化工学报 ›› 2025, Vol. 76 ›› Issue (10): 5213-5224.DOI: 10.11949/0438-1157.20250417
赵海霞1,4( ), 刘洋2, 王雷1,4, 高凤凤3, 郭志远1,4, 张盼盼1,4, 汪婧1,4, 纪志永1,4(
), 刘洋2, 王雷1,4, 高凤凤3, 郭志远1,4, 张盼盼1,4, 汪婧1,4, 纪志永1,4( )
)
收稿日期:2025-04-20
修回日期:2025-05-29
出版日期:2025-10-25
发布日期:2025-11-25
通讯作者:
纪志永
作者简介:赵海霞(1998—),女,硕士研究生,3344046314@qq.com
基金资助:
Haixia ZHAO1,4( ), Yang LIU2, Lei WANG1,4, Fengfeng GAO3, Zhiyuan GUO1,4, Panpan ZHANG1,4, Jing WANG1,4, Zhiyong JI1,4(
), Yang LIU2, Lei WANG1,4, Fengfeng GAO3, Zhiyuan GUO1,4, Panpan ZHANG1,4, Jing WANG1,4, Zhiyong JI1,4( )
)
Received:2025-04-20
Revised:2025-05-29
Online:2025-10-25
Published:2025-11-25
Contact:
Zhiyong JI
摘要:
油气田采出水是油气开采过程中产生的废水,含锂、溴等高附加值矿物资源。目前,溶存锂、溴提取需分步进行,效率较低、操作较为烦琐。因此,开发同步提取技术,对实现锂、溴的高效、绿色、低成本资源化提取与回收具有重要意义。本文分别采用高温固相法和水热法制备LiMn2O4(LMO)和BiOBr(BOB)电极活性材料,进而构建LMO/BOB电极体系;对无膜同步分极吸附和有膜同步分极脱附的操作电压进行调控,并对两电极间活性材料质量比进行优化匹配。优化条件下,3 h达到吸附平衡,Li+吸附容量24.13 mg/g、Br-吸附容量88.13 mg/g;3 h脱附达到平衡,基于单位质量LMO电极,单次分极脱附可得到2.70 mmol LiBr溶液。研究结果为油气田采出水中锂和溴的同步提取与回收提供了方法和数据参考。
中图分类号:
赵海霞, 刘洋, 王雷, 高凤凤, 郭志远, 张盼盼, 汪婧, 纪志永. 电化学法油气田采出水同步提锂溴及制溴化锂[J]. 化工学报, 2025, 76(10): 5213-5224.
Haixia ZHAO, Yang LIU, Lei WANG, Fengfeng GAO, Zhiyuan GUO, Panpan ZHANG, Jing WANG, Zhiyong JI. Electrochemical method for synchronous extraction of lithium and bromine from oil and gas field produced water to lithium bromide[J]. CIESC Journal, 2025, 76(10): 5213-5224.
| 共存离子 | 浓度/(mg/L) |
|---|---|
| Li+ | 47 |
| Na+ | 62347 |
| K+ | 2766 |
| Mg2+ | 472 |
| Ca2+ | 2816 |
| Cl- | 107715 |
| 1936 | |
| Br- | 543 |
表1 某高氯油气田采出水组成成分
Table 1 Composition in high-chlorine produced water of oil and gas field
| 共存离子 | 浓度/(mg/L) |
|---|---|
| Li+ | 47 |
| Na+ | 62347 |
| K+ | 2766 |
| Mg2+ | 472 |
| Ca2+ | 2816 |
| Cl- | 107715 |
| 1936 | |
| Br- | 543 |
| 电极体系 | 全称 | 简称 |
|---|---|---|
| 单独提锂体系 | LiMn2O4/Li1-x Mn2O4 | LMO/L1-x MO |
| 单独提溴体系 | BiOBr/BiOBr1-x | BOB/BOB1-x |
| 同步分极提锂、溴体系 | Li1-x Mn2O4/BiOBr1-x | L1-x MO/BOB1-x |
表2 不同电极体系的简称
Table 2 Abbreviations of different electrochemical systems
| 电极体系 | 全称 | 简称 |
|---|---|---|
| 单独提锂体系 | LiMn2O4/Li1-x Mn2O4 | LMO/L1-x MO |
| 单独提溴体系 | BiOBr/BiOBr1-x | BOB/BOB1-x |
| 同步分极提锂、溴体系 | Li1-x Mn2O4/BiOBr1-x | L1-x MO/BOB1-x |
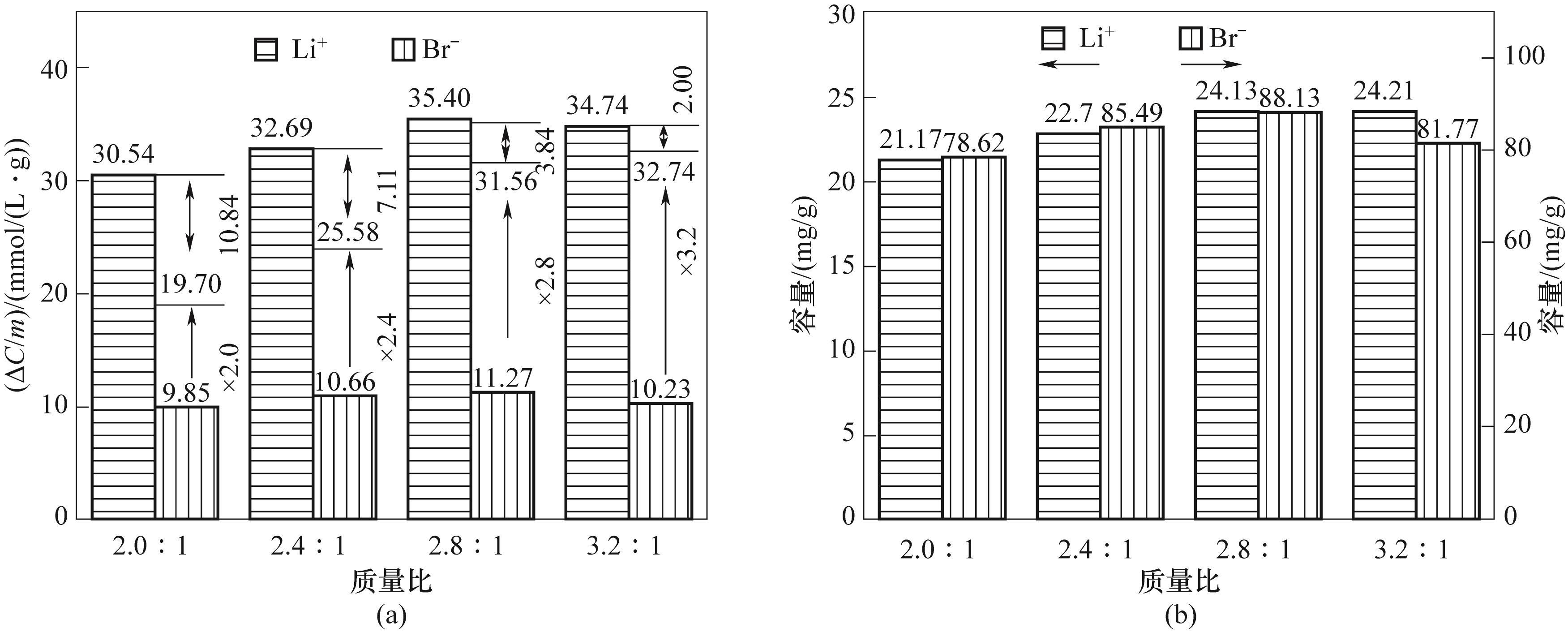
图9 LMO/BOB体系中Li+和Br-对应的吸附浓度变化量与活性材料质量的比值(a)和Li+和Br-的吸附容量(b)
Fig.9 The corresponding change in adsorption concentration of Li+ and Br-versus the mass of the active material (a) and the adsorption capacity of Li⁺ and Br- (b) in LMO/BOBsystem
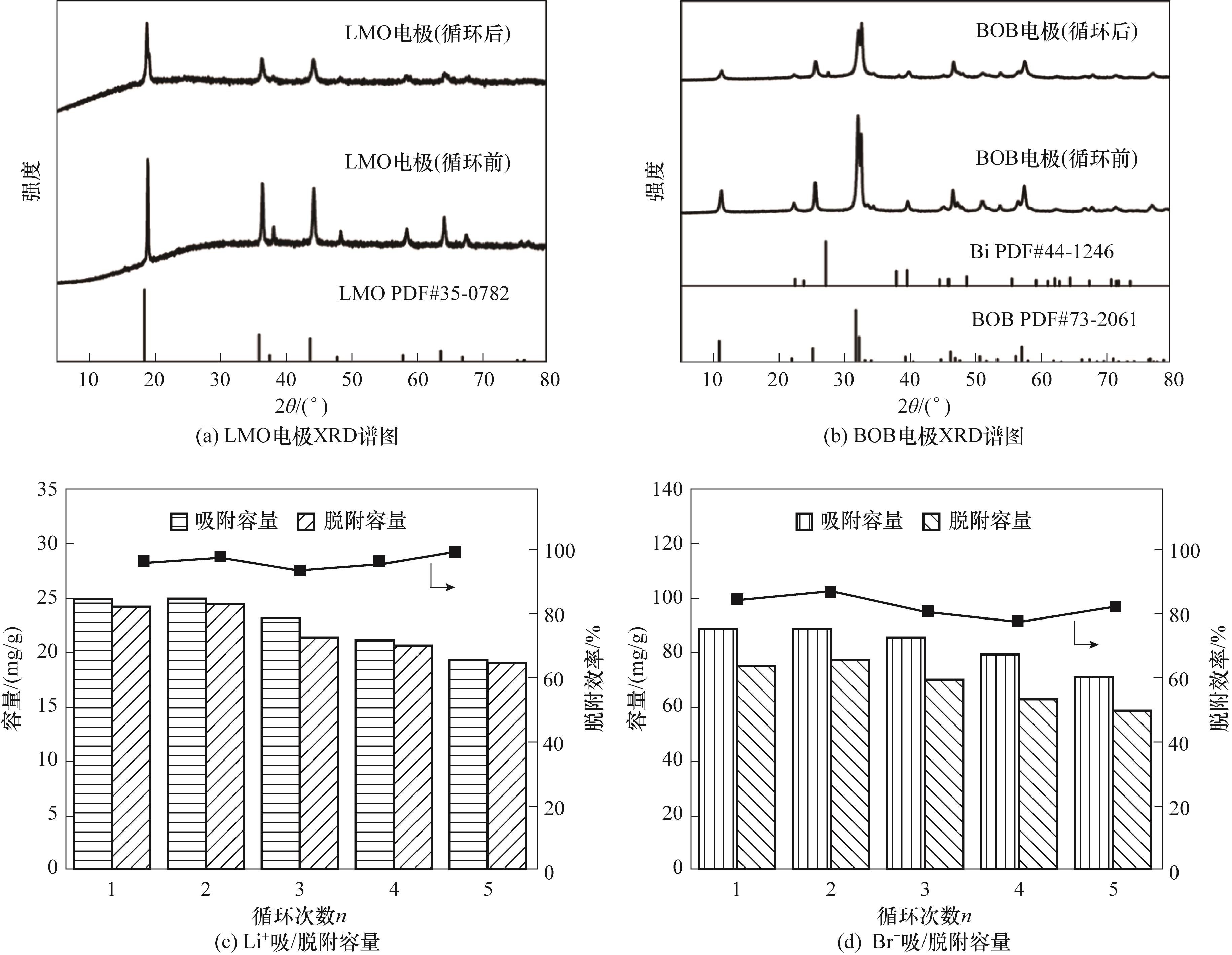
图11 在LMO/BOB电极体系5次循环中电极的XRD谱图和吸/脱附容量及脱附效率变化
Fig.11 XRD patterns of the electrodes and adsorption/desorption capacity and desorption efficiency change during the 5 cycles in the LMO/BOB electrode system
| 电极体系 | Li+吸附容量/ (mg/g) | Br-吸附容量/ (mg/g) | 时间/min | 文献 |
|---|---|---|---|---|
| B-LMO//Ag | 20.60 | — | 60 | [ |
| H-LMOE | 11.93 | — | 10 | [ |
| CNTS/QCS/BiOBr | — | 78.71 | 120 | [ |
| BiOBr/PVDF/CB | — | 67.10 | 240 | [ |
| BiOBr | 9.52(Cs+) | 53.28 | 240 | [ |
| LMO/L1-x MO | 18.11 | — | 180 | 本工作 |
| BOB/BOB1-x | — | 105.02 | 180 | 本工作 |
| LMO/BOB | 24.13 | 88.13 | 180 | 本工作 |
表3 不同电极体系对Li+和Br-的吸附性能比较
Table 3 Comparison of Li+ and Br- adsorption performance in different electrochemical systems
| 电极体系 | Li+吸附容量/ (mg/g) | Br-吸附容量/ (mg/g) | 时间/min | 文献 |
|---|---|---|---|---|
| B-LMO//Ag | 20.60 | — | 60 | [ |
| H-LMOE | 11.93 | — | 10 | [ |
| CNTS/QCS/BiOBr | — | 78.71 | 120 | [ |
| BiOBr/PVDF/CB | — | 67.10 | 240 | [ |
| BiOBr | 9.52(Cs+) | 53.28 | 240 | [ |
| LMO/L1-x MO | 18.11 | — | 180 | 本工作 |
| BOB/BOB1-x | — | 105.02 | 180 | 本工作 |
| LMO/BOB | 24.13 | 88.13 | 180 | 本工作 |
| [1] | Vera M L, Torres W R, Galli C I, et al. Environmental impact of direct lithium extraction from brines[J]. Nature Reviews Earth & Environment, 2023, 4: 149-165. |
| [2] | 蒋晨啸, 陈秉伦, 张东钰, 等. 我国盐湖锂资源分离提取进展[J]. 化工学报, 2022, 73(2): 481-503. |
| Jiang C X, Chen B L, Zhang D Y, et al. Progress in isolating lithium resources from China salt lake brine[J]. CIESC Journal, 2022, 73(2): 481-503. | |
| [3] | 杨婷, 张红梅, 张帆, 等. CNT-GO掺杂LiMn2O4膜电极的制备及其提锂性能[J]. 高校化学工程学报, 2023, 37(5): 832-839. |
| Yang T, Zhang H M, Zhang F, et al. Preparation and lithium extraction performance of CNT-GO doped LiMn2O4 film electrode[J]. Journal of Chemical Engineering of Chinese Universities, 2023, 37(5): 832-839. | |
| [4] | 张晓, 纪志永, 汪婧, 等. 电氧化法地下卤水提溴探究及条件优化[J]. 化工学报, 2021, 72(4): 2123-2131. |
| Zhang X, Ji Z Y, Wang J, et al. Exploration and optimization of extraction process of bromine from underground brine by electrooxidation[J]. CIESC Journal, 2021, 72(4): 2123-2131. | |
| [5] | Marín P E, Milian Y, Ushak S, et al. Lithium compounds for thermochemical energy storage: a state-of-the-art review and future trends[J]. Renewable and Sustainable Energy Reviews, 2021, 149: 111381. |
| [6] | Cabeza L F, Gutierrez A, Barreneche C, et al. Lithium in thermal energy storage: a state-of-the-art review[J]. Renewable and Sustainable Energy Reviews, 2015, 42: 1106-1112. |
| [7] | Tang L Y, Lu W J, Li X F. Electrolytes for bromine-based flow batteries: challenges, strategies, and prospects[J]. Energy Storage Materials, 2024, 70: 103532. |
| [8] | Shakoor N, Adeel M, Ahmad M A, et al. Reimagining safe lithium applications in the living environment and its impacts on human, animal, and plant system[J]. Environmental Science and Ecotechnology, 2023, 15: 100252. |
| [9] | Shtangeeva I, Niemelä M, Perämäki P, et al. Phytoextration of bromine from contaminated soil[J]. Journal of Geochemical Exploration, 2017, 174: 21-28. |
| [10] | Yang C H, Zhu B, Zhan M J, et al. Lithium in cancer therapy: friend or foe?[J]. Cancers, 2023, 15(4): 1095. |
| [11] | Villegas-Vázquez E Y, Quintas-Granados L I, Cortés H, et al. Lithium: a promising anticancer agent[J]. Life, 2023, 13(2): 537. |
| [12] | Pei W, Cheng Q, Jiao S, et al. Performance evaluation of the electrodialysis regenerator for the lithium bromide solution with high concentration in the liquid desiccant air-conditioning system[J]. Energy, 2019, 187: 115928. |
| [13] | Baudino L, Santos C, Pirri C F, et al. Recent advances in the lithium recovery from water resources: from passive to electrochemical methods[J]. Advanced Science, 2022, 9(27): 2201380. |
| [14] | Wang J, Fang J W, Ji Z Y, et al. Preparation of a novel hollow porous LiMn2O4 film electrode (H-LMOE) and its improved performance for lithium extraction[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110878. |
| [15] | Wang T Y, An X W, Wang P F, et al. Simultaneous selective separation and enrichment of bromine and cesium ions by electrochemically switched amphoteric ion membrane[J]. Desalination, 2024, 581: 117594. |
| [16] | Li M C, Zhao J Y, Li Y R, et al. Enhanced adsorption of cesium ions by electrochemically switched ion exchange method: based on surface-synthetic Na2Ti3O7 nanotubes[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 579: 123712. |
| [17] | Zhang B L, Du X, Hao X G, et al. A novel potential-triggered SBA-15/PANI/PSS composite film for selective removal of lead ions from wastewater[J]. Journal of Solid State Electrochemistry, 2018, 22(8): 2473-2483. |
| [18] | Wang Z D, Feng Y T, Hao X G, et al. An intelligent displacement pumping film system: a new concept for enhancing heavy metal ion removal efficiency from liquid waste[J]. Journal of Hazardous Materials, 2014, 274: 436-442. |
| [19] | Ji W W, Niu J J, Zhang W, et al. An electroactive ion exchange hybrid film with collaboratively-driven ability for electrochemically-mediated selective extraction of chloride ions[J]. Chemical Engineering Journal, 2022, 427: 130807. |
| [20] | Cheng Y J, Wang J, Luo J H, et al. BiOI with inherent photo/electric biactivity recovery I- by novel photoassisted electrochemically switched ion exchange technology[J]. Industrial & Engineering Chemistry Research, 2022, 61(26): 9394-9404. |
| [21] | Ye D N, Gao F F, Zeng G L, et al. An electroactive BiOBr/PVDF/CB film electrode for electrochemical extraction of bromine ions from brines[J]. Industrial & Engineering Chemistry Research, 2023, 62(22): 8882-8892. |
| [22] | Song X Y, Niu J J, Yan W J, et al. An electroactive BiOBr@PPy hybrid film with synergistic effect for electrochemically switched capture of bromine ions from aqueous solutions[J]. Separation and Purification Technology, 2022, 290: 120845. |
| [23] | Zhou G L, Chen L L, Li X W, et al. Construction of truncated-octahedral LiMn2O4 for battery-like electrochemical lithium recovery from brine[J]. Green Energy & Environment, 2023, 8(4): 1081-1090. |
| [24] | Zeng J S, Li M S, Li X F, et al. A novel coating onto LiMn2O4 cathode with increased lithium ion battery performance[J]. Applied Surface Science, 2014, 317: 884-891. |
| [25] | 郭志远, 张帆, 纪志永, 等. 选择性电渗析提锂技术的研究进展[J]. 河北工业大学学报, 2022, 51(6): 1-9. |
| Guo Z Y, Zhang F, Ji Z Y, et al. Research and prospect of lithium extraction by selective electrodialysis[J]. Journal of Hebei University of Technology, 2022, 51(6): 1-9. | |
| [26] | Jin W, Hu M Q, Sun Z, et al. Simultaneous and precise recovery of lithium and boron from salt lake brine by capacitive deionization with oxygen vacancy-rich CoP/Co3O4-graphene aerogel[J]. Chemical Engineering Journal, 2021, 420: 127661. |
| [27] | Zhao M Y, Ji Z Y, Zhang Y G, et al. Study on lithium extraction from brines based on LiMn2O4/Li1- x Mn2O4 by electrochemical method[J]. Electrochimica Acta, 2017, 252: 350-361. |
| [28] | Wu Y, Cao C B, Zhang J T, et al. Hierarchical LiMn2O4 hollow cubes with exposed{111}planes as high-power cathodes for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(30): 19567-19572. |
| [29] | Guo Z Y, Ji Z Y, Chen Q B, et al. Prefractionation of LiCl from concentrated seawater/salt lake brines by electrodialysis with monovalent selective ion exchange membranes[J]. Journal of Cleaner Production, 2018, 193: 338-350. |
| [30] | Zhang L, Ma Z, Sun H D, et al. A novel CNTs/QCS/BiOBr composite membrane with electron-ion transfer channel for Br- recovery in ESIX process[J]. Journal of Colloid and Interface Science, 2023, 646: 784-793. |
| [31] | Gu J, Zhou G L, Chen L L, et al. Particle size control and electrochemical lithium extraction performance of LiMn2O4 [J]. Journal of Electroanalytical Chemistry, 2023, 940: 117487. |
| [1] | SUSIAL P., RODRIGUEZ-HENRIQUEZ J.J., SOSA-ROSARIO A., RIOS-SANTANA R.. 乙酸乙酯与CnH2n+1OH (n=1,2,3)双系统在0.3 MPa下的气液平衡[J]. Chinese Journal of Chemical Engineering, 2012, 20(4): 723-730. |
| [2] | L.S. Chan, W.H. Cheung, S.J. Allen, G. McKay. 竹制活性炭处理酸性染料废水吸附等温线模型的误差分析[J]. CIESC Journal, 2012, 20(3): 535-542. |
| [3] | 王道广, 李志宝. 三水碳酸镁在Li + Na + K + NH4 + Mg + Cl + H2O体系中溶解度的全组分化学模拟[J]. CIESC Journal, 2012, 20(2): 267-276. |
| [4] | 王建立, 朱建军, 宋辰兴, 张兴. 3ω法测量纳米流体热导率 [J]. 化工学报, 2011, 62(S1): 42-47. |
| [5] | 马维刚, 王海东, 张兴, 王玮. 多晶金薄膜电子-声子耦合的实验研究 [J]. 化工学报, 2011, 62(S1): 48-53. |
| [6] | 崔福庆, 何雅玲, 程泽东, 李东, 陶于兵. 有压腔式吸热器内辐射传播过程的Monte Carlo模拟 [J]. 化工学报, 2011, 62(S1): 60-65. |
| [7] | 何奎, 汪双凤, 黄间珍. 气液两相流在微小T型三通中的相分配特性 [J]. 化工学报, 2011, 62(S1): 92-96. |
| [8] | 刘绩伟, 吴玉庭, 马重芳. 管肋式空间辐射器设计 [J]. 化工学报, 2011, 62(S1): 103-107. |
| [9] | 李旺, 王情愿, 李瑞龙, 李超, 宇波, 张劲军, 代鹏飞. 大型浮顶油罐温度场数值模拟 [J]. 化工学报, 2011, 62(S1): 108-112. |
| [10] | 胡朝发, 贾力. 脉动热管气液塞振荡运动模型 [J]. 化工学报, 2011, 62(S1): 113-117. |
| [11] | 张艾萍, 谢立强, 徐志明. 超声空化对换热器协同场的影响 [J]. 化工学报, 2011, 62(S1): 130-133. |
| [12] | 曾庆, 郭印诚, 牛振祺, 林文漪. 填料塔中氨水吸收二氧化碳的传质性能 [J]. 化工学报, 2011, 62(S1): 146-150. |
| [13] | 褚燕彬, 曹柳林, 王晶. 利用组合B样条神经网络实现对间歇反应过程的动态建模 [J]. 化工学报, 2011, 62(S1): 157-162. |
| [14] | 吴海波, 方岩雄, 纪红兵. 助溶剂促进的β-环糊精与肉桂醛的包结性能 [J]. 化工学报, 2011, 62(S1): 168-173. |
| [15] | 任楠, 王涛, 吴玉庭, 马重芳. 混合碳酸盐的DSC测量与比热容分析 [J]. 化工学报, 2011, 62(S1): 197-202. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号