CIESC Journal ›› 2020, Vol. 71 ›› Issue (6): 2628-2642.DOI: 10.11949/0438-1157.20200043
• Reviews and monographs • Previous Articles Next Articles
Yating ZHANG1,2( ),Bochao ZHANG1,Jianlan ZHANG1,Keke LI1,Yongqiang DANG1,Yingfeng DUAN1
),Bochao ZHANG1,Jianlan ZHANG1,Keke LI1,Yongqiang DANG1,Yingfeng DUAN1
Received:2020-01-13
Revised:2020-04-08
Online:2020-06-05
Published:2020-06-05
Contact:
Yating ZHANG
张亚婷1,2( ),张博超1,张建兰1,李可可1,党永强1,段瑛峰1
),张博超1,张建兰1,李可可1,党永强1,段瑛峰1
通讯作者:
张亚婷
作者简介:张亚婷(1972—),女,教授,基金资助:CLC Number:
Yating ZHANG, Bochao ZHANG, Jianlan ZHANG, Keke LI, Yongqiang DANG, Yingfeng DUAN. Research progress in “bottom-up” chemical synthesis of nanographenes[J]. CIESC Journal, 2020, 71(6): 2628-2642.
张亚婷, 张博超, 张建兰, 李可可, 党永强, 段瑛峰. “自下而上”化学合成纳米石墨烯的研究进展[J]. 化工学报, 2020, 71(6): 2628-2642.
Add to citation manager EndNote|Ris|BibTeX
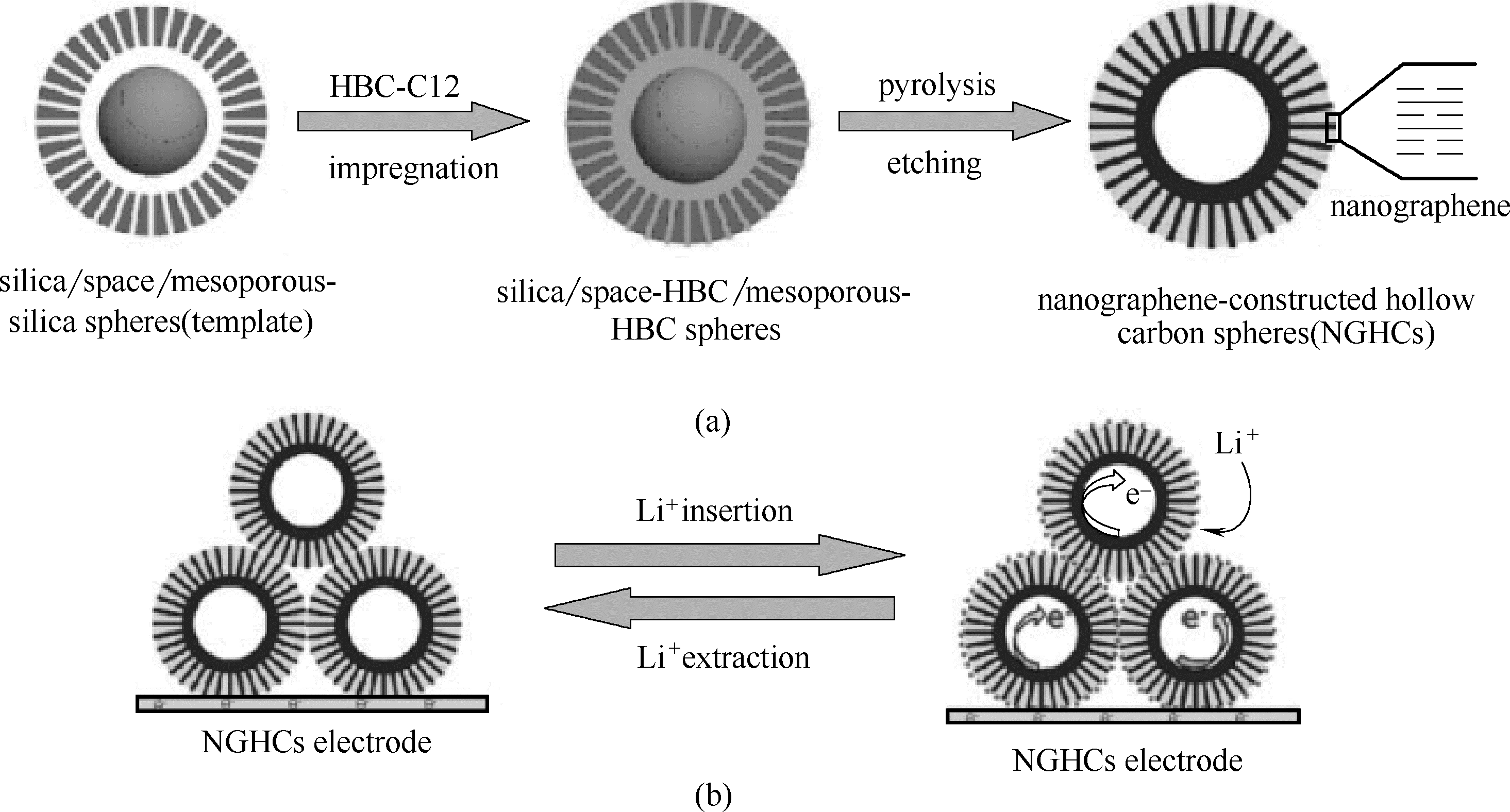
Fig.23 Schematic diagram of structure of NGHC (a) and diffusion of lithium ions and electrons during discharge and charging of NGHCs electrodes (b) [81]
| 1 | Novoselov K S, Geim A K, Morozov S V, et al. Two-dimensional gas of massless Dirac fermions in graphene[J]. Nature, 2005, 438: 197-200. |
| 2 | Westervelt R M. Graphene nanoelectronics[J]. Science, 2008, 320(5874): 324-325. |
| 3 | Schedin F, Geim A K, Morozov S V, et al. Detection of individual gas molecules adsorbed on graphene[J]. Nature Materials, 2007, 6(9): 652-655. |
| 4 | Dua V, Surwade S P, Ammu S, et al. All‐organic vapor sensor using inkjet‐printed reduced graphene oxide[J]. Angewandte Chemie, 2010, 122(12): 2200-2203. |
| 5 | Scheuermann G M, Rumi L, Steurer P, et al. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki-Miyaura coupling reaction[J]. Journal of the American Chemical Society, 2009, 131(23): 8262-8270. |
| 6 | 张亚婷, 李可可, 任邵昭, 等. 煤基石墨烯宏观体的制备及其在CO2光催化还原过程中的应用[J]. 新型炭材料, 2015, 30(6): 539-544. |
| Zhang Y T, Li K K, Ren S Z, et al. Fabrication and electrochemical capacitive performance of PANI/coal-based three-dimensional graphene[J]. New Carbon Materials, 2015, 30(6): 539-544. | |
| 7 | Dreyer D R, Bielawski C W. Carbocatalysis: heterogeneous carbons finding utility in synthetic chemistry[J]. Chemical Science, 2011, 2(7): 1233-1240. |
| 8 | Pumera M. Graphene-based nanomaterials for energy storage[J]. Energy & Environmental Science, 2011, 4(3): 668-674. |
| 9 | Zhang Y T, Zhang K B, Jia K L, et al. Preparation of coal-based graphene quantum dots/α-Fe2O3 nanocomposites and their lithium-ion storage properties[J]. Fuel, 2019, 241: 646-652. |
| 10 | Sun Y, Wu Q, Shi G. Graphene based new energy materials[J]. Energy & Environmental Science, 2011, 4(4): 1113-1132. |
| 11 | Xu W, Ling X, Xiao J, et al. Surface enhanced Raman spectroscopy on a flat graphene surface[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(24): 9281-9286. |
| 12 | 张亚婷, 李景凯, 刘国阳, 等. MnO2/煤基石墨烯纳米复合材料的制备及其电化学性能[J]. 新型炭材料, 2016, 31(5): 545-549. |
| Zhang Y T, Li J K, Liu G Y, et al. Preparation of MnO2/coal-based graphene composites for supercapacitors[J]. New Carbon Materials, 2016, 31(5): 545-549. | |
| 13 | Schwierz F. Graphene transistors[J]. Nature Nanotechnology, 2010, 5(7): 487-496. |
| 14 | Chen L, Hernandez Y, Feng X, et al. From nanographene and graphene nanoribbons to graphene sheets: chemical synthesis[J]. Angewandte Chemie International Edition, 2012, 51(31): 7640-7654. |
| 15 | Rieger R, Müllen K. Forever young: polycyclic aromatic hydrocarbons as model cases for structural and optical studies[J]. Journal of Physical Organic Chemistry, 2010, 23(4): 315-325. |
| 16 | Liu J, Nilantha P, Shi Z Q, et al. Molecular-based design and emerging applications of nanoporous carbon spheres[J]. Nature Materials, 2015, 14: 763–774. |
| 17 | Dai Y, Liu Y, Ding K, et al. A short review of nanographenes: structures, properties and applications[J]. Molecular Physics, 2018, 116(7/8): 987-1002. |
| 18 | Narita A, Wang Y X, Feng X L, et al. New advances in nanographene chemistry[J]. Chemical Society Reviews, 2015, 44: 6616-6643. |
| 19 | Wang X Y, Yao X L, Müllen K. Polycyclic aromatic hydrocarbons in the graphene era[J]. Science China Chemistry, 2019, 62(9): 1099-1144 |
| 20 | Zhang Y T, Li K K, Ren S Z, et al. Coal-derived graphene quantum dots produced by ultrasonic physical tailoring and their capacity for Cu(Ⅱ) detection[J]. ACS Sustainable Chemistry & Engineering, 2019,7(11): 9793-9799. |
| 21 | Helge S, Balagi P, David J J, et al. Hexa-peri-hexabenzocoronene in organic electronics[J]. Pure and Applied Chemistry, 2012, 84(4): 1047-1067. |
| 22 | Clar E, Ironside C T, Zander M. The electronic interaction between benzenoid rings in condensed aromatic hydrocarbons. 1: 12-2: 3-4: 5-6: 7-8: 9-10: 11-hexabenzocoronene, 1: 2-3: 4-5: 6-10: 11-tetrabenzoanthanthrene, and 4: 5-6: 7-11: 12-13: 14-tetrabenzoperopyrene[J]. Journal of the Chemical Society, 1959: 142-147. |
| 23 | Halleux A, Martin R H, King G S D. Synthèses dans la série des dérivés polycycliques aromatiques hautement condensés. L'hexabenzo‐1, 12; 2, 3; 4, 5; 6, 7; 8, 9; 10, 11‐coronène, le tétrabenzo‐4, 5; 6, 7; 11, 12; 13, 14‐péropyrène et le tétrabenzo‐1, 2; 3, 4; 8, 9; 10, 11‐bisanthène[J]. Helvetica Chimica Acta, 1958, 41(5): 1177-1183. |
| 24 | Hendel W, Khan Z H, Schmidt W. Hexa-peri-benzocoronene, a candidate for the origin of the diffuse interstellar visible absorption bands[J]. Tetrahedron, 1986, 42(4): 1127-1134. |
| 25 | Wu J, Pisula W, Müllen K. Graphenes as potential material for electronics[J]. Chemical Reviews, 2007, 107(3): 718-747. |
| 26 | Yang X, Dou X, Müllen K. Efficient synthesis of symmetrically and unsymmetrically substituted hexaphenylbenzene analogues by Suzuki–Miyaura coupling reactions[J]. Chemistry-An Asian Journal, 2008, 3(4): 759-766. |
| 27 | Feng X, Wu J, Enkelmann V, et al. Hexa-peri-hexabenzocoronenes by efficient oxidative cyclodehydrogenation: the role of the oligophenylene precursors[J]. Organic Letters, 2006, 8(6): 1145-1148. |
| 28 | Weiss K, Beernink G, Dötz F, et al. Templateffekte bei der Herstellung polycyclischer aromatischer Kohlenwasserstoffe: Cyclodehydrierung und Planarisierung eines Hexaphenylbenzols an einer Kupferoberfläche[J]. Angewandte Chemie, 1999, 111(24): 3974-3978. |
| 29 | Simpson C D, Mattersteig G, Martin K, et al. Nanosized molecular propellers by cyclodehydrogenation of polyphenylene dendrimers[J]. Journal of the American Chemical Society, 126(10): 3139-3147. |
| 30 | Dötz F, Brand J D, Ito S, et al. Synthesis of large polycyclic aromatic hydrocarbons: variation of size and periphery[J]. Journal of the American Chemical Society, 2000, 122(32): 7707-7717. |
| 31 | Simpson C D, Brand J D, Berresheim A J, et al. Synthesis of a giant 222 carbon graphite sheet[J]. Chemical-A Eurpean Journal, 2002, 8(6): 1424-1429. |
| 32 | Rodríguez-Lojo D, Peña D, Pérez D, et al. Aryne-mediated syntheses of structurally related acene derivatives[J]. Organic Biomolecular Chemistry, 2010, 8(15): 3386-3388. |
| 33 | Schuler B, Collazos S, Gross L, et al. From perylene to a 22-ring aromatic hydrocarbon in one-pot[J]. Angewandte Chemie, 2014, 126(34): 9150-9152. |
| 34 | Romero C, Peña D, Pérez D, et al. Synthesis of extended triphenylenes by palladium‐catalyzed [2+2+2] cycloaddition of triphenylynes[J]. Chemical-A Eurpean Journal, 2006, 12(22): 5677-5684. |
| 35 | Rüdiger E C, Porz M, Schaffroth M, et al. Synthesis of soluble, alkyne-substituted trideca- and hexadeca-starphenes[J]. Chemical-A Eurpean Journal, 2014, 20(40): 12725-12728. |
| 36 | Xiao S, Myers M, Miao Q, et al. Molecular wires from contorted aromatic compounds[J]. Angewandte Chemie International Edition, 2005, 44(45): 7390-7394. |
| 37 | Feng X, Pisula W, Müllen K. Large polycyclic aromatic hydrocarbons: synthesis and discotic organization[J]. Pure and Applied Chemistry, 2009, 81(12): 2203-2224. |
| 38 | Huang P Y, Ruiz-Vargas C S, van der Zande A M, et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts[J]. Nature, 2011, 469(7330): 389-392. |
| 39 | Kurasch S, Kotakoski J, Lehtinen O, et al. Atom-by-atom observation of grain boundary migration in graphene[J]. Nano Letters, 2012, 12(6): 3168-3173. |
| 40 | Scott L T. Methods for the chemical synthesis of fullerenes[J]. Angew. Chem. Int. Ed., 2004, 43, 4994–5007. |
| 41 | Yamamoto K, Harada T, Nakazaki M, et al. Synthesis and characterization of [7]circulene[J]. Journal of the American Chemical Society, 1983, 105(24): 7171-7172. |
| 42 | Yamamoto K, Saitho Y, Iwaki D, et al. [7.7]circulene, a molecule shaped like a figure of eight [J]. Angewandte Chemie International Edition, 1991, 30(9): 1173-1174. |
| 43 | Luo J, Xu X, Mao R, et al. Curved polycyclic aromatic molecules that are π-isoelectronic to hexabenzocoronene[J]. Journal of the American Chemical Society, 2012, 134(33): 13796-13803. |
| 44 | Kawasumi K, Zhang Q, Segawa Y, et al. A grossly warped nanographene and the consequences of multiple odd-membered-ring defects[J]. Nature Chemistry, 2013, 5(9): 739-744. |
| 45 | Feng C N, Kuo M Y, Wu Y T. Synthesis, structural analysis, and properties of [8]circulenes[J]. Angewandte Chemie, 2013, 125(30): 7945-7948. |
| 46 | Sakamoto Y, Suzuki T. Tetrabenzo [8]circulene: aromatic saddles from negatively curved graphene [J]. Journal of the American Chemical Society, 2013, 135(38): 14074-14077. |
| 47 | Jiang W, Li Y, Wang Z. Heteroarenes as high performance organic semiconductors[J]. Chemical Society Reviews, 2013, 42(14): 6113-6127. |
| 48 | Stępień M, Gońka E, Żyła M, et al. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: synthetic routes, properties, and applications[J]. Chemical Reviews. 2017, 117: 3479-3716. |
| 49 | Wang X Y, Yao X L, Narita A, et al. Heteroatom-doped nanographenes with structural precision[J]. Accounts of Chemical Research, 2019, 52: 2491-2505. |
| 50 | Takase M, Enkelmann V, Sebastiani D, et al. Annularly fused hexapyrrolohexaazacoronenes: an extended π system with multiple interior nitrogen atoms displays stable oxidation states[J]. Angewandte Chemie, 2007, 119(29): 5620-5623. |
| 51 | Takase M, Narita T, Fujita W, et al. Pyrrole-fused azacoronene family: the influence of replacement with dialkoxybenzenes on the optical and electronic properties in neutral and oxidized states[J]. Journal of the American Chemical Society, 2013, 135(21): 8031-8040. |
| 52 | Draper S M, Gregg D J, Madathil R. Heterosuperbenzenes: a new family of nitrogen-functionalized, graphitic molecules[J]. Journal of the American Chemical Society, 2002, 124(14): 3486-3487. |
| 53 | Wei J, Han B, Guo Q, et al. 1, 5, 9‐Triazacoronenes: a family of polycyclic heteroarenes synthesized by a threefold Pictet-Spengler reaction[J]. Angewandte Chemie International Edition, 2010, 49(44): 8209-8213. |
| 54 | Berger R, Giannakopoulos A, Ravat P, et al. Synthesis of nitrogen-doped zigzag‐edge peripheries: dibenzo-9a-azaphenalene as repeating unit[J]. Angewandte Chemie International Edition, 2014, 53(39): 10520-10524. |
| 55 | Martin C J, Gil B, Perera S D, et al. Thienyl directed polyaromatic C—C bond fusions: S-doped hexabenzocoronenes[J]. Chemical Communications, 2011, 47(12): 3616-3618. |
| 56 | Chiu C Y, Kim B, Gorodetsky A A, et al. Shape-shifting in contorted dibenzotetrathienocoronenes[J]. Chemical Science, 2011, 2: 1480-1486. |
| 57 | Chen L, Puniredd S R, Tan Y Z, et al. Hexathienocoronenes: synthesis and self-organization[J]. Journal of the American Chemical Society, 2012, 134(43): 17869-17872. |
| 58 | Chernichenko K Y, Sumerin V V, Shpanchenko R V, et al. “Sulflower”: a new form of carbon sulfide[J]. Angewandte Chemie International Edition, 2006, 45(44): 7367-7370. |
| 59 | Lu R Q, Zhou Y N, Yan X Y, et al. Thiophene-fused bowl-shaped polycyclic aromatics with a dibenzo [a, g] corannulene core for organic field-effect transistors[J]. Chemical Communications, 2015, 51(9): 1681-1684. |
| 60 | Feng X, Wu J, Ai M, et al. Triangle-shaped polycyclic aromatic hydrocarbons[J]. Angewandte Chemie, 2007, 119(17): 3093-3096. |
| 61 | Escande A, Ingleson M J. Fused polycyclic aromatics incorporating boron in the core: fundamentals and applications[J]. Chemical Communications, 2015, 51(29): 6257-6274. |
| 62 | Wang X Y, Wang J Y, Pei J. BN heterosuperbenzenes: synthesis and properties[J]. Chemical-A Eurpean Journal, 2015, 21(9): 3528-3539. |
| 63 | Pisula W, Feng X, Müllen K. Charge-carrier transporting graphene-type molecules[J]. Chemistry of Materials, 2010, 23(3): 554-567. |
| 64 | Sergeyev S, Pisula W, Geerts Y H. Discotic liquid crystals: a new generation of organic semiconductors[J]. Chemical Society Reviews, 2007, 36(12): 1902-1929. |
| 65 | Ito S, Wehmeier M, Brand J D, et al. Synthesis and self‐assembly of functionalized hexa‐peri‐hexabenzocoronenes[J]. Chemical-A Eurpean Journal, 2000, 6(23): 4327-4342. |
| 66 | Wu J, Watson M D, Müllen K. The versatile synthesis and self-assembly of star-type hexabenzocoronenes[J]. Angewandte Chemie International Edition, 2003, 42(43): 5329-5333. |
| 67 | Wu J, Watson M D, Zhang L, et al. Hexakis (4-iodophenyl)-peri-hexabenzocoronene—a versatile building block for highly ordered discotic liquid crystalline materials[J]. Journal of the American Chemical Society, 2004, 126(1): 177-186. |
| 68 | Wu J, Li J, Kolb U, et al. A water-soluble hexa-peri-hexabenzocoronene: synthesis, self-assembly and role as template for porous silica with aligned nanochannels[J]. Chemical Communications, 2006, (1): 48-50. |
| 69 | Wu J, Baumgarten M, Debije M G, et al. Arylamine-substituted hexa-peri-hexabenzocoronenes: facile synthesis and their potential applications as “coaxial” hole-transport materials[J]. Angewandte Chemie International Edition, 2004, 43: 5331-5335. |
| 70 | Tan Y Z, Yang B, Parvez K, et al. Atomically precise edge chlorination of nanographenes and its application in graphene nanoribbons[J]. Nature Communications, 2013, 4: 2646. |
| 71 | Mkhalid I A I, Barnard J H, Marder T B, et al. C- H activation for the construction of C- B bonds[J]. Chemical Reviews, 2009, 110(2): 890-931. |
| 72 | Yamaguchi R, Hiroto S, Shinokubo H. Synthesis of oxygen-substituted hexa-peri-hexabenzocoronenes through Ir-catalyzed direct borylation[J]. Organic Letters, 2012, 14(10): 2472-2475. |
| 73 | Yamaguchi R, Ito S, Lee B S, et al. Functionalization of hexa-peri-hexabenzocoronenes: investigation of the substituent effects on a superbenzene[J]. Chemistry-An Asian Journal, 2013, 8(1): 178-190. |
| 74 | He Y, Yamamoto Y, Jin W, et al. Hexabenzocoronene graphitic nanotube appended with dithienylethene pendants: photochromism for the modulation of photoconductivity[J]. Advanced Materials, 2010, 22: 829. |
| 75 | Hill J P, Jin W, Kosaka A, et al. Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube[J]. Science, 2004, 304: 1481-1483. |
| 76 | Jin W, Fukushima T, Kosaka A, et al. Controlled self-assembly triggered by olefin metathesis: cross-linked graphitic nanotubes from an amphiphilic hexa-peri-hexabenzocoronene[J]. Journal of the American Chemical Society, 2005, 127: 8284-8285 |
| 77 | Zhang C, Liu Y, Xiong X Q, et al. Three-dimensional nanographene based on triptycene: synthesis and its application in fluorescence imaging[J]. Organic Letters, 2012, 23: 5912-5915. |
| 78 | Yin M, Shen J, Pisula W, et al. Functionalization of self-assembled hexa-peri-hexabenzocoronene fibers with peptides for bioprobing[J]. Journal of the American Chemical Society, 2009, 131: 14618. |
| 79 | Hill J P, Jin W, Kosaka A, et al. Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube[J]. Science, 2004, 304(5676): 1481-1483. |
| 80 | Yamamoto Y, Fukushima T, Suna Y, et al. Photoconductive coaxial nanotubes of molecularly connected electron donor and acceptor layers[J]. Science, 2006, 314(5806): 1761-1764. |
| 81 | Yang S B, Feng X L, Zhi L J, et al. Nanographene-constructed hollow carbon spheres and their favorable electroactivity with respect to lithium storage[J]. Advanced Materails, 2010, 22: 838-842 |
| 82 | Amann A, Smith D. Breath analysis for clincal diagnosis and therapeutic monitoring[C]//Conference Breath Gas Analysis for Medical Diagnostics. Dornbirn, Austria, 2004. |
| 83 | Amann A, Spanel P, Smith D. Breath analysis: the approach towards clinical applications[J]. Mini-reviews in Medicinal Chemistry, 2007, 7: 115. |
| 84 | Zilberman Y, Tisch U, Pisula W, et al. Spongelike structures of hexa-peri-hexabenzocoronene derivatives enhance the sensitivity of chemiresistive carbon nanotubes to nonpolar volatile organic compounds of cancer[J]. Langmuir, 2009, 25: 5411-5416. |
| [1] | LIN Zhanglin, ZHANG Yan, WANG Xu, LIU Peng. Recent advances in synthetic biology [J]. CIESC Journal, 2015, 66(8): 2863-2871. |
| [2] | LI Zhaohua, CHU Youqun, MA Chun'an. Indirect electrochemical synthesis of 1,4-naphthoquinone mediated by Ce3+/Ce4+ [J]. CIESC Journal, 2013, 64(1): 334-339. |
| [3] | MA Qiangqiang,ZHAO Guangrong. Research progress in L-DOPA synthesis [J]. Chemical Industry and Engineering Progree, 2013, 32(06): 1367-1371. |
| [4] | MA Feng,YANG Xiaoyong,CHEN Minghui. Preparation of expanded graphite by K-THF-GIC microwave expanding [J]. , 2010, 29(9): 1715-. |
| [5] | QI Ji1,NING Guiling2,LIU Junlong3,WANG Chen1. Recent research progress in vanadium dioxide powder [J]. , 2010, 29(8): 1513-. |
| [6] | WANG Shiquan, LI Guohua, DU Guodong, JIANG Xueya, FENG Chuanqi, GUO Zaiping, KIM Seung-Joo. Hydrothermal Synthesis of Molybdenum Disulfide for Lithium Ion Battery Applications [J]. , 2010, 18(6): 910-913. |
| [7] | DONG Guangxin,JIANG Jiahuan. Recent progress in microfluidic mixing-based synthesis of micro/nanoparticles [J]. , 2010, 29(11): 2026-. |
| [8] | NIAN Baoyi,XU Gang,YANG Lirong. Advance in chemical synthesis and chiral resolution of l-menthol [J]. , 2006, 25(4): 401-. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||