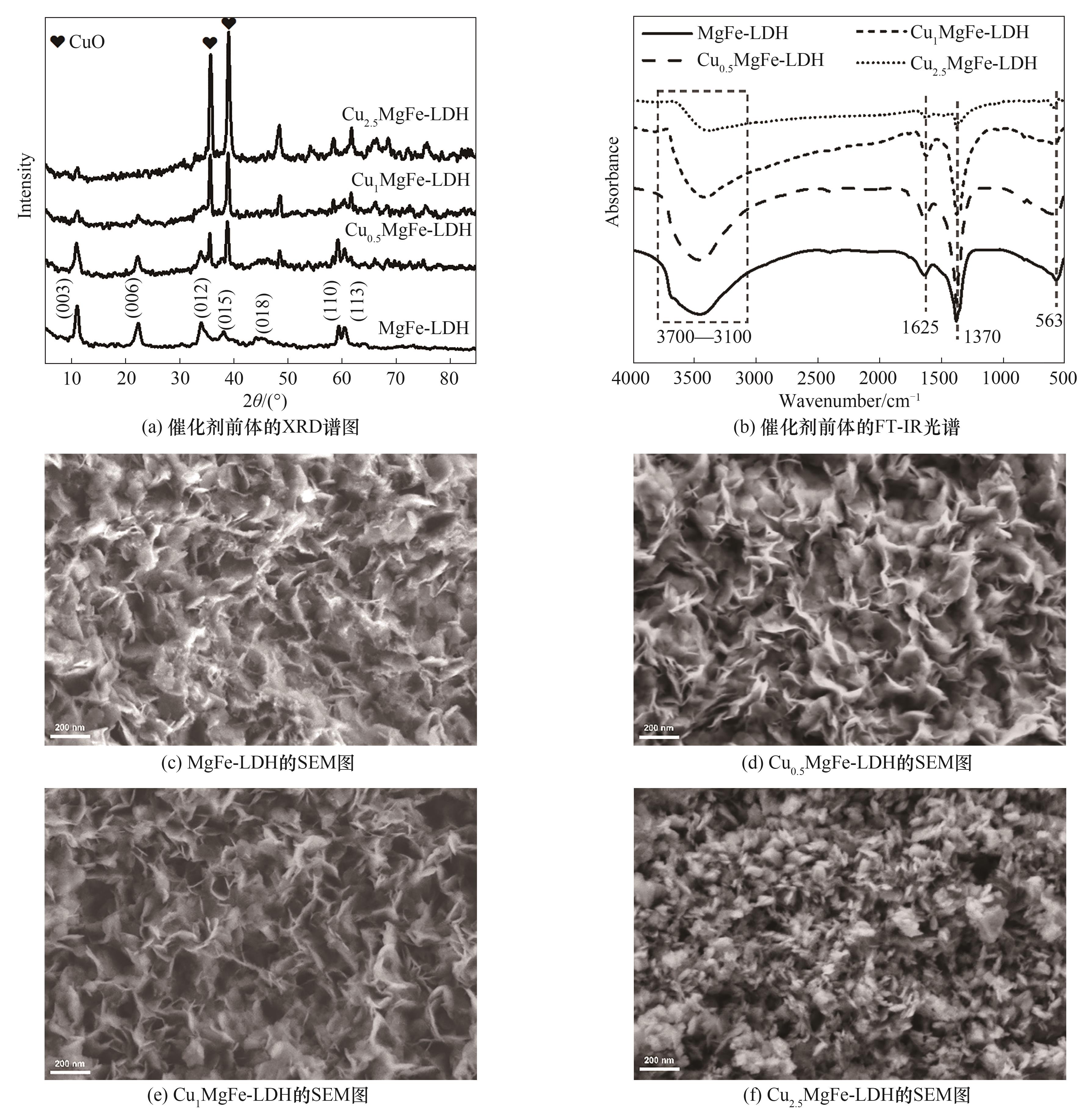
图1 催化剂前体的XRD谱图、FT-IR光谱以及MgFe-LDH、Cu0.5MgFe-LDH、Cu1MgFe-LDH、Cu2.5MgFe-LDH的扫描电镜图
Fig.1 XRD patterns and FT-IR spectra of series precursors, and SEM images of MgFe-LDH, Cu0.5MgFe-LDH, Cu1MgFe-LDH, Cu2.5MgFe-LDH

图2 不同催化剂的NO x 转化率、甲醇转化率、N2选择性、CO2选择性[反应条件:500 mg/L NO x,500 mg/L NH3,750 mg/L甲醇,5% O2和N2为平衡气,GHSV=30000 ml/(g·h)]
Fig.2 NO x conversion, methanol conversion,N2 selectivity,CO2 selectivity over different catalysts [reaction conditions: 500 mg/L NO x, 500 mg/L NH3, 750 mg/L methanol, 5% O2 and N2 as balance, GHSV=30000 ml/(g·h)]
| 催化剂 | 比表面积/ (m2/g) | 孔径/nm | 孔容/(cm3/g) |
|---|---|---|---|
| MgFe-LDO | 62.6 | 8.8 | 0.2 |
| Cu0.5MgFe-LDO | 38.1 | 16.4 | 0.2 |
| Cu1MgFe-LDO | 31.8 | 11.8 | 0.1 |
| Cu2.5MgFe-LDO | 19.7 | 22.6 | 0.1 |
表1 催化剂的比表面积、孔径和孔体积
Table 1 The specific surface area, pore diameter and pore volume of catalysts
| 催化剂 | 比表面积/ (m2/g) | 孔径/nm | 孔容/(cm3/g) |
|---|---|---|---|
| MgFe-LDO | 62.6 | 8.8 | 0.2 |
| Cu0.5MgFe-LDO | 38.1 | 16.4 | 0.2 |
| Cu1MgFe-LDO | 31.8 | 11.8 | 0.1 |
| Cu2.5MgFe-LDO | 19.7 | 22.6 | 0.1 |
| 催化剂 | NH3相对 吸附量 | NH3脱附比例/% | |
|---|---|---|---|
| 弱酸 | 强酸 | ||
| MgFe-LDO | 1.0 | 100 | - |
| Cu0.5MgFe-LDO | 0.7 | 30 | 70 |
| Cu1MgFe-LDO | 0.6 | 34 | 66 |
| Cu2.5MgFe-LDO | 0.2 | 96 | 4 |
表2 催化剂的氨脱附结果
Table 2 NH3 desorption results of catalysts
| 催化剂 | NH3相对 吸附量 | NH3脱附比例/% | |
|---|---|---|---|
| 弱酸 | 强酸 | ||
| MgFe-LDO | 1.0 | 100 | - |
| Cu0.5MgFe-LDO | 0.7 | 30 | 70 |
| Cu1MgFe-LDO | 0.6 | 34 | 66 |
| Cu2.5MgFe-LDO | 0.2 | 96 | 4 |
| 催化剂 | 表面元素物种/% | 总氧物种脱附量 | ||
|---|---|---|---|---|
| Oɑ | Oβ | Oγ | ||
| Mg3Fe1-LDO | 28.8 | 1.8 | 69.4 | 1 |
| Cu0.5MgFe-LDO | 37.7 | 60.5 | 1.8 | 0.8 |
| Cu1Mg2Fe1-LDO | 43.1 | 49.5 | 7.4 | 0.6 |
| Cu2.5Mg0.5Fe1-LDO | 32.7 | 67.3 | — | 0.5 |
表3 各催化剂的O2-TPD数据分析
Table 3 Analysis of O2-TPD data for various catalysts
| 催化剂 | 表面元素物种/% | 总氧物种脱附量 | ||
|---|---|---|---|---|
| Oɑ | Oβ | Oγ | ||
| Mg3Fe1-LDO | 28.8 | 1.8 | 69.4 | 1 |
| Cu0.5MgFe-LDO | 37.7 | 60.5 | 1.8 | 0.8 |
| Cu1Mg2Fe1-LDO | 43.1 | 49.5 | 7.4 | 0.6 |
| Cu2.5Mg0.5Fe1-LDO | 32.7 | 67.3 | — | 0.5 |
| 催化剂 | 相对浓度比/% | ||
|---|---|---|---|
| Cu+/(Cu++Cu2+) | Fe3+/(Fe3++Fe2+) | Oβ/(Oα+Oβ) | |
| MgFe-LDO | — | 40 | 58 |
| Cu0.5MgFe-LDO | 51 | 61 | 71 |
| Cu1MgFe-LDO | 56 | 59 | 51 |
| Cu2.5MgFe-LDO | 65 | 58 | 50 |
表4 各催化剂的XPS分析
Table 4 XPS analysis of different catalysts
| 催化剂 | 相对浓度比/% | ||
|---|---|---|---|
| Cu+/(Cu++Cu2+) | Fe3+/(Fe3++Fe2+) | Oβ/(Oα+Oβ) | |
| MgFe-LDO | — | 40 | 58 |
| Cu0.5MgFe-LDO | 51 | 61 | 71 |
| Cu1MgFe-LDO | 56 | 59 | 51 |
| Cu2.5MgFe-LDO | 65 | 58 | 50 |
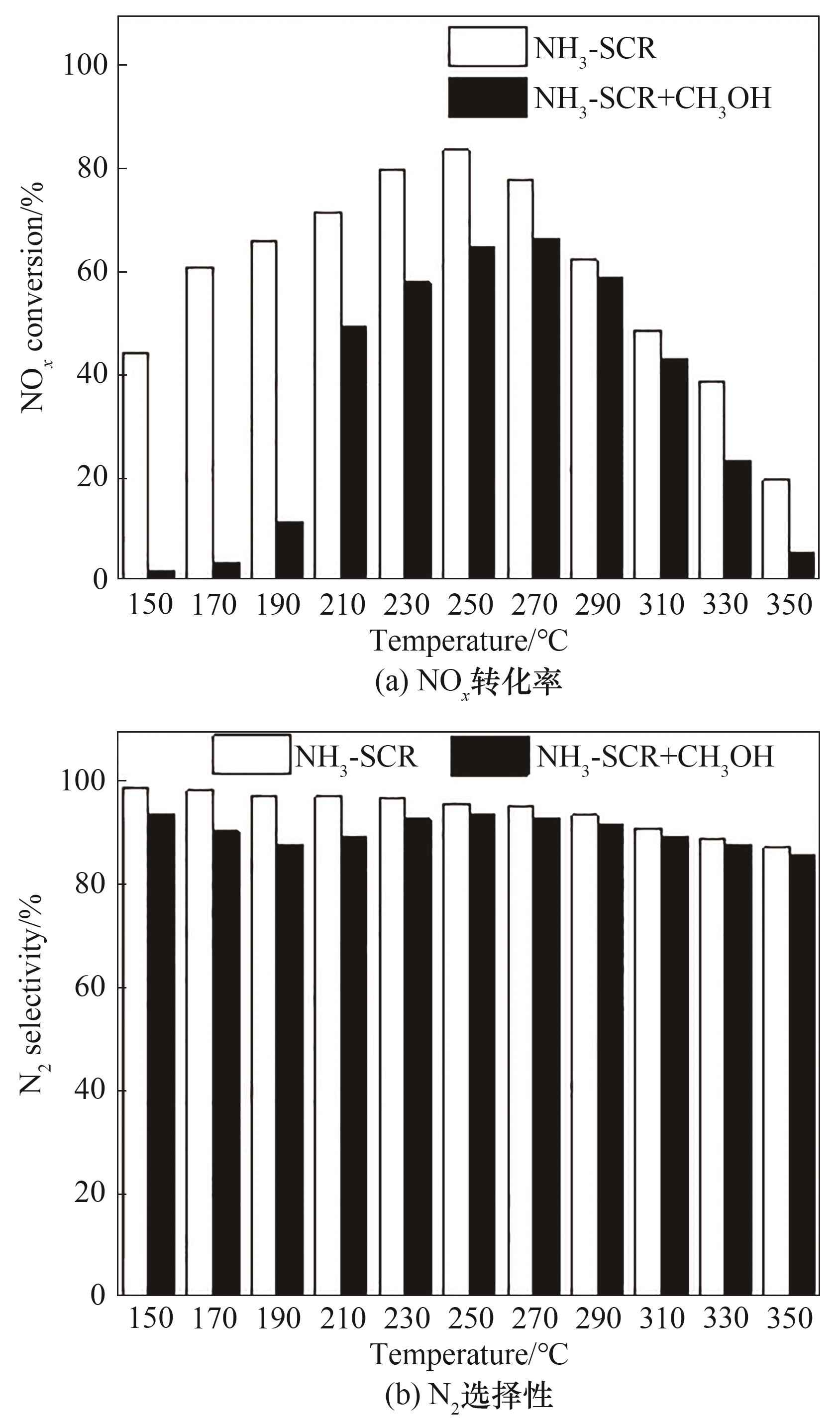
图9 甲醇对NO x 转化率和N2选择性的影响[反应条件:500 mg/L NO x, 500 mg/L NH3, 750 mg/L甲醇,5% O2和N2为平衡气,GHSV=30000ml/(g·h)]
Fig.9 Effects of methanol on NO x conversion and N2 selectivity [reaction condition: 500 mg/L NO, 500 mg/L NH3, 750 mg/L methanol, 5% O2 and N2 as balance, GHSV=30000 ml/(g·h)]
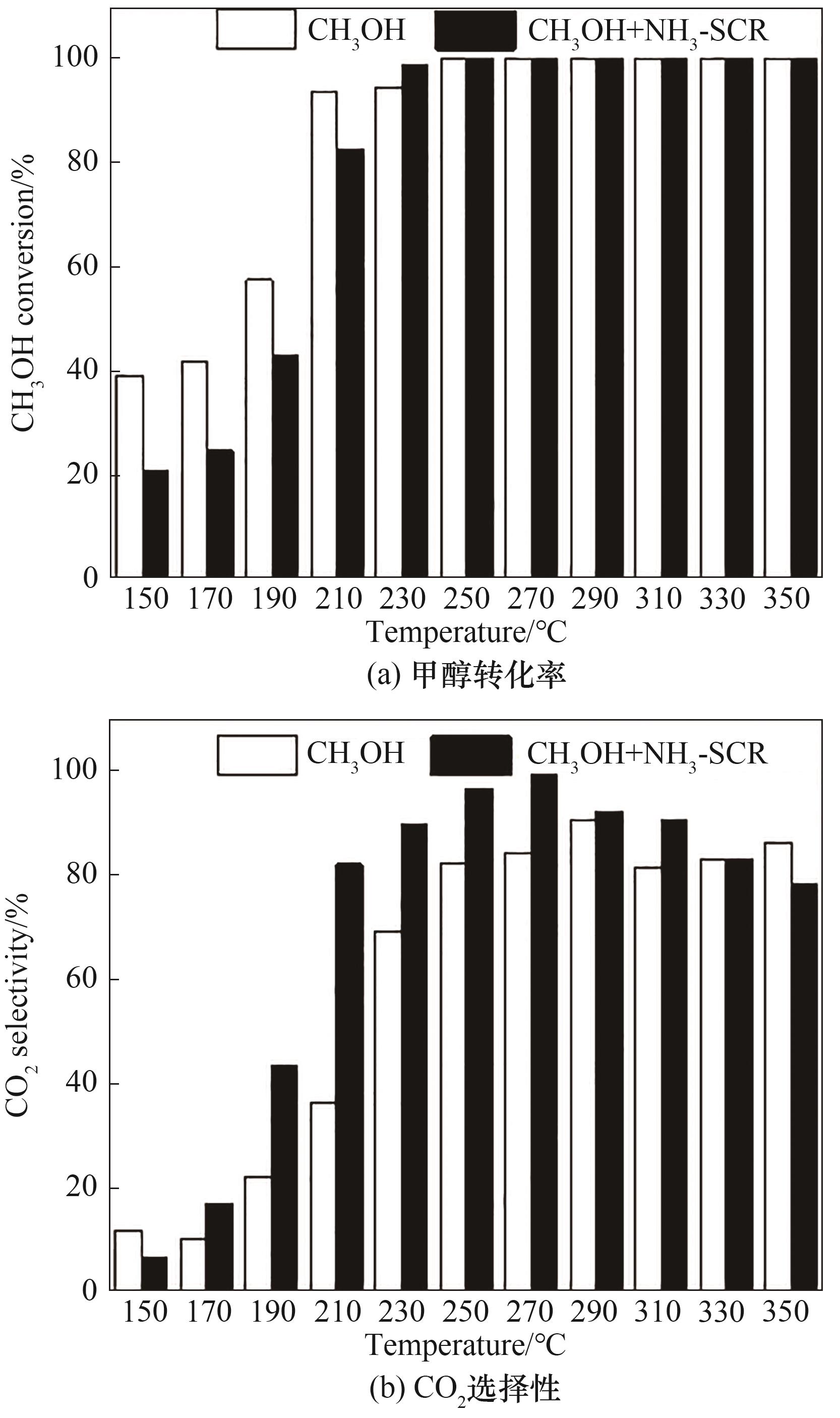
图10 SCR气氛对甲醇转化率、CO2选择性的影响[反应条件:500 mg/L NO x,500 mg/L NH3,750 mg/L甲醇,5% O2和N2为平衡气,GHSV=30000 ml/(g·h)]
Fig.10 Effects of SCR gas components on methanol conversion and CO2 selectivity [reaction condition: 500 mg/L NO, 500 mg/L NH3,750 mg/L methanol, 5% O2 and N2 as balance, GHSV = 30000 ml/(g·h)]
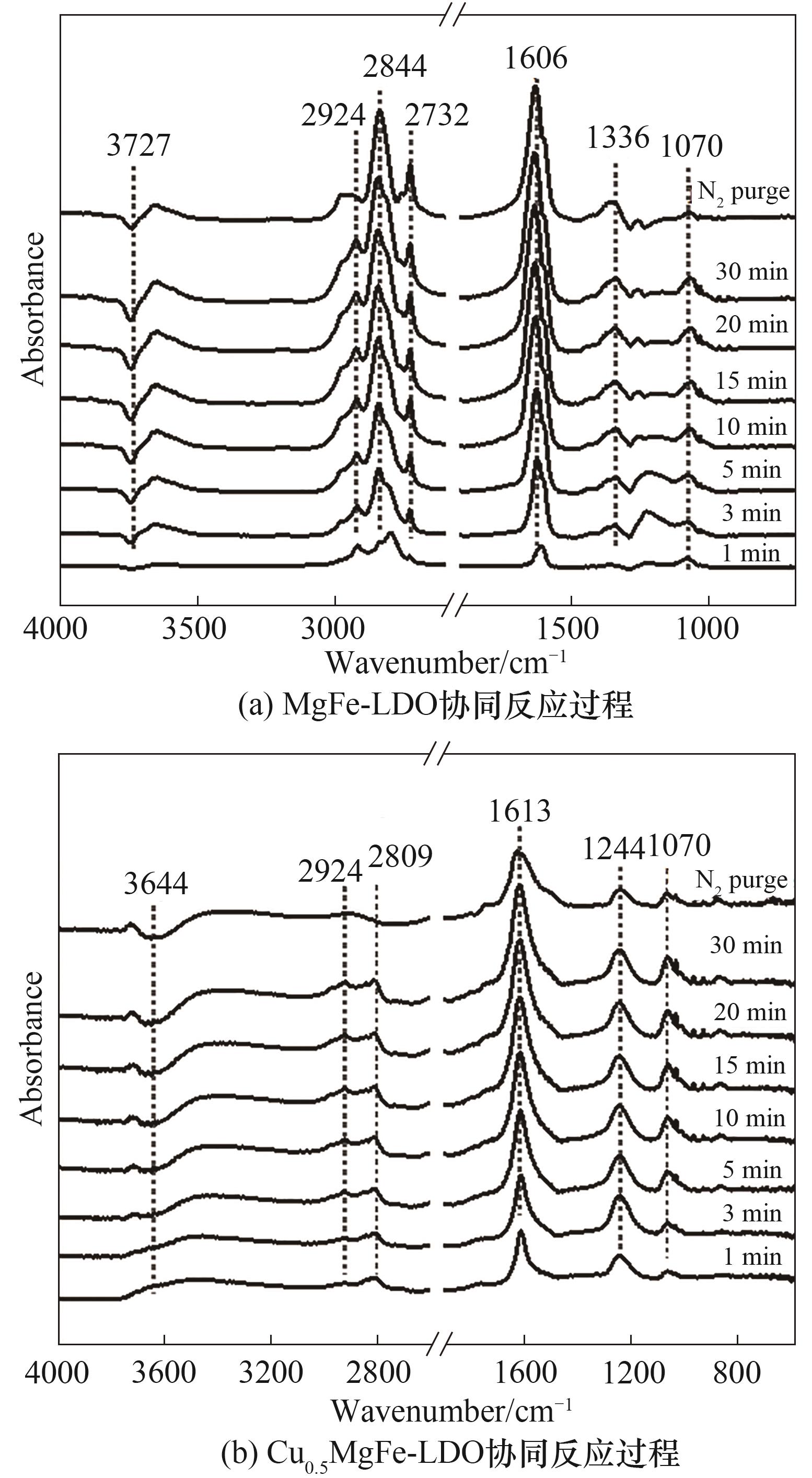
图11 协同反应过程的原位DRIFTs光谱(反应条件: 500 mg/L NO x, 500 mg/L NH3, 750 mg/L甲醇, 5% O2和N2)
Fig.11 In situ DRIFTs spectra of synergistic reaction process (reaction condition: 500 mg/L NO x, 500 mg/L NH3, 750 mg/L methanol, 5% O2 and N2)
| 1 | Ou J M, Yuan Z B, Zheng J Y, et al. Ambient ozone control in a photochemically active region: short-term despiking or long-term attainment?[J]. Environmental Science & Technology, 2016, 50(11): 5720-5728. |
| 2 | Lu C Y, Wey M Y. Simultaneous removal of VOC and NO by activated carbon impregnated with transition metal catalysts in combustion flue gas[J]. Fuel Processing Technology, 2007, 88(6): 557-567. |
| 3 | Zheng F, Liu C J, Ma X J, et al. Review on NH3-SCR for simultaneous abating NO x and VOCs in industrial furnaces: catalysts’ composition, mechanism, deactivation and regeneration[J]. Fuel Processing Technology, 2023, 247: 107773. |
| 4 | Kouraichi R, Delgado J J, López-Castro J D, et al. Deactivation of Pt/MnO x -CeO2 catalysts for the catalytic wet oxidation of phenol: formation of carbonaceous deposits and leaching of manganese[J]. Catalysis Today, 2010, 154(3/4): 195-201. |
| 5 | Long Y P, Su Y T, Xue Y H, et al. V2O5-WO3/TiO2 catalyst for efficient synergistic control of NO x and chlorinated organics: insights into the arsenic effect[J]. Environmental Science & Technology, 2021, 55(13): 9317-9325. |
| 6 | Pan H, Chen Z H, Ma M D, et al. Mutual inhibition mechanism of simultaneous catalytic removal of NO x and toluene on Mn-based catalysts[J]. Journal of Colloid and Interface Science, 2022, 607(Pt 2): 1189-1200. |
| 7 | Jiang W Y, Yu Y L, Bi F, et al. Synergistic elimination of NO x and chloroaromatics on a commercial V2O5-WO3/TiO2 catalyst: byproduct analyses and the SO2 effect[J]. Environmental Science & Technology, 2019, 53(21): 12657-12667. |
| 8 | Wang D, Chen Q Z, Zhang X, et al. Multipollutant control (MPC) of flue gas from stationary sources using SCR technology: a critical review[J]. Environmental Science & Technology, 2021, 55(5): 2743-2766. |
| 9 | Liang Q M, Li J, He H, et al. Effects of SO2 on the low temperature selective catalytic reduction of NO by NH3 over CeO2-V2O5-WO3/TiO2 catalysts[J]. Frontiers of Environmental Science & Engineering, 2017, 11(4): 4. |
| 10 | 高凤雨, 刘恒恒, 姚小龙, 等. 球形表面富锰Mn x Co3- x O4- η 尖晶石型催化剂选择性催化还原NO x 研究[J]. 物理化学学报, 2023, 39(9): 136-148. |
| Gao F Y, Liu H H, Yao X L, et al. Spherical Mn x Co3- x O4- η spinel with Mn-enriched surface as high-efficiency catalysts for low-temperature selective catalytic reduction of NO x by NH3 [J]. Acta Physico-Chimica Sinica, 2023, 39(9): 136-148. | |
| 11 | Li Y B, Han S H, Zhang L P, et al. Manganese-based catalysts for indoor volatile organic compounds degradation with low energy consumption and high efficiency[J]. Transactions of Tianjin University, 2022, 28(1): 53-66. |
| 12 | Shi Y J, Guo X L, Wang Y Y, et al. New insight into the design of highly dispersed Pt supported CeO2-TiO2 catalysts with superior activity for VOCs low-temperature removal[J]. Green Energy & Environment, 2023, 8(6): 1654-1663. |
| 13 | Gallastegi-Villa M, Aranzabal A, Boukha Z, et al. Role of surface vanadium oxide coverage support on titania for the simultaneous removal of o-dichlorobenzene and NO x from waste incinerator flue gas[J]. Catalysis Today, 2015, 254: 2-11. |
| 14 | Wu X, Meng H, Du Y L, et al. Insight into Cu2O/CuO collaboration in the selective catalytic reduction of NO with NH3: enhanced activity and synergistic mechanism[J]. Journal of Catalysis, 2020, 384: 72-87. |
| 15 | 于艳科, 耿梦荞, 魏德胜, 等. 钾对CuSO4/TiO2脱硝催化剂的失活效应[J]. 物理化学学报, 2023, 39(4): 76-84. |
| Yu Y K, Geng M Q, Wei D S, et al. Effect of potassium on the performance of a CuSO4/TiO2 catalyst used in the selective catalytic reduction of NO x by NH3 [J]. Acta Physico-Chimica Sinica, 2023, 39(4): 76-84. | |
| 16 | Ghodselahi T, Vesaghi M A, Shafiekhani A, et al. XPS study of the Cu@Cu2O core-shell nanoparticles[J]. Applied Surface Science, 2008, 255(5): 2730-2734. |
| 17 | Fang D, Qi K, Li F X, et al. Excellent sulfur tolerance performance over Fe-SO4/TiO2 catalysts for NH3-SCR: influence of sulfation and Fe-based sulfates[J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 107038. |
| 18 | Han L P, Gao M, Feng C, et al. Fe2O3-CeO2@Al2O3 nanoarrays on Al-mesh as SO2-tolerant monolith catalysts for NO x reduction by NH3 [J]. Environmental Science & Technology, 2019, 53(10): 5946-5956. |
| 19 | Delimaris D, Ioannides T. VOC oxidation over CuO-CeO2 catalysts prepared by a combustion method[J]. Applied Catalysis B: Environmental, 2009, 89(1/2): 295-302. |
| 20 | Cocuzza C, Sartoretti E, Novara C, et al. Copper-manganese oxide catalysts prepared by solution combustion synthesis for total oxidation of VOCs[J]. Catalysis Today, 2023, 423: 114292. |
| 21 | Zhang J, Qu H X. Low-temperature selective catalytic reduction of NO x with NH3 over Fe-Cu mixed oxide/ZSM-5 catalysts containing Fe2CuO4 phase[J]. Research on Chemical Intermediates, 2015, 41(7): 4961-4975. |
| 22 | Pan D, Ge S S, Tian J Y, et al. Research progress in the field of adsorption and catalytic degradation of sewage by hydrotalcite-derived materials[J]. Chemical Record, 2020, 20(4): 355-369. |
| 23 | Palomares A. Using the “memory effect” of hydrotalcites for improving the catalytic reduction of nitrates in water[J]. Journal of Catalysis, 2004, 221(1): 62-66. |
| 24 | He L M, Cheng H Y, Liang G F, et al. Effect of structure of CuO/ZnO/Al2O3 composites on catalytic performance for hydrogenation of fatty acid ester[J]. Applied Catalysis A: General, 2013, 452: 88-93. |
| 25 | Wu X F, Liu J N, Liu L L, et al. Superior CuMgFe mixed oxide catalysts engineered by tuning the redox cycle for enhancing NO x removal performance[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108824. |
| 26 | Wu X F, Liu J N, Liu X Z, et al. Fabrication of carbon doped Cu-based oxides as superior NH3-SCR catalysts via employing sodium dodecyl sulfonate intercalating CuMgAl-LDH[J]. Journal of Catalysis, 2022, 407(1): 265-280. |
| 27 | 杜冬冬, 刘欢, 马若愚, 等. 基于分子间作用力组装的镁铝水滑石光稳定剂及其性能研究[J]. 化工学报, 2021, 72(6): 3095-3104. |
| Du D D, Liu H, Ma R Y, et al. Performance of MgAl layered double hydroxides light stabilizer assembled via intermolecular forces[J]. CIESC Journal, 2021, 72(6): 3095-3104. | |
| 28 | 张因, 郭健健, 任欢杰, 等. 插层阴离子对以类水滑石为前体Ni-Al2O3催化剂催化乙酰丙酸加氢性能的影响[J]. 化工学报, 2020, 71(8): 3614-3624. |
| Zhang Y, Guo J J, Ren H J, et al. Effect of intercalation anions on catalytic performance of hydrotalcite-like precursor Ni-Al2O3 catalyst for levulinic acid hydrogenation[J]. CIESC Journal, 2020, 71(8): 3614-3624. | |
| 29 | Wu X, Feng Y L, Du Y L, et al. Enhancing DeNO x performance of CoMnAl mixed metal oxides in low-temperature NH3-SCR by optimizing layered double hydroxides (LDHs) precursor template[J]. Applied Surface Science, 2019, 467/468: 802-810. |
| 30 | Wu M, Wang X Y, Dai Q G, et al. Low temperature catalytic combustion of chlorobenzene over Mn-Ce-O/γ-Al2O3 mixed oxides catalyst[J]. Catalysis Today, 2010, 158(3/4): 336-342. |
| 31 | Li J L, Zhang S H, Chen Y, et al. A novel three-dimensional hierarchical CuAl layered double hydroxide with excellent catalytic activity for degradation of methyl orange[J]. RSC Advances, 2017, 7(46): 29051-29057. |
| 32 | Dong C L, Yuan X T, Wang X, et al. Rational design of cobalt-chromium layered double hydroxide as a highly efficient electrocatalyst for water oxidation[J]. Journal of Materials Chemistry A, 2016, 4(29): 11292-11298. |
| 33 | Chen L J, Sun B, Wang X D, et al. 2D ultrathin nanosheets of Co-Al layered double hydroxides prepared in l-asparagine solution: enhanced peroxidase-like activity and colorimetric detection of glucose[J]. Journal of Materials Chemistry. B, 2013, 1(17): 2268-2274. |
| 34 | Huang Y Z, Li F, Zhang X, et al. Cu vacancy engineering on facet dependent CuO to enhance water oxidation efficiency[J]. International Journal of Hydrogen Energy, 2022, 47(15): 9261-9272. |
| 35 | Chen W, Yang S, Liu H, et al. Single-atom Ce-modified α-Fe2O3 for selective catalytic reduction of NO with NH3 [J]. Environmental Science & Technology, 2022, 56(14): 10442-10453. |
| 36 | 陈银飞, 刘华彦. MgFe氧化物催化氧化吸附SO2的研究[J]. 宁夏大学学报(自然科学版), 2001, 22(2): 178-180. |
| Chen Y F, Liu H Y. Study on the MgFe complex oxides for SO2 catalytic oxidative adsorption[J]. Journal of Ningxia University (Natural Science Edition), 2001, 22(2): 178-180. | |
| 37 | Li S D, Wang H S, Li W M, et al. Effect of Cu substitution on promoted benzene oxidation over porous CuCo-based catalysts derived from layered double hydroxide with resistance of water vapor[J]. Applied Catalysis B: Environmental, 2015, 166: 260-269. |
| 38 | Yang L, Wang P C, Yao L, et al. Copper doping promotion on Ce/CAC-CNT catalysts with high sulfur dioxide tolerance for low-temperature NH3-SCR[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(2): 987-997. |
| 39 | Zhang T, Qiu F, Chang H Z, et al. Identification of active sites and reaction mechanism on low-temperature SCR activity over Cu-SSZ-13 catalysts prepared by different methods[J]. Catalysis Science & Technology, 2016, 6(16): 6294-6304. |
| 40 | Wu X, Feng Y L, Liu X Z, et al. Redox & acidity optimizing of LDHs-based CoMnAl mixed oxides for enhancing NH3-SCR performance[J]. Applied Surface Science, 2019, 495: 143513. |
| 41 | Shi Y J, Kong F Z, Wan J, et al. Synergistic effect of ZSM-5 zeolite in Pt-CeO2-TiO2/ZSM-5 catalysts for highly efficient catalytic oxidation of VOCs[J]. Industrial & Engineering Chemistry Research, 2023, 62(8): 3546-3556. |
| 42 | Chmielarz L, Węgrzyn A, Wojciechowska M, et al. Selective catalytic oxidation (SCO) of ammonia to nitrogen over hydrotalcite originated Mg-Cu-Fe mixed metal oxides[J]. Catalysis Letters, 2011, 141(9): 1345-1354. |
| 43 | Yang M, Li S, Deng Y M, et al. Effect of Fe-loading in iron-based catalysts for the CH4 decomposition to H2 and nanocarbons[J]. Journal of Environmental Management, 2023, 346: 118999. |
| 44 | Wang B, Yang G P, Yang Q L, et al. Fabrication of nanohybrid Spinel@CuO catalysts for propane oxidation: modified spinel and enhanced activity by temperature-dependent acid sites[J]. ACS Applied Materials & Interfaces, 2021, 13(23): 27106-27118. |
| 45 | Chen Z, Fan C, Pang L, et al. Direct synthesis of submicron Cu-SAPO-34 as highly efficient and robust catalyst for selective catalytic reduction of NO by NH3 [J]. Applied Surface Science, 2018, 448: 671-680. |
| 46 | Wang Y, Yang D Y, Li S Z, et al. Layered copper manganese oxide for the efficient catalytic CO and VOCs oxidation[J]. Chemical Engineering Journal, 2019, 357: 258-268. |
| 47 | Ben Soltan W, Peng J B, Cao Z G, et al. Bimetallic Fe-Mn loaded H-ZSM-5 zeolites for excellent VOCs catalytic oxidation at low-temperatures: synergistic effects and catalytic mechanisms[J]. Chemical Engineering Journal, 2023, 475: 146251. |
| 48 | Morales J, Espinos J P, Caballero A, et al. XPS study of interface and ligand effects in supported Cu2O and CuO nanometric particles[J]. The Journal of Physical Chemistry. B, 2005, 109(16): 7758-7765. |
| 49 | He Z, Lin H Q, He P, et al. Effect of boric oxide doping on the stability and activity of a Cu-SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol[J]. Journal of Catalysis, 2011, 277(1): 54-63. |
| 50 | Scheffe J R, Francés A, King D M, et al. Atomic layer deposition of iron(Ⅲ) oxide on zirconia nanoparticles in a fluidized bed reactor using ferrocene and oxygen[J]. Thin Solid Films, 2009, 517(6): 1874-1879. |
| 51 | Hu H, Cai S X, Li H R, et al. In situ DRIFTs investigation of the low-temperature reaction mechanism over Mn-doped Co3O4 for the selective catalytic reduction of NO x with NH3 [J]. The Journal of Physical Chemistry C, 2015, 119(40): 22924-22933. |
| 52 | Li Q, Yang H S, Qiu F M, et al. Promotional effects of carbon nanotubes on V2O5/TiO2 for NO x removal[J]. Journal of Hazardous Materials, 2011, 192(2): 915-921. |
| 53 | Zhu Y Y, Zhou F Y, Wang X Q, et al. Reaction behaviors of NO x and methanol simultaneous abatement over a ceria-based NH3-SCR catalyst at low-medium temperatures[J]. The Journal of Physical Chemistry C, 2021, 125(27): 14666-14674. |
| 54 | Bethke K A, Li C, Kung M C, et al. The role of NO2 in the reduction of NO by hydrocarbon over Cu-ZrO2 and Cu-ZSM-5 catalysts[J]. Catalysis Letters, 1995, 31(2): 287-299. |
| 55 | Bertinchamps F, Treinen M, Blangenois N, et al. Positive effect of NO on the performances of VO/TiO-based catalysts in the total oxidation abatement of chlorobenzene[J]. Journal of Catalysis, 2005, 230(2): 493-498. |
| 56 | Jiang Z Y, Jing M Z, Feng X B, et al. Stabilizing platinum atoms on CeO2 oxygen vacancies by metal-support interaction induced interface distortion: mechanism and application[J]. Applied Catalysis B: Environmental, 2020, 278: 119304. |
| 57 | Creci S, Wang X T, Carlsson P A, et al. Methoxy ad-species in MFI zeotypes during methane exposure and methanol desorption followed by in situ IR spectroscopy[J]. Catalysis Today, 2021, 369: 123-128. |
| 58 | Keresszegi C, Ferri D, Mallat T, et al. Unraveling the surface reactions during liquid-phase oxidation of benzyl alcohol on Pd/Al2O3: an in situ ATR-IR study[J]. The Journal of Physical Chemistry B, 2005, 109(2): 958-967. |
| 59 | Collins S E, Briand L E, Gambaro L A, et al. Adsorption and decomposition of methanol on gallium oxide polymorphs[J]. The Journal of Physical Chemistry C, 2008, 112(38): 14988-15000. |
| 60 | Barreau M, Tarot M L, Duprez D, et al. Remarkable enhancement of the selective catalytic reduction of NO at low temperature by collaborative effect of ethanol and NH3 over silver supported catalyst[J]. Applied Catalysis B: Environmental, 2018, 220: 19-30. |
| 61 | Nusrat Mafy N, Muhibur Rahman M, Mollah M Y A, et al. Temperature perturbation on hydrogen bonding in aqueous solutions at different amide concentrations[J]. ChemistrySelect, 2016, 1(18): 5789-5800. |
| 62 | Wang X Q, Zhu Y Y, Liu Y, et al. Tailoring the simultaneous abatement of methanol and NO x on Sb-Ce-Zr catalysts via copper modification[J]. Frontiers of Environmental Science & Engineering, 2022, 16(10): 130. |
| [1] | 白天昊, 王晓雯, 杨梦滋, 段新伟, 米杰, 武蒙蒙. 类水滑石衍生锌基氧化物高温煤气脱硫过程中COS释放行为及其抑制研究[J]. 化工学报, 2023, 74(4): 1772-1780. |
| [2] | 周微, 王福烨, 贺宁, 于海斌, 马新宾, 刘家旭. Cu/SSZ-13催化剂脱硝活性中心与催化性能构效关系的研究[J]. 化工学报, 2022, 73(2): 672-680. |
| [3] | 谢玉仙, 刘涛, 苏胜, 刘利军, 钟毓秀, 马智伟, 许凯, 汪一, 胡松, 向军. 工业窑炉烟气氧含量对钒钛系催化剂NH3-SCR脱硝反应的影响[J]. 化工学报, 2022, 73(10): 4410-4418. |
| [4] | 杨润农,余林,赵向云,杨晓波,高梓寒,傅广赢,姜久兴,练纬琳,刘武源,范群. 无模板法合成的Phi分子筛在NO选择性催化还原中的应用[J]. 化工学报, 2020, 71(12): 5578-5588. |
| [5] | 卫彩云, 谭静静, 夏晓丽, 赵永祥. 焙烧温度对CuMgAl催化剂催化糠醇加氢制戊二醇的影响[J]. 化工学报, 2019, 70(4): 1409-1419. |
| [6] | 程爱华, 钱大鹏. 棉花模板Zn/Ti/Fe-LDO吸附水中硝酸盐机制[J]. 化工学报, 2018, 69(12): 5283-5291. |
| [7] | 赵士林, 段钰锋, 丁艳军, 谷小兵, 杜明生, 姚婷, 陈聪, 刘猛, 吕剑虹. 320MW燃煤电厂痕量元素的分布、脱除及排放特性[J]. 化工学报, 2017, 68(7): 2910-2917. |
| [8] | 张帅, 张一科, 呼日勒朝克图, 甄彬, 韩明汉. 甲醇氧化制甲醛铁钼催化剂表面结构与活性[J]. 化工学报, 2016, 67(9): 3678-3683. |
| [9] | 胡斌, 刘勇, 杨春敏, 侯大伟, 袁竹林, 杨林军. 化学团聚促进电除尘脱除烟气中PM2.5和SO3[J]. 化工学报, 2016, 67(9): 3902-3909. |
| [10] | 廖永进, 张亚平, 余岳溪, 李娟, 郭婉秋, 汪小蕾. MnOx/WO3/TiO2低温选择性催化还原NOx机理的原位红外研究[J]. 化工学报, 2016, 67(12): 5031-5039. |
| [11] | 高巍, 于心玉, 赵建忠, 程文萍, 杨建国. 金属离子对MgAlX复合氧化物类FCC硫转移剂性能的影响[J]. 化工学报, 2013, 64(4): 1438-1443. |
| [12] | 王天雷,刘梅堂,马鸿文. 层层自组装技术制备类水滑石基新型薄膜材料的研究进展[J]. 化工进展, 2013, 32(07): 1584-1590. |
| [13] | 陆 敏,刘 媛,李树白,文 艺,刘承先. 类水滑石催化剂催化合成碳酸二丙酯[J]. 化工进展, 2013, 32(05): 1070-1073. |
| [14] | 孙金陆1,2,甄卫军1,2,李 进1,2. LDHs材料的结构、性质及其应用研究进展[J]. 化工进展, 2013, 32(03): 610-616. |
| [15] | 程翔, 黄新瑞, 王兴祖, 孙德智. ZnAlLa类水滑石对污泥脱水液中磷酸根的吸附 [J]. 化工学报, 2010, 61(4): 955-962. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号