化工学报 ›› 2024, Vol. 75 ›› Issue (11): 3987-4004.DOI: 10.11949/0438-1157.20240445
庞茂斌( ), 徐子昂, 甄翊含, 许琴, 林董澄, 刘京, 王保国(
), 徐子昂, 甄翊含, 许琴, 林董澄, 刘京, 王保国( )
)
收稿日期:2024-04-23
修回日期:2024-06-12
出版日期:2024-11-25
发布日期:2024-12-26
通讯作者:
王保国
作者简介:庞茂斌(1999—),男,博士研究生,pmb21@mails.tsinghua.edu.cn
基金资助:
Maobin PANG( ), Zi’ang XU, Yihan ZHEN, Qin XU, Dongcheng LIN, Jing LIU, Baoguo WANG(
), Zi’ang XU, Yihan ZHEN, Qin XU, Dongcheng LIN, Jing LIU, Baoguo WANG( )
)
Received:2024-04-23
Revised:2024-06-12
Online:2024-11-25
Published:2024-12-26
Contact:
Baoguo WANG
摘要:
碱性离子膜(阴离子交换膜)作为电解水制氢、二氧化碳还原等电化学过程的关键材料,在传递离子、分隔阴阳两极和阻隔气体渗透方面具有重要的应用价值。现有碱性离子膜起源于电渗析过程,其离子传导率偏低,无法满足电化学过程对高电流密度、高稳定性的需求。针对电解水制氢过程对高通量、低电阻、低能耗的需求,从膜内离子传递过程出发,结合氢氧根传递特点分析满足综合性能需求的碱性离子膜结构特征,重点阐述膜内强化离子传递策略,归纳分析最新研究成果,分类讨论交联、定向排列、微相分离以及构筑微孔等具体策略,指明高性能碱性离子膜研究路径,促进以电解水制氢为代表的电化学反应器技术发展。
中图分类号:
庞茂斌, 徐子昂, 甄翊含, 许琴, 林董澄, 刘京, 王保国. 碱性离子膜内强化离子传递策略及研究进展[J]. 化工学报, 2024, 75(11): 3987-4004.
Maobin PANG, Zi’ang XU, Yihan ZHEN, Qin XU, Dongcheng LIN, Jing LIU, Baoguo WANG. Recent progress of strategies for enhancing ion transport in anion exchange membranes[J]. CIESC Journal, 2024, 75(11): 3987-4004.
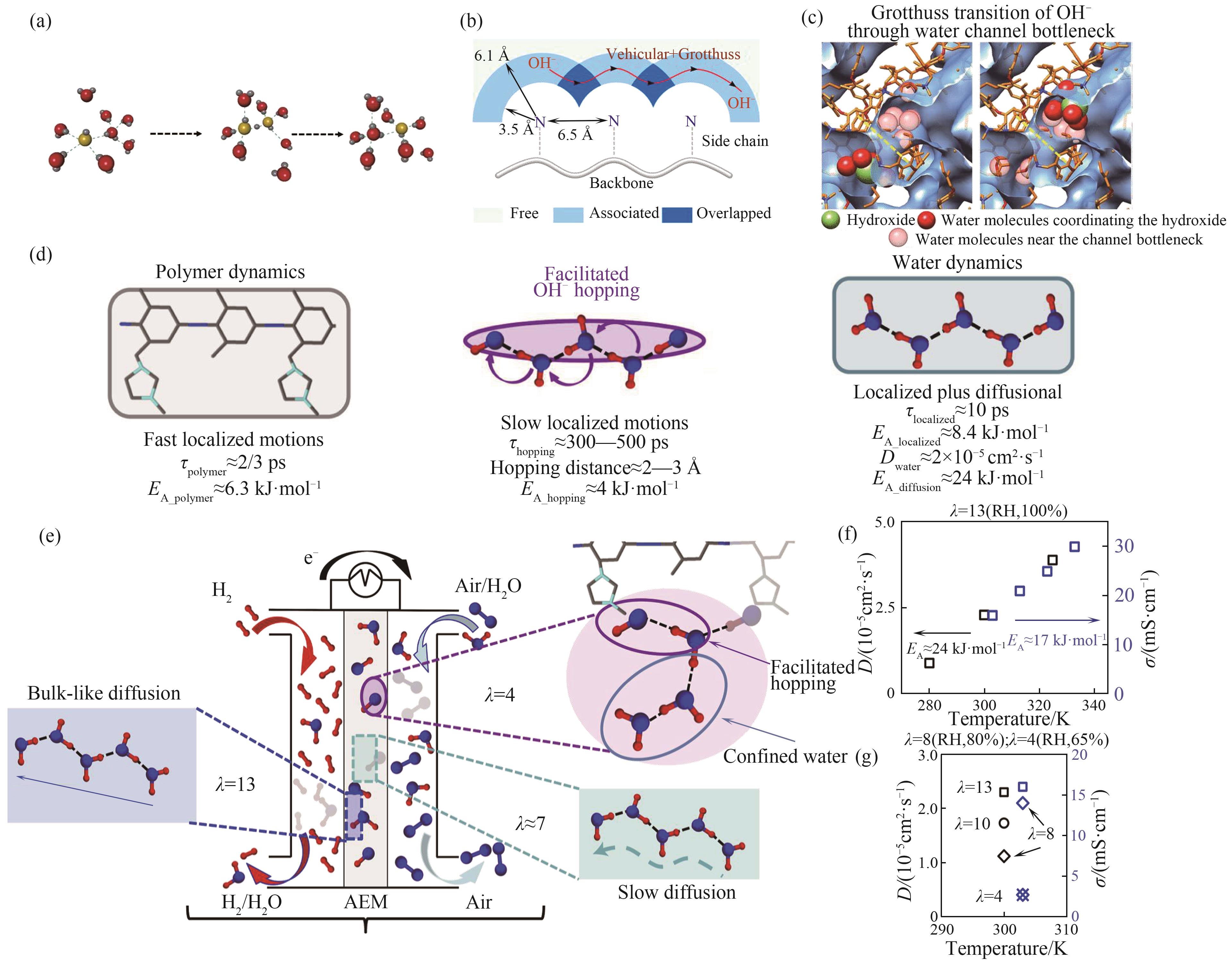
图2 氢氧根传递机制研究进展[32-35](a) 氢氧根Grotthuss机制;(b) AEM内氢氧根传递;(c) 水通道“瓶颈”处氢氧根传递;(d) QENS测试结果;(e)~(g)膜内氢氧根传递行为解耦
Fig.2 Progress of hydroxide ion transfer mechanism[32-35](a) Grotthuss mechanism of hydroxide; (b) Hydroxide transfers in AEM; (c) Hydroxide transfers at “bottleneck” of water channel; (d) QENS test results; (e)—(g) Decoupling of hydroxyl transfer behavior in membranes
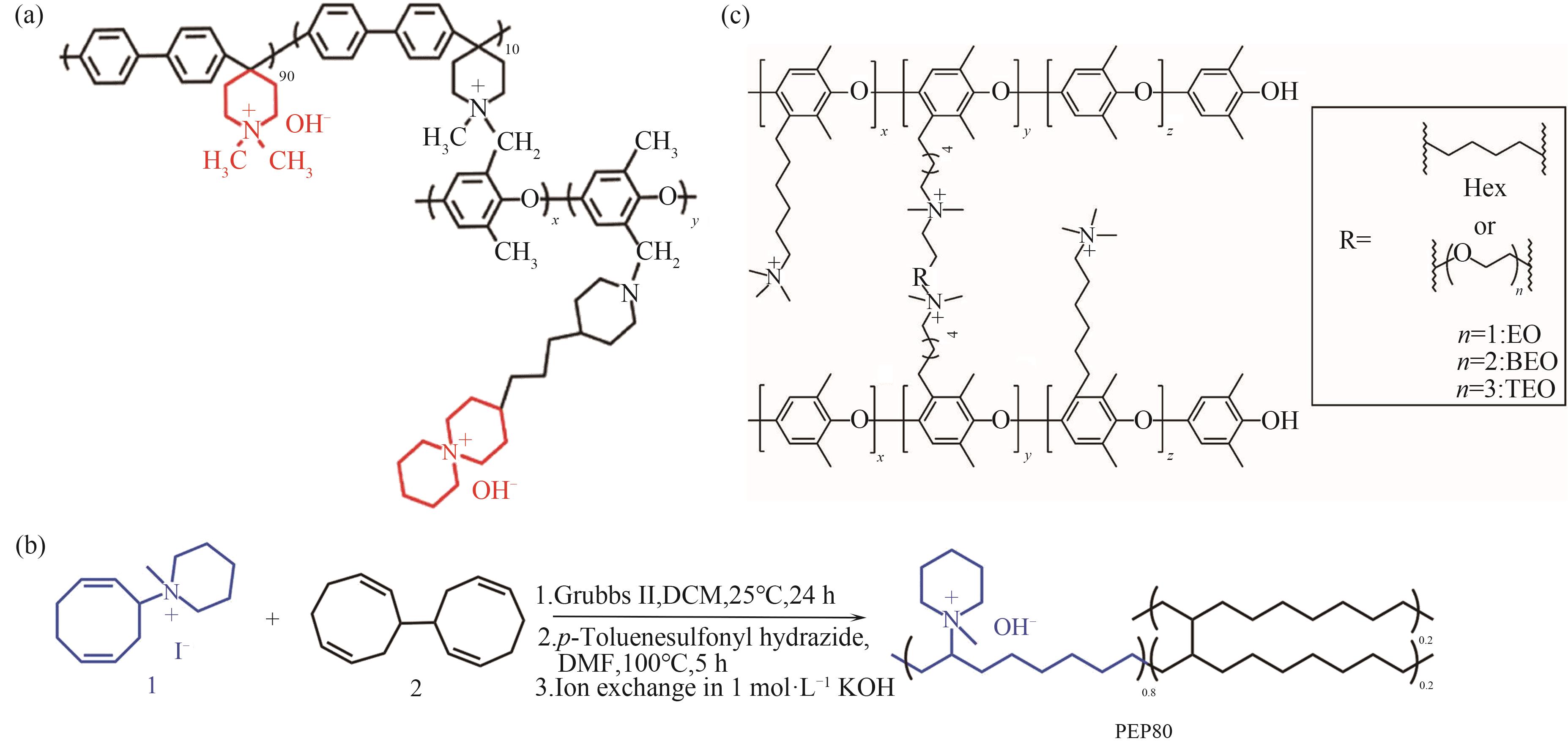
图4 交联策略常见结构示意图[45,54-55](a) PBP-ASU-PPO结构; (b) PEP80结构及合成路径; (c) PPO-EO/BEO/TEO结构
Fig.4 Schematic illustration of common structures of cross-linking strategies[45,54-55](a) PBP-ASU-PPO structure; (b) PEP80 structure and synthesis pathway; (c) PO-EO/BEO/TEO structure
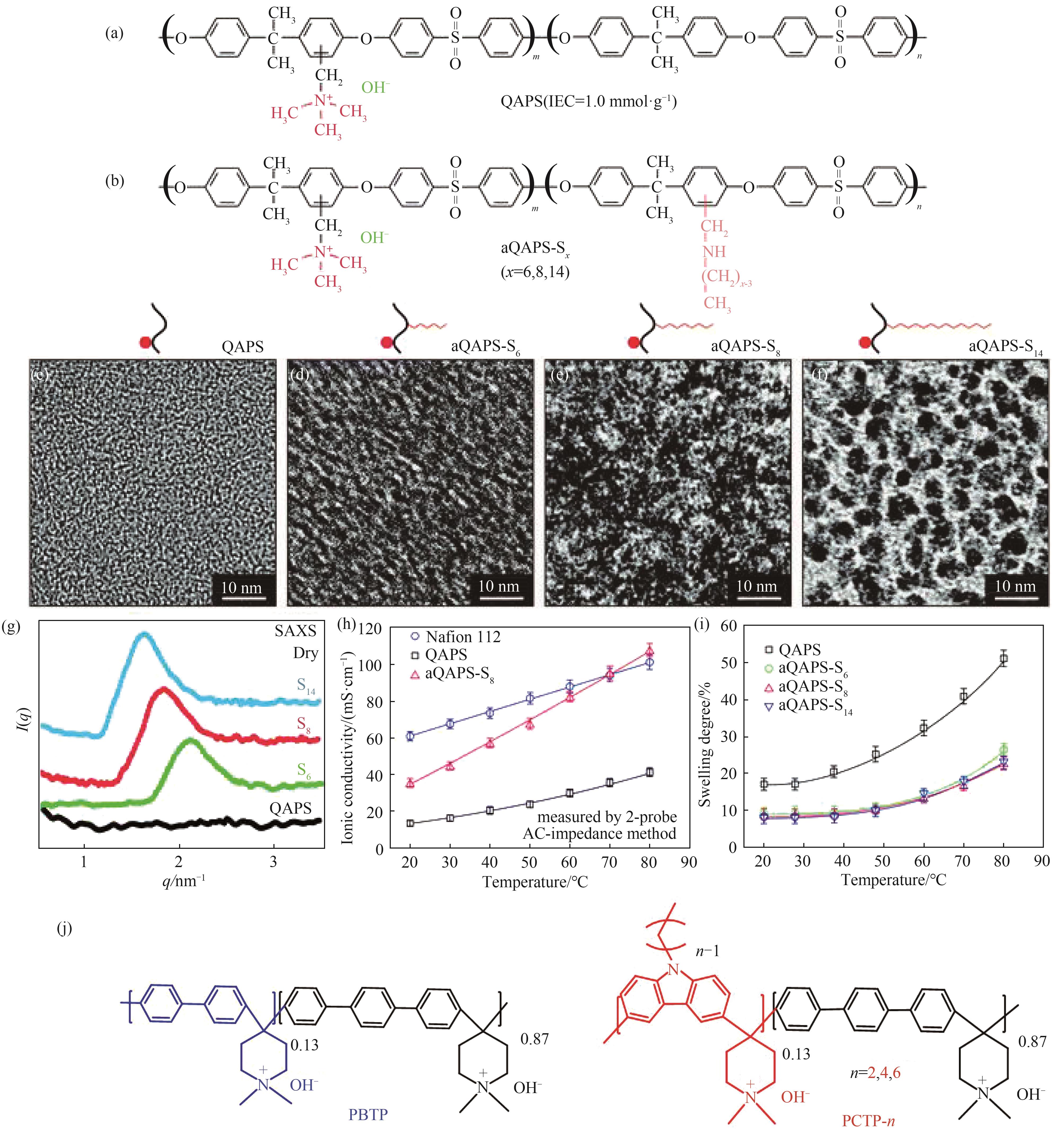
图5 QAPS结构微相分离策略示意图[59-60](a),(b) aQAPS-S x 结构; (c)~(f) TEM图像;(g)SAXS图像;(h) 离子传导率与温度关系;(i) 溶胀率与温度关系;(j) PBTP及PCTP-n结构
Fig.5 Schematic illustration of microphase separation for QAPS structure[59-60](a),(b) Structure of aQAPS-S x; (c)—(f) TEM images; (g) SAXS image; (h) Relation between ionic conductivity and temperature; (i) Relation between swelling degree and temperature; (j) Structure of PBTP and PCTP-n
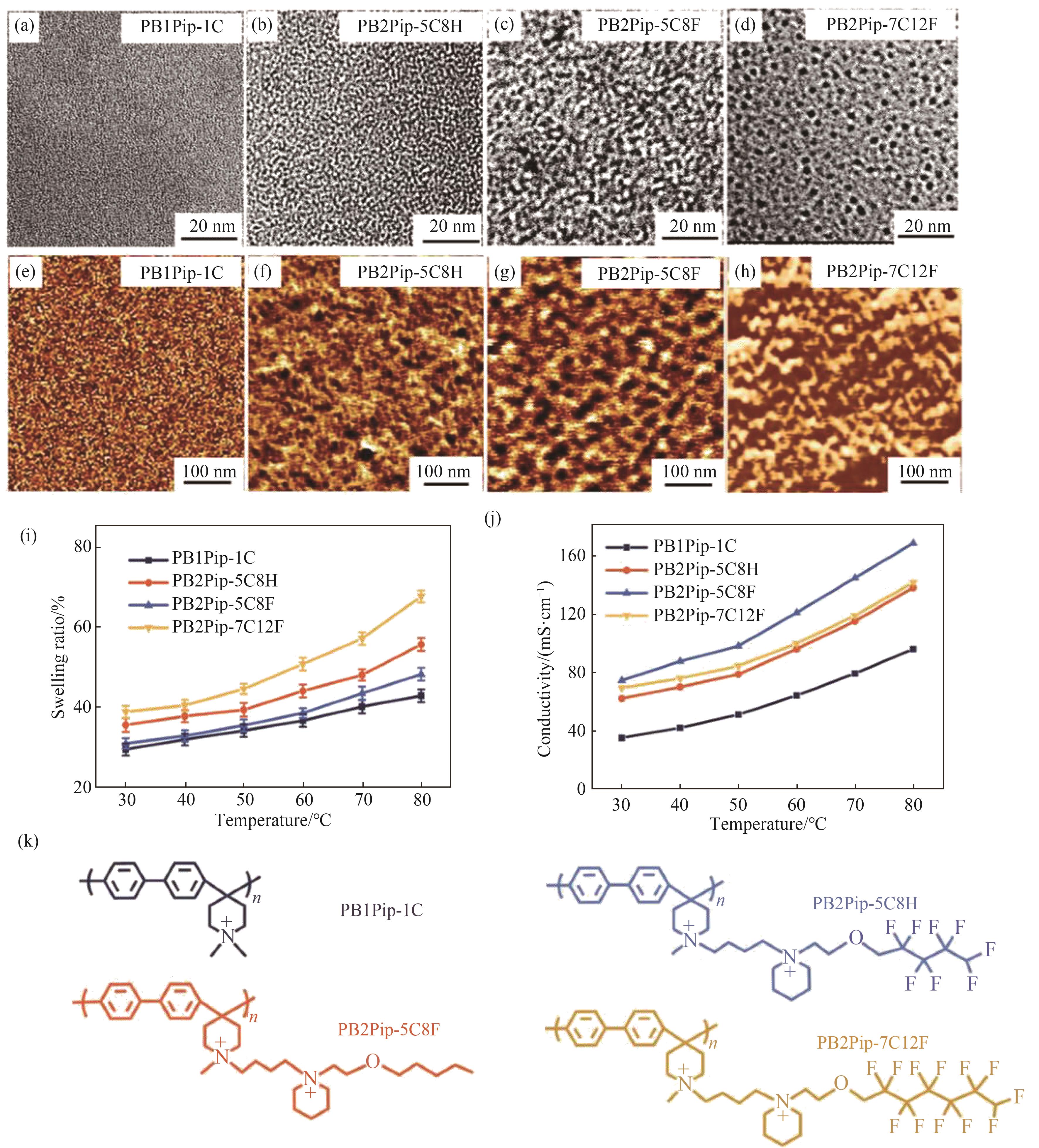
图6 PBnPip结构微相分离策略示意图[46](a)~(d) TEM图像;(e)~(h) AFM图像;(i) 溶胀率与温度的关系;(j) 离子传导率与温度的关系;(k) 分子结构
Fig.6 Schematic illustration of microphase separation for PBnPip structure[46](a)—(d) TEM images; (e)—(h) AFM image; (i) Relationship between swelling rate and temperature; (j) Relation between conductivity and temperature; (k) Molecular structure

图7 多重交联结构PXm-Tn用于高效传递氢氧根[62](a) 分子结构及合成路径; (b) 有序/无序通道下氢氧根传递能垒对比
Fig.7 Multiple cross-linked PXm-Tn for OH- transportation highway[62](a) Molecular structure and synthetic pathways; (b) Hydroxyl transfer barrier comparison in ordered/disordered channels
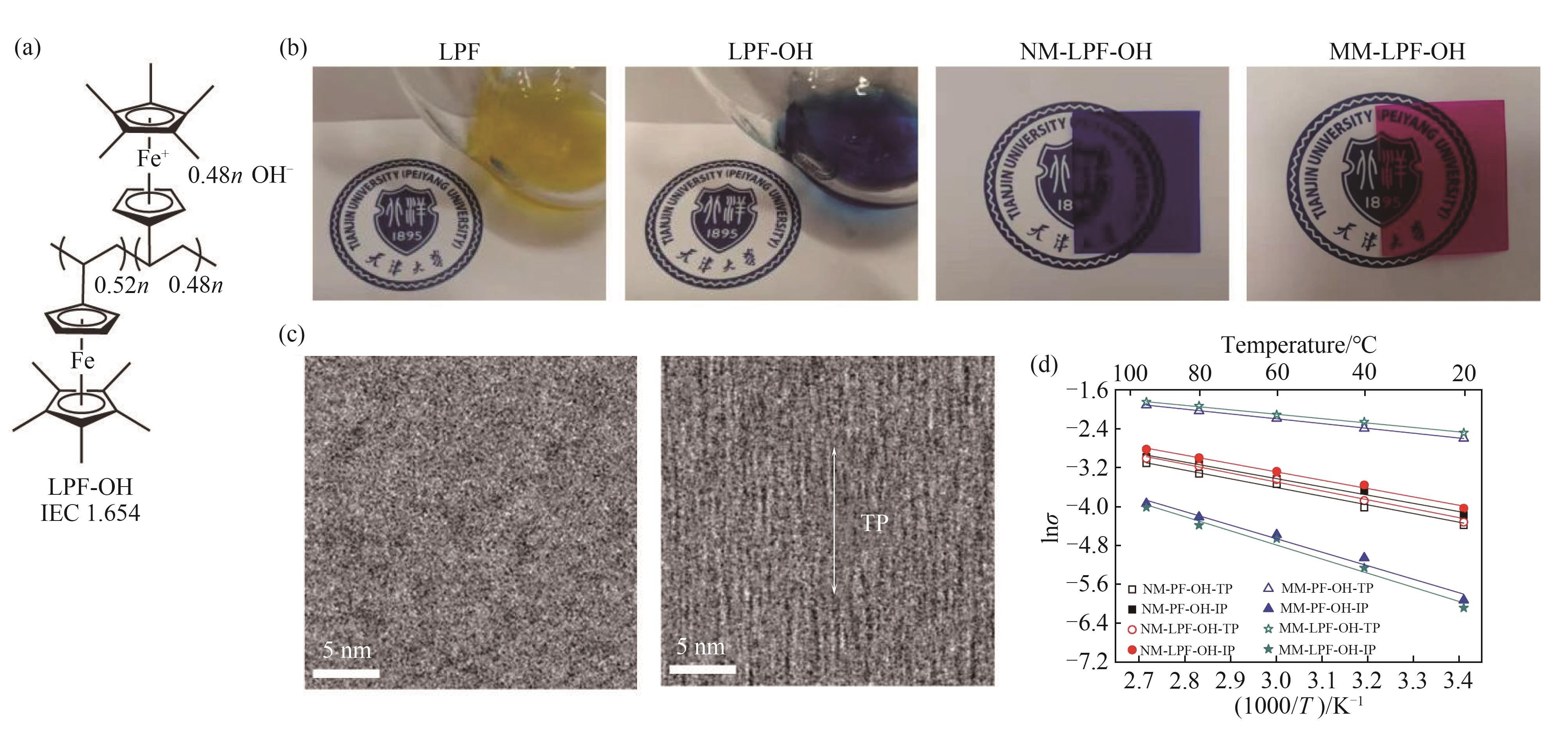
图8 磁场定向构筑稳定二茂铁基离子交换膜[63](a) 分子结构;(b) 筑膜液及膜材料图像;(c) 磁场对膜材料TEM图像的影响;(d) 筑膜条件对膜材料离子传导率的影响
Fig.8 Magnetic-field-oriented mixed-valence-stabilized ferrocenium AEM[63](a) Molecular structure; (b) Images of film building fluid and membranes; (c) Influence of magnetic field on TEM images;(d) Influence of different conditions on conductivity of membranes
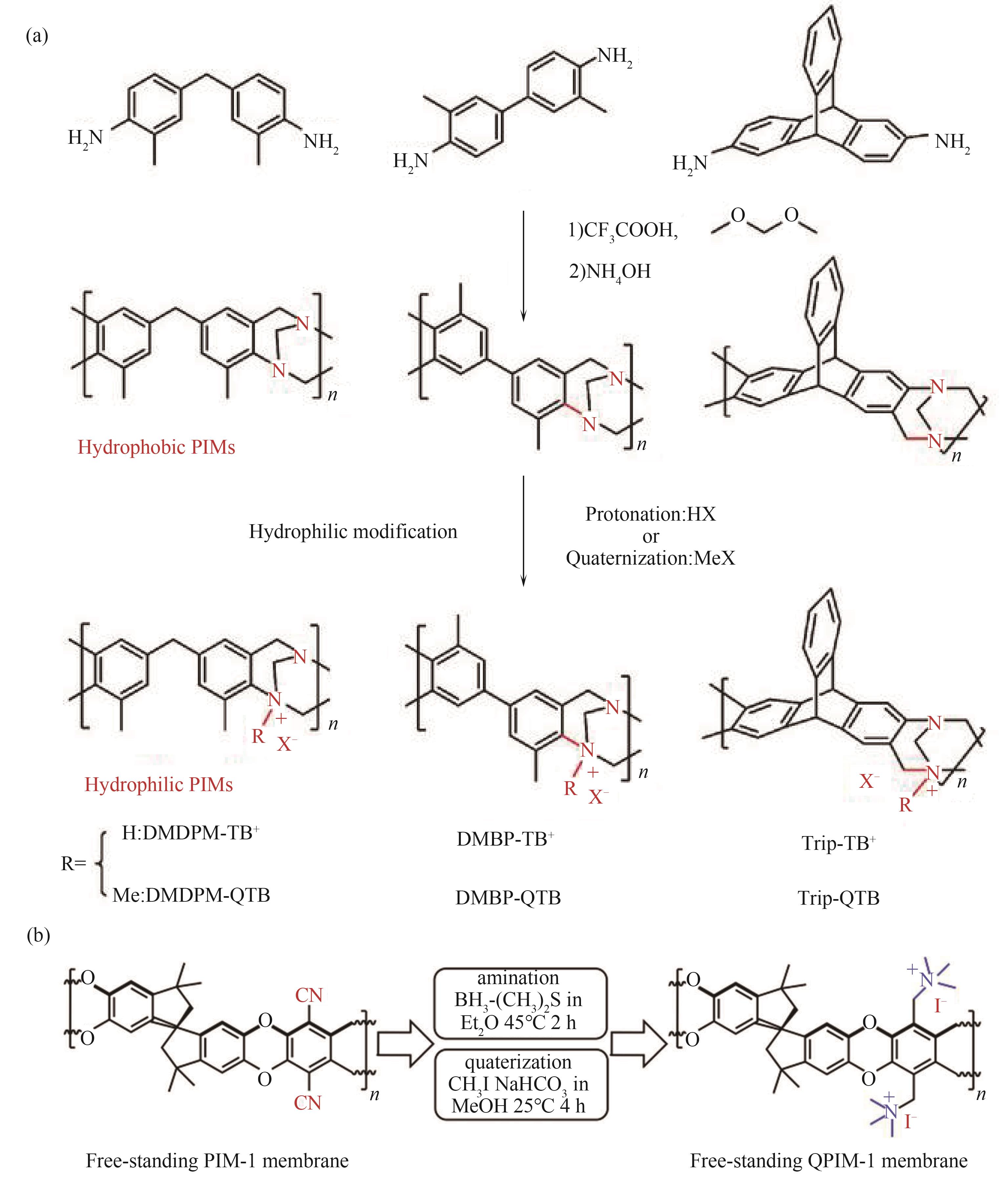
图9 PIM类碱性离子膜合成路径[68,71](a) Tröger’s base类AEM合成路线;(b) 基于PIM-1结构的AEM改性路线
Fig.9 Synthetic route of AEM from positively charged PIMs[68,71](a) Tröger’s base AEM synthesis route; (b) AEM modification route based on PIM-1 structure
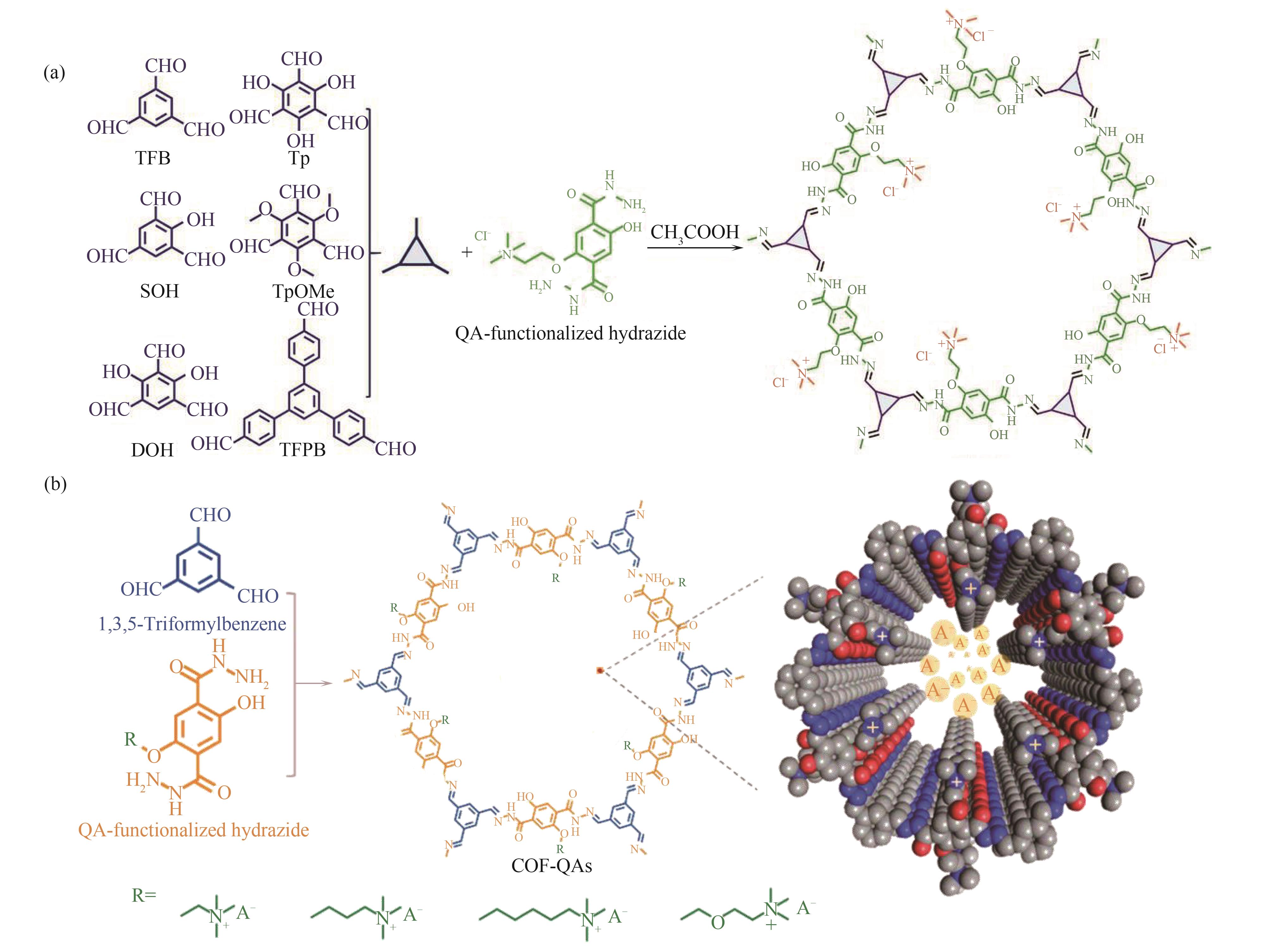
图10 COFs材料合成路径[78-79](a) 醛单体调节COF-QAs结构;(b) 侧链长度调节COF-QAs结构
Fig.10 Synthetic route of COFs[78-79](a) Structures of COF-QAs regulated by aldehyde monomers; (b) COF-QAs structure regulated by length side chains
| 强化策略 | AEMs名称 | IEC/(mmol·g-1) | 水含量(80℃)/% | 溶胀率(80℃)/% | OH型离子传导率(80℃)/(mS·cm-1) | 机械强度/ MPa | 文献 |
|---|---|---|---|---|---|---|---|
| 高IEC情况交联抑制溶胀 | 10% PBP-ASU-PPO | 2.61 | 140 | 25 | 128 | 32 | [ |
| PEP80-20PS | 4.05 | 256 | 50.2 | 354.3 | 1.1 | [ | |
| x-BEO-PPO | 2.23 | 101.8 | 18.18 | 132 | 27 | [ | |
| 微相分离构建局部传递通道 | PCTP-2 | 2.83 | 54.2 | 19.8 | 135 | 42 | [ |
| aQAPS-S8 | 1 | 21 | 105 | 12.1 | [ | ||
| PB2Pip-5C8F | 2.53 | 101.4(30℃) | 48.3 | 168.5 | 37 | [ | |
| 定向排列提升通道利用率 | PX75-T50 | 0.91 | 7.9 | 2.6 | 111.6 | 172 | [ |
| MM-LPF-OH | 1.654 | 37 | 7(TP) 28(IP) | 160(95℃,TP) 18(95℃,IP) | 22 | [ | |
| 微孔构筑刚性传递通道 | BPPO-MPC60 | 0.62 | 3.65 | <2 | 182 | [ | |
| DMBP-QTB | 0.82 | 36(30℃) | <5 | 164.4 | [ | ||
| COF-QA-2 | 2.38 | 81 | 20 | 212 | 50 | [ | |
| COF-SDQA | 2.70 | 77.7 | 11.71 | 329.4 | 27 | [ | |
| 其他策略 | Chitosan-Cu | 1.6 | 56 | 67(室温) | 112 | [ | |
| AAEM3d-e·2OH- | 0.68 | 18.4(60℃) | 7.5(60℃) | 189 | [ |
表1 强化离子传递策略的代表AEMs
Table 1 Representative AEMs of reinforcement strategies for enhancing ion transport
| 强化策略 | AEMs名称 | IEC/(mmol·g-1) | 水含量(80℃)/% | 溶胀率(80℃)/% | OH型离子传导率(80℃)/(mS·cm-1) | 机械强度/ MPa | 文献 |
|---|---|---|---|---|---|---|---|
| 高IEC情况交联抑制溶胀 | 10% PBP-ASU-PPO | 2.61 | 140 | 25 | 128 | 32 | [ |
| PEP80-20PS | 4.05 | 256 | 50.2 | 354.3 | 1.1 | [ | |
| x-BEO-PPO | 2.23 | 101.8 | 18.18 | 132 | 27 | [ | |
| 微相分离构建局部传递通道 | PCTP-2 | 2.83 | 54.2 | 19.8 | 135 | 42 | [ |
| aQAPS-S8 | 1 | 21 | 105 | 12.1 | [ | ||
| PB2Pip-5C8F | 2.53 | 101.4(30℃) | 48.3 | 168.5 | 37 | [ | |
| 定向排列提升通道利用率 | PX75-T50 | 0.91 | 7.9 | 2.6 | 111.6 | 172 | [ |
| MM-LPF-OH | 1.654 | 37 | 7(TP) 28(IP) | 160(95℃,TP) 18(95℃,IP) | 22 | [ | |
| 微孔构筑刚性传递通道 | BPPO-MPC60 | 0.62 | 3.65 | <2 | 182 | [ | |
| DMBP-QTB | 0.82 | 36(30℃) | <5 | 164.4 | [ | ||
| COF-QA-2 | 2.38 | 81 | 20 | 212 | 50 | [ | |
| COF-SDQA | 2.70 | 77.7 | 11.71 | 329.4 | 27 | [ | |
| 其他策略 | Chitosan-Cu | 1.6 | 56 | 67(室温) | 112 | [ | |
| AAEM3d-e·2OH- | 0.68 | 18.4(60℃) | 7.5(60℃) | 189 | [ |
| 强化策略 | 优点 | 缺点 |
|---|---|---|
| 交联 | 提升IEC及抑制溶胀率 | 交联程度有限(<5%) IEC利用程度下降 成本提升(工序增加) |
| 微相分离 | 局部传递通道阻力降低 | 微相分离程度难以控制 材料均一性问题 亲水区域局部溶胀较高 |
| 定向排列 | 传递通道利用效率提升 | 适用范围较小(结构特殊性) 成本提升(工序烦琐) |
| 微孔构筑 | 水环境浓度提升 刚性通道传递阻力下降 | 结构适配性不足(稳定性、功能基团种类) 机械强度(高分子链非紧密堆积) |
| IEC过低限制离子传导率进一步提升 |
表2 强化离子传递策略对比
Table 2 Comparison of reinforcement strategies for enhancing ion transport
| 强化策略 | 优点 | 缺点 |
|---|---|---|
| 交联 | 提升IEC及抑制溶胀率 | 交联程度有限(<5%) IEC利用程度下降 成本提升(工序增加) |
| 微相分离 | 局部传递通道阻力降低 | 微相分离程度难以控制 材料均一性问题 亲水区域局部溶胀较高 |
| 定向排列 | 传递通道利用效率提升 | 适用范围较小(结构特殊性) 成本提升(工序烦琐) |
| 微孔构筑 | 水环境浓度提升 刚性通道传递阻力下降 | 结构适配性不足(稳定性、功能基团种类) 机械强度(高分子链非紧密堆积) |
| IEC过低限制离子传导率进一步提升 |
| 1 | Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future[J]. Nature, 2012, 488(7411): 294-303. |
| 2 | Heide D, von Bremen L, Greiner M, et al. Seasonal optimal mix of wind and solar power in a future, highly renewable Europe[J]. Renewable Energy, 2010, 35(11): 2483-2489. |
| 3 | Li D G, Park E J, Zhu W L, et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers[J]. Nature Energy, 2020, 5: 378-385. |
| 4 | Ulleberg Ø, Nakken T, Eté A. The wind/hydrogen demonstration system at Utsira in Norway: evaluation of system performance using operational data and updated hydrogen energy system modeling tools[J]. International Journal of Hydrogen Energy, 2010, 35(5): 1841-1852. |
| 5 | Bergen A, Pitt L, Rowe A, et al. Transient electrolyser response in a renewable-regenerative energy system[J]. International Journal of Hydrogen Energy, 2009, 34(1): 64-70. |
| 6 | Zeng K, Zhang D K. Recent progress in alkaline water electrolysis for hydrogen production and applications[J]. Progress in Energy and Combustion Science, 2010, 36(3): 307-326. |
| 7 | Carmo M, Fritz D L, Mergel J, et al. A comprehensive review on PEM water electrolysis[J]. International Journal of Hydrogen Energy, 2013, 38(12): 4901-4934. |
| 8 | Diaz L A, Coppola R E, Abuin G C, et al. Alkali-doped polyvinyl alcohol-polybenzimidazole membranes for alkaline water electrolysis[J]. Journal of Membrane Science, 2017, 535: 45-55. |
| 9 | Pavel C C, Cecconi F, Emiliani C, et al. Highly efficient platinum group metal free based membrane-electrode assembly for anion exchange membrane water electrolysis[J]. Angewandte Chemie International Edition, 2014, 53(5): 1378-1381. |
| 10 | Xu T W. Ion exchange membranes: state of their development and perspective[J]. Journal of Membrane Science, 2005, 263(1/2): 1-29. |
| 11 | Xiong P, Zhang L Y, Chen Y Y, et al. A chemistry and microstructure perspective on ion-conducting membranes for redox flow batteries[J]. Angewandte Chemie International Edition, 2021, 60(47): 24770-24798. |
| 12 | Ran J, Wu L, He Y B, et al. Ion exchange membranes: new developments and applications[J]. Journal of Membrane Science, 2017, 522: 267-291. |
| 13 | Nawn G, Vezzù K, Cavinato G, et al. Opening doors to future electrochemical energy devices: the anion-conducting polyketone polyelectrolytes[J]. Advanced Functional Materials, 2018, 28(29): 1706522. |
| 14 | Varcoe J R, Atanassov P, Dekel D R, et al. Anion-exchange membranes in electrochemical energy systems[J]. Energy & Environmental Science, 2014, 7(10): 3135-3191. |
| 15 | Ran J, Wu L, Ru Y F, et al. Anion exchange membranes (AEMs) based on poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) and its derivatives[J]. Polymer Chemistry, 2015, 6(32): 5809-5826. |
| 16 | 尹卓毓, 吴洪, 姜忠义. 阴离子交换膜离子传导率与耐碱稳定性研究进展[J].膜科学与技术, 2023, 43(6): 112-127. |
| Yin Z Y, Wu H, Jiang Z Y. Progress in the research of ionic conductivity and alkaline stability of anion exchange membranes[J].Membrane Science and Technology, 2023, 43(6): 112-127. | |
| 17 | Ziv N, Dekel D R. A practical method for measuring the true hydroxide conductivity of anion exchange membranes[J]. Electrochemistry Communications, 2018, 88: 109-113. |
| 18 | 葛倩倩, 葛亮, 汪耀明, 等. 离子交换膜的发展态势与应用展望[J].化工进展, 2016, 35(6): 1774-1785. |
| Ge Q Q, Ge L, Wang Y M, et al. Perspective in ion exchange membranes[J].Chemical Industry and Engineering Progress, 2016, 35(6): 1774-1785. | |
| 19 | Abbasi R, Setzler B P, Lin S S, et al. A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers[J]. Advanced Materials, 2019, 31(31): 1805876. |
| 20 | Vincent I, Bessarabov D. Low cost hydrogen production by anion exchange membrane electrolysis: a review[J]. Renewable and Sustainable Energy Reviews, 2018, 81: 1690-1704. |
| 21 | 王培灿, 万磊, 徐子昂, 等. 碱性膜电解水制氢技术现状与展望[J].化工学报, 2021, 72(12): 6161-6175. |
| Wang P C, Wan L, Xu Z A, et al. Hydrogen production based-on anion exchange membrane water electrolysis: a critical review and perspective[J].CIESC Journal, 2021, 72(12): 6161-6175. | |
| 22 | Xu Z A, Wan L, Liao Y W, et al. Anisotropic anion exchange membranes with extremely high water uptake for water electrolysis and fuel cells[J]. Journal of Materials Chemistry A, 2021, 9(41): 23485-23496. |
| 23 | Wan L, Xu Z A, Xu Q, et al. Key components and design strategy of the membrane electrode assembly for alkaline water electrolysis[J]. Energy & Environmental Science, 2023, 16(4): 1384-1430. |
| 24 | Song W J, Peng K, Xu W, et al. Upscaled production of an ultramicroporous anion-exchange membrane enables long-term operation in electrochemical energy devices[J]. Nature Communications, 2023, 14: 2732. |
| 25 | Zeng M Y, He X Y, Wen J, et al. N-methylquinuclidinium-based anion exchange membrane with ultrahigh alkaline stability[J]. Advanced Materials, 2023, 35(51): 2306675. |
| 26 | Cohen M H, Turnbull D. Molecular transport in liquids and glasses[J]. The Journal of Chemical Physics, 1959, 31(5): 1164-1169. |
| 27 | Zielinski J M, Duda J L. Predicting polymer/solvent diffusion coefficients using free-volume theory[J]. AIChE Journal, 1992, 38(3): 405-415. |
| 28 | 徐晖, 王绍宁, 郑俊民. 用自由体积理论描述扩散现象[J].中国药剂学杂志 (网络版), 2004, 2(1): 7-13. |
| Xu H, Wang S N, Zheng J M. Description of the diffusion phenomenon using free volume theory[J].Chinese Journal of Pharmaceutics (Online Edition), 2004, 2(1): 7-13. | |
| 29 | Ramírez S C, Paz R R. New Trends in Ion Exchange Studies[M]. Istanbul: Selcan Karakuş, 2018: 51-70. |
| 30 | Thampan T, Malhotra S, Tang H, et al. Modeling of conductive transport in proton-exchange membranes for fuel cells[J]. Journal of the Electrochemical Society, 2000, 147(9): 3242. |
| 31 | Wang Z X, Chen K, Han J H, et al. Anion exchange membranes for fuel cells: equilibrium water content and conductivity characterization[J]. Advanced Functional Materials, 2023, 33(40): 2303857. |
| 32 | Tuckerman M E, Marx D, Parrinello M. The nature and transport mechanism of hydrated hydroxide ions in aqueous solution[J]. Nature, 2002, 417: 925-929. |
| 33 | Chen C, Tse Y L S, Lindberg G E, et al. Hydroxide solvation and transport in anion exchange membranes[J]. Journal of the American Chemical Society, 2016, 138(3): 991-1000. |
| 34 | Dong D P, Zhang W W, van Duin A C T, et al. Grotthuss versus vehicular transport of hydroxide in anion-exchange membranes: insight from combined reactive and nonreactive molecular simulations[J]. The Journal of Physical Chemistry Letters, 2018, 9(4): 825-829. |
| 35 | Foglia F, Berrod Q, Clancy A J, et al. Disentangling water, ion and polymer dynamics in an anion exchange membrane[J]. Nature Materials, 2022, 21: 555-563. |
| 36 | Perrin J C, Lyonnard S, Volino F. Quasielastic neutron scattering study of water dynamics in hydrated nafion membranes[J]. The Journal of Physical Chemistry C, 2007, 111(8): 3393-3404. |
| 37 | Lyonnard S, Berrod Q, Brüning B A, et al. Perfluorinated surfactants as model charged systems for understanding the effect of confinement on proton transport and water mobility in fuel cell membranes. A study by QENS[J]. The European Physical Journal Special Topics, 2010, 189(1): 205-216. |
| 38 | Berrod Q, Hanot S, Guillermo A, et al. Water sub-diffusion in membranes for fuel cells[J]. Scientific Reports, 2017, 7(1): 8326. |
| 39 | Park J S, Park S H, Yim S D, et al. Performance of solid alkaline fuel cells employing anion-exchange membranes[J]. Journal of Power Sources, 2008, 178(2): 620-626. |
| 40 | Yang Y X, Li P, Zheng X B, et al. Anion-exchange membrane water electrolyzers and fuel cells[J]. Chemical Society Reviews, 2022, 51(23): 9620-9693. |
| 41 | Du N Y, Roy C, Peach R, et al. Anion-exchange membrane water electrolyzers[J]. Chemical Reviews, 2022, 122(13): 11830-11895. |
| 42 | Chen N J, Long C, Li Y X, et al. High-performance layered double hydroxide/poly(2,6-dimethyl-1,4-phenylene oxide) membrane with porous sandwich structure for anion exchange membrane fuel cell applications[J]. Journal of Membrane Science, 2018, 552: 51-60. |
| 43 | Fan J T, Zhu H, Li R, et al. Layered double hydroxide-polyphosphazene-based ionomer hybrid membranes with electric field-aligned domains for hydroxide transport[J]. Journal of Materials Chemistry A, 2014, 2(22): 8376-8385. |
| 44 | Chen N J, Hu C, Wang H H, et al. Poly(alkyl-terphenyl piperidinium) ionomers and membranes with an outstanding alkaline-membrane fuel-cell performance of 2.58 W·cm-2 [J]. Angewandte Chemie International Edition, 2021, 60(14): 7710-7718. |
| 45 | Chen N J, Lu C R, Li Y X, et al. Robust poly(aryl piperidinium)/N-spirocyclic poly(2,6-dimethyl-1,4-phenyl) for hydroxide-exchange membranes[J]. Journal of Membrane Science, 2019, 572: 246-254. |
| 46 | Xu G D, Pan J, Zou X Y, et al. High-performance poly(biphenyl piperidinium) type anion exchange membranes with interconnected ion transfer channels: cooperativity of dual cations and fluorinated side chains[J]. Advanced Functional Materials, 2023, 33(35): 2302364. |
| 47 | 徐子昂, 万磊, 刘凯, 等. 高稳定碱性离子膜分子设计研究进展[J]. 化工学报, 2021, 72(8): 3891-3906. |
| Xu Z A, Wan L, Liu K, et al. Recent progress of molecular design for highly stable alkaline anion exchange membranes[J]. CIESC Journal, 2021, 72(8): 3891-3906. | |
| 48 | Olsson J S, Pham T H, Jannasch P. Poly(arylene piperidinium) hydroxide ion exchange membranes: synthesis, alkaline stability, and conductivity[J]. Advanced Functional Materials, 2018, 28(2): 1702758. |
| 49 | Li D G, Matanovic I, Lee A S, et al. Phenyl oxidation impacts the durability of alkaline membrane water electrolyzer[J]. ACS Applied Materials & Interfaces, 2019, 11(10): 9696-9701. |
| 50 | Li D G, Chung H T, Maurya S, et al. Impact of ionomer adsorption on alkaline hydrogen oxidation activity and fuel cell performance[J]. Current Opinion in Electrochemistry, 2018, 12: 189-195. |
| 51 | Gilliam R J, Graydon J W, Kirk D W, et al. A review of specific conductivities of potassium hydroxide solutions for various concentrations and temperatures[J]. International Journal of Hydrogen Energy, 2007, 32(3): 359-364. |
| 52 | Pan J, Chen C, Zhuang L, et al. Designing advanced alkaline polymer electrolytes for fuel cell applications[J]. Accounts of Chemical Research, 2012, 45(3): 473-481. |
| 53 | Bai T T, Cong M Y, Jia Y X, et al. Preparation of self-crosslinking anion exchange membrane with acid block performance from side-chain type polysulfone[J]. Journal of Membrane Science, 2020, 599: 117831. |
| 54 | Chen H H, Bang K T, Tian Y, et al. Poly(ethylene piperidinium)s for anion exchange membranes[J]. Angewandte Chemie International Edition, 2023, 62(38): e202307690. |
| 55 | Sung S, Mayadevi T S, Min K, et al. Crosslinked PPO-based anion exchange membranes: the effect of crystallinity versus hydrophilicity by oxygen-containing crosslinker chain length[J]. Journal of Membrane Science, 2021, 619: 118774. |
| 56 | Wu L, Pan Q, Varcoe J R, et al. Thermal crosslinking of an alkaline anion exchange membrane bearing unsaturated side chains[J]. Journal of Membrane Science, 2015, 490: 1-8. |
| 57 | Wang L Z, Hickner M A. Low-temperature crosslinking of anion exchange membranes[J]. Polymer Chemistry, 2014, 5(8): 2928-2935. |
| 58 | Yang B W, Zhang C M. Progress in constructing high-performance anion exchange membrane: molecular design, microphase controllability and in-device property[J]. Chemical Engineering Journal, 2023, 457: 141094. |
| 59 | Pan J, Chen C, Li Y, et al. Constructing ionic highway in alkaline polymer electrolytes[J]. Energy & Environmental Science, 2014, 7(1): 354-360. |
| 60 | Cai Z H, Gao X L, Gao W T, et al. Effect of hydrophobic side chain length on poly(carbazolyl terphenyl piperidinium) anion exchange membranes[J]. ACS Applied Energy Materials, 2022, 5(8): 10165-10176. |
| 61 | Zhang A R, Li L, Ma L L, et al. Soft template promoted microphase separation in anion exchange membrane of electrodialysis[J]. Journal of Membrane Science, 2022, 658: 120758. |
| 62 | Kim Y, Wang Y M, France-Lanord A, et al. Ionic highways from covalent assembly in highly conducting and stable anion exchange membrane fuel cells[J]. Journal of the American Chemical Society, 2019, 141(45): 18152-18159. |
| 63 | Liu X, Xie N, Xue J D, et al. Magnetic-field-oriented mixed-valence-stabilized ferrocenium anion-exchange membranes for fuel cells[J]. Nature Energy, 2022, 7: 329-339. |
| 64 | Zuo P P, Xu Z A, Zhu Q, et al. Ion exchange membranes: constructing and tuning ion transport channels[J]. Advanced Functional Materials, 2022, 32(52): 2207366. |
| 65 | Li Z Q, Guo J, Zheng J F, et al. A microporous polymer with suspended cations for anion exchange membrane fuel cells[J]. Macromolecules, 2020, 53(24): 10998-11008. |
| 66 | Yang Z J, Liu Y Z, Guo R, et al. Highly hydroxide conductive ionomers with fullerene functionalities[J]. Chemical Communications, 2016, 52(13): 2788-2791. |
| 67 | Zhang H Q, Zhang Y, Zhang F, et al. Enhancing side chain swing ability by novel all-carbon twisted backbone for high performance anion exchange membrane at relatively low IEC level[J]. Journal of Membrane Science Letters, 2021, 1(2): 100007. |
| 68 | Yang Z J, Guo R, Malpass-Evans R, et al. Highly conductive anion-exchange membranes from microporous Tröger's base polymers[J]. Angewandte Chemie International Edition, 2016, 55(38): 11499-11502. |
| 69 | Inoue K, Ishiwari F, Fukushima T. Selective synthesis of diazacyclooctane-containing flexible ladder polymers with symmetrically or unsymmetrically substituted side chains[J]. Polymer Chemistry, 2020, 11(22): 3690-3694. |
| 70 | Ishiwari F, Takeuchi N, Sato T, et al. Rigid-to-flexible conformational transformation: an efficient route to ring-opening of a Tröger's base-containing ladder polymer[J]. ACS Macro Letters, 2017, 6(7): 775-780. |
| 71 | Huang T, Zhang J F, Pei Y B, et al. Mechanically robust microporous anion exchange membranes with efficient anion conduction for fuel cells[J]. Chemical Engineering Journal, 2021, 418: 129311. |
| 72 | Ishiwari F, Sato T, Yamazaki H, et al. An anion-conductive microporous membrane composed of a rigid ladder polymer with a spirobiindane backbone[J]. Journal of Materials Chemistry A, 2016, 4(45): 17655-17659. |
| 73 | Yang Z Z, Guo W, Mahurin S M, et al. Surpassing Robeson upper limit for CO2/N2 separation with fluorinated carbon molecular sieve membranes[J]. Chem, 2020, 6(3): 631-645. |
| 74 | Xin L, Zhang D Z, Qu K, et al. Zr-MOF-enabled controllable ion sieving and proton conductivity in flow battery membrane[J]. Advanced Functional Materials, 2021, 31(42): 2104629. |
| 75 | Wu B, Ge L, Yu D B, et al. Cationic metal-organic framework porous membranes with high hydroxide conductivity and alkaline resistance for fuel cells[J]. Journal of Materials Chemistry A, 2016, 4(38): 14545-14549. |
| 76 | Khan N A, Zhang R N, Wu H, et al. Solid-vapor interface engineered covalent organic framework membranes for molecular separation[J]. Journal of the American Chemical Society, 2020, 142(31): 13450-13458. |
| 77 | Shen J L, Zhang R N, Su Y L, et al. Polydopamine-modulated covalent organic framework membranes for molecular separation[J]. Journal of Materials Chemistry A, 2019, 7(30): 18063-18071. |
| 78 | Kong Y, He X Y, Wu H, et al. Tight covalent organic framework membranes for efficient anion transport via molecular precursor engineering[J]. Angewandte Chemie International Edition, 2021, 60(32): 17638-17646. |
| 79 | He X Y, Yang Y, Wu H, et al. De novo design of covalent organic framework membranes toward ultrafast anion transport[J]. Advanced Materials, 2020, 32(36): 2001284. |
| 80 | Kong Y, Lyu B H, Fan C Y, et al. Manipulation of cationic group density in covalent organic framework membranes for efficient anion transport[J]. Journal of the American Chemical Society, 2023, 145(51): 27984-27992. |
| 81 | Wu M L, Zhang X, Zhao Y, et al. A high-performance hydroxide exchange membrane enabled by Cu2+-crosslinked chitosan[J]. Nature Nanotechnology, 2022, 17(6): 629-636. |
| 82 | Ge X L, He Y B, Guiver M D, et al. Alkaline anion-exchange membranes containing mobile ion shuttles[J]. Advanced Materials, 2016, 28(18): 3467-3472. |
| 83 | Ge X L, He Y B, Zhang K Y, et al. Fast bulky anion conduction enabled by free shuttling phosphonium cations[J]. Research, 2021, 2021: 9762709. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 余后川, 任腾, 张宁, 姜晓滨, 代岩, 张晓鹏, 鲍军江, 贺高红. 二维氧化石墨烯膜离子选择性传递调控的研究进展[J]. 化工学报, 2023, 74(1): 303-312. |
| [3] | 朱嫣然, 葛亮, 李兴亚, 徐铜文. 三相结构离子交换膜的构筑及应用研究[J]. 化工学报, 2022, 73(6): 2397-2414. |
| [4] | 黄盼, 练成, 刘洪来. 基于模拟退火算法的真实多孔电极中热-质传递的研究[J]. 化工学报, 2022, 73(6): 2529-2542. |
| [5] | 徐子昂, 万磊, 刘凯, 王保国. 高稳定碱性离子膜分子设计研究进展[J]. 化工学报, 2021, 72(8): 3891-3906. |
| [6] | 祝海涛, 杨波, 吴雅琴, 高从堦. 电渗析脱盐过程离子传递现象的数值模拟[J]. 化工学报, 2020, 71(8): 3518-3526. |
| [7] | 常明,陈爱平,何洪波,马磊,李春忠. 层层组装修饰Ni片阳极的光催化辅助电解水制氢[J]. 化工学报, 2012, 63(7): 2195-2201. |
| [8] | 朱玉婵, 任占冬, 刘晔, 张智勇. 氧化电解水杀菌特性及其对肉品杀菌作用 [J]. 化工学报, 2009, 60(10): 2583-2589. |
| [9] | 吕宏凌;王保国 . 高分子聚合物中溶剂扩散系数的预测 [J]. CIESC Journal, 2006, 57(1): 6-12. |
| [10] | 王保国; 山口猛央; 中尾真一. 均相玻璃态高分子中溶剂扩散系数的数学模型 [J]. CIESC Journal, 2002, 53(4): 338-343. |
| [11] | 蒋文华; 韩世钧. 3种芳香烃在聚乙烯膜中的无限稀释扩散系数 [J]. CIESC Journal, 2002, 53(3): 285-289. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号