CIESC Journal ›› 2024, Vol. 75 ›› Issue (4): 1153-1166.DOI: 10.11949/0438-1157.20231326
• Reviews and monographs • Previous Articles Next Articles
Xiaojie JU( ), Wanlu SONG, Chenyu ZHOU, Qiutong SHEN, Yutian LIAO, Jueying GONG, Liangyin CHU
), Wanlu SONG, Chenyu ZHOU, Qiutong SHEN, Yutian LIAO, Jueying GONG, Liangyin CHU
Received:2023-12-12
Revised:2024-01-23
Online:2024-06-06
Published:2024-04-25
Contact:
Xiaojie JU
巨晓洁( ), 宋婉璐, 周宸宇, 沈秋彤, 廖雨田, 龚珏颖, 褚良银
), 宋婉璐, 周宸宇, 沈秋彤, 廖雨田, 龚珏颖, 褚良银
通讯作者:
巨晓洁
作者简介:巨晓洁(1980—),女,博士,教授,juxiaojie@scu.edu.cn
基金资助:CLC Number:
Xiaojie JU, Wanlu SONG, Chenyu ZHOU, Qiutong SHEN, Yutian LIAO, Jueying GONG, Liangyin CHU. Advances in drug nanodelivery systems for the treatment of intracellular bacterial infections[J]. CIESC Journal, 2024, 75(4): 1153-1166.
巨晓洁, 宋婉璐, 周宸宇, 沈秋彤, 廖雨田, 龚珏颖, 褚良银. 纳米药物载体用于治疗胞内菌感染的研究进展[J]. 化工学报, 2024, 75(4): 1153-1166.
Add to citation manager EndNote|Ris|BibTeX
| 类型 | 优点 | 缺点 | 典型体系 | 杀菌率 |
|---|---|---|---|---|
| 聚合物纳米颗粒 | 稳定性高;易修饰 | 少部分具有细胞毒性; 溶剂残留 | 天然聚合物纳米颗粒、合成聚合物 纳米颗粒[ | 高[ |
| 生物源性纳米颗粒 | 生物相容性良好;可降解; 靶向性好 | 稳定性较差 | 外泌体、细胞膜纳米颗粒、细菌囊泡[ | 极高[ |
| 脂质体与脂质纳米颗粒 | 生物相容性良好;不良反应少; 易制备;靶向性强 | SLN载药量低 | 脂质体、SLN、NLC[ | 极高[ |
| 无机纳米颗粒 | 形状多样;易制备;易修饰 | 可降解性较差 | 金属和金属氧化物纳米颗粒、量子点、 无机非金属纳米颗粒[ | 高[ |
Table 1 Nanodelivery systems for intracellular bacteria therapy
| 类型 | 优点 | 缺点 | 典型体系 | 杀菌率 |
|---|---|---|---|---|
| 聚合物纳米颗粒 | 稳定性高;易修饰 | 少部分具有细胞毒性; 溶剂残留 | 天然聚合物纳米颗粒、合成聚合物 纳米颗粒[ | 高[ |
| 生物源性纳米颗粒 | 生物相容性良好;可降解; 靶向性好 | 稳定性较差 | 外泌体、细胞膜纳米颗粒、细菌囊泡[ | 极高[ |
| 脂质体与脂质纳米颗粒 | 生物相容性良好;不良反应少; 易制备;靶向性强 | SLN载药量低 | 脂质体、SLN、NLC[ | 极高[ |
| 无机纳米颗粒 | 形状多样;易制备;易修饰 | 可降解性较差 | 金属和金属氧化物纳米颗粒、量子点、 无机非金属纳米颗粒[ | 高[ |
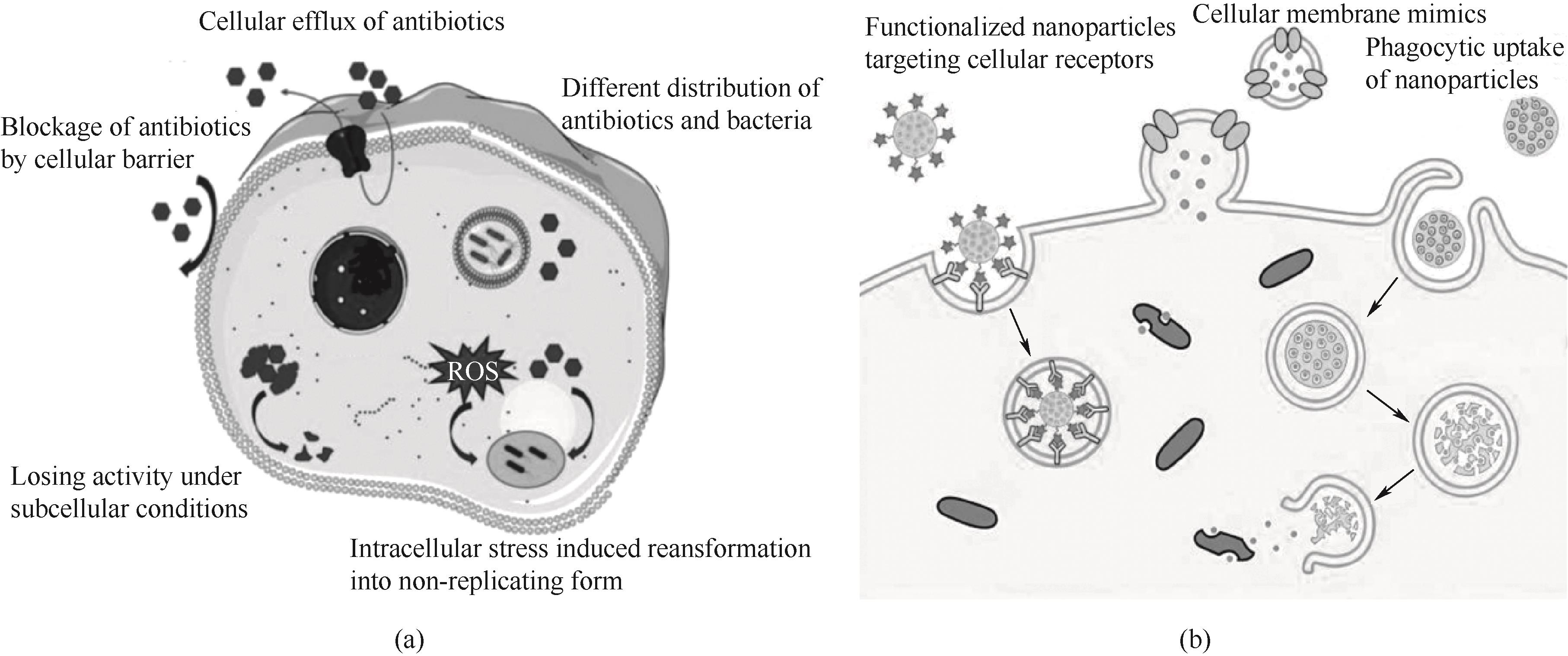
Fig.2 (a) Challenges in the antibiotic treatment of intracellular bacterial infections[60]; (b) Enhancement of internalization mechanisms of drugs by infected cells[7]
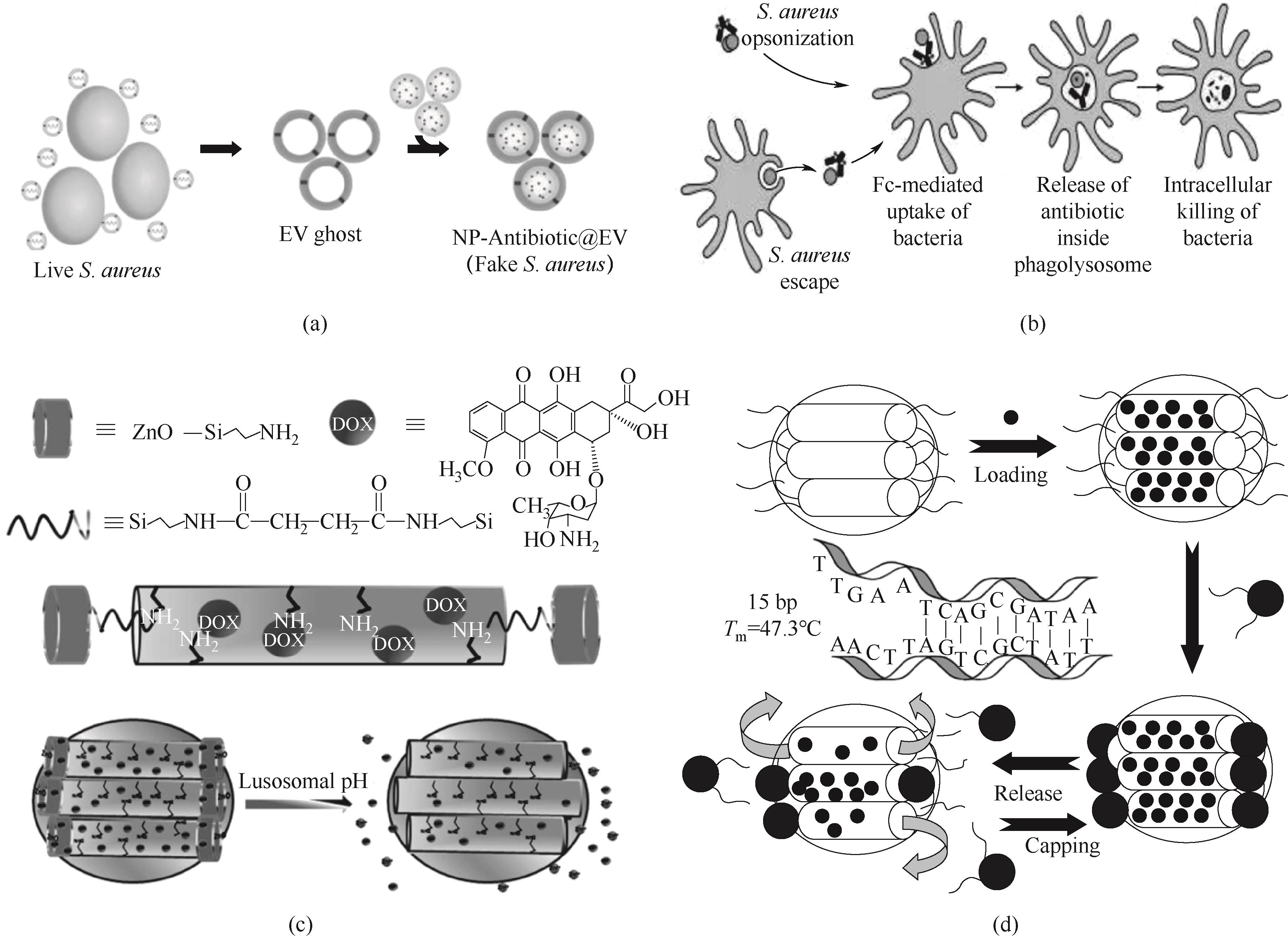
Fig.3 (a) Schematic diagram of preparation of drug nanodelivery system for the treatment of intracellular Staphylococcus aureus infection[83]; (b) Schematic diagram of the action mechanism of AAC[94]; (c) Schematic diagram of synthesis and pH-responsive drug release process of ZnO@MSNs-DOX[96]; (d) Schematic diagram of the release mechanism of magnetic MSNs[98]
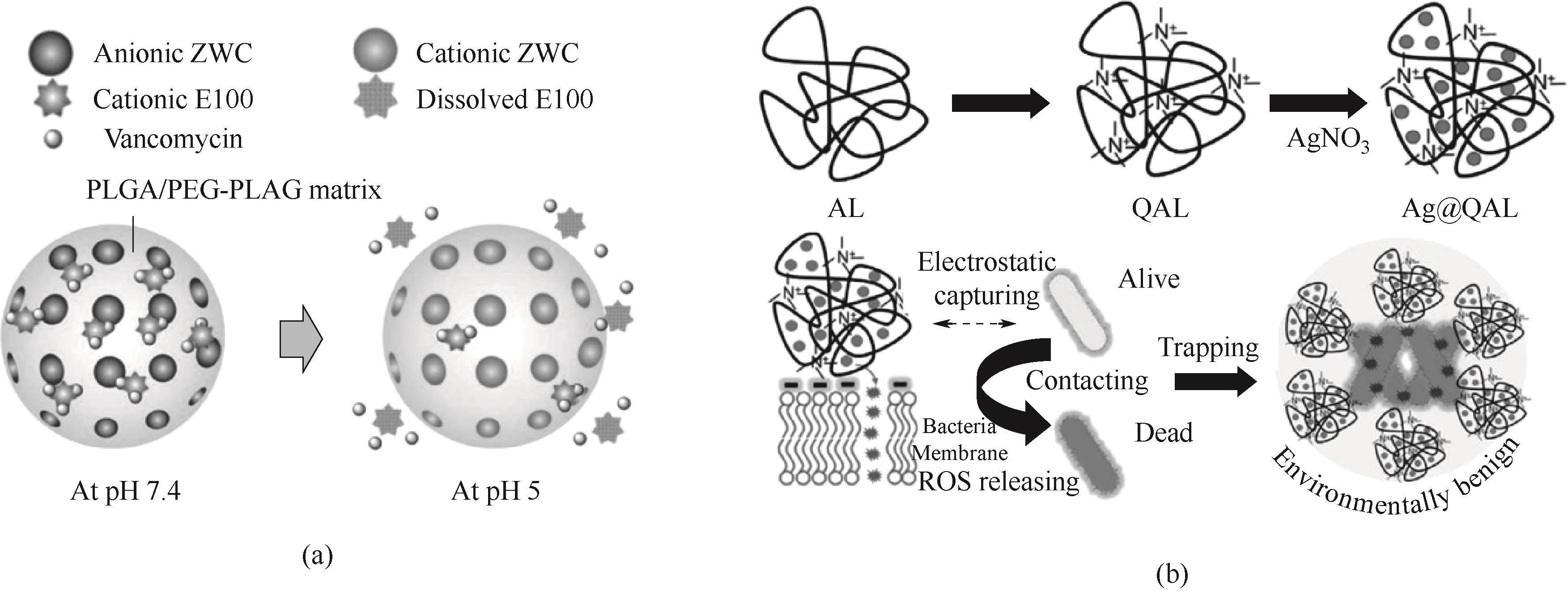
Fig.4 (a) Schematic diagram of pH-sensitive release of vancomycin from polymer nanoparticles[103]; (b) Schematic diagram of the synthesis route and antibacterial process of Ag@QAL[109]
| 4 | Fu A K, Yao B Q, Dong T T, et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer[J]. Cell, 2022, 185(8): 1356-1372. |
| 5 | Galeano Niño J L, Wu H R, LaCourse K D, et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer[J]. Nature, 2022, 611: 810-817. |
| 6 | LaCourse K D, Zepeda-Rivera M, Kempchinsky A G, et al. The cancer chemotherapeutic 5-fluorouracil is a potent Fusobacterium nucleatum inhibitor and its activity is modified by intratumoral microbiota[J]. Cell Reports, 2022, 41(7): 111625. |
| 7 | Subramaniam S, Joyce P, Thomas N, et al. Bioinspired drug delivery strategies for repurposing conventional antibiotics against intracellular infections[J]. Advanced Drug Delivery Reviews, 2021, 177: 113948. |
| 8 | Chen G Y, Roy I, Yang C H, et al. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy[J]. Chemical Reviews, 2016, 116(5): 2826-2885. |
| 9 | Huh A J, Kwon Y J. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era[J]. Journal of Controlled Release, 2011, 156(2): 128-145. |
| 10 | Tang L, Zhang A N, Zhang Z Y, et al. Multifunctional inorganic nanomaterials for cancer photoimmunotherapy[J]. Cancer Communications, 2022, 42(2): 141-163. |
| 11 | Barroso L, Viegas C, Vieira J, et al. Lipid-based carriers for food ingredients delivery[J]. Journal of Food Engineering, 2021, 295: 110451. |
| 12 | Lam S J, Wong E H H, Boyer C, et al. Antimicrobial polymeric nanoparticles[J]. Progress in Polymer Science, 2018, 76: 40-64. |
| 13 | Rösler A, Vandermeulen G W M, Klok H A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers[J]. Advanced Drug Delivery Reviews, 2012, 64: 270-279. |
| 14 | Griffiths G, Nyström B, Sable S B, et al. Nanobead-based interventions for the treatment and prevention of tuberculosis[J]. Nature Reviews Microbiology, 2010, 8: 827-834. |
| 15 | Xiong M H, Li Y J, Bao Y, et al. Bacteria-responsive multifunctional nanogel for targeted antibiotic delivery[J]. Advanced Materials, 2012, 24(46): 6175-6180. |
| 16 | 冯晴晴, 张天鲛, 赵潇, 等. 合成纳米生物学——合成生物学与纳米生物学的交叉前沿[J]. 合成生物学, 2022, 3(2): 260-278. |
| Feng Q Q, Zhang T J, Zhao X, et al. Synthetic nanobiology—fusion of synthetic biology and nanobiology[J]. Synthetic Biology Journal, 2022, 3(2): 260-278. | |
| 17 | Zahid A A, Chakraborty A, Luo W, et al. Tailoring the inherent properties of biobased nanoparticles for nanomedicine[J]. ACS Biomaterials Science & Engineering, 2023, 9(7): 3972-3986. |
| 18 | Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles[J]. Annual Review of Cell and Developmental Biology, 2014, 30: 255-289. |
| 19 | Mathieu M, Martin-Jaular L, Lavieu G, et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication[J]. Nature Cell Biology, 2019, 21: 9-17. |
| 20 | Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function[J]. Nature Reviews Immunology, 2002, 2: 569-579. |
| 21 | Ibrahim A, Marbán E. Exosomes: fundamental biology and roles in cardiovascular physiology[J]. Annual Review of Physiology, 2016, 78: 67-83. |
| 22 | Kanada M, Bachmann M H, Hardy J W, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(12): E1433-E1442. |
| 23 | Alvarez-Erviti L, Seow Y, Yin H F, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes[J]. Nature Biotechnology, 2011, 29: 341-345. |
| 24 | Yang X H, Shi G M, Guo J, et al. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus [J]. International Journal of Nanomedicine, 2018, 13: 8095-8104. |
| 25 | He Y C, Cong C, Li L, et al. Sequential intra-intercellular delivery of nanomedicine for deep drug-resistant solid tumor penetration[J]. ACS Applied Materials & Interfaces, 2020, 12(8): 8978-8988. |
| 26 | Nokhodchi A, Ghafourian T, Mohammadi G. Nanotechnology tools for efficient antibacterial delivery to Salmonella [M]//Salmonella — A Diversified Superbug. London: InTech, 2012. |
| 27 | Puglia C, Bonina F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals[J]. Expert Opinion on Drug Delivery, 2012, 9(4): 429-441. |
| 28 | Haider M, Abdin S M, Kamal L, et al. Nanostructured lipid carriers for delivery of chemotherapeutics: a review[J]. Pharmaceutics, 2020, 12(3): 288. |
| 29 | Viegas C, Patrício A B, Prata J M, et al. Solid lipid nanoparticles vs. nanostructured lipid carriers: a comparative review[J]. Pharmaceutics, 2023, 15(6): 1593. |
| 30 | Xie S Y, Yang F, Tao Y F, et al. Enhanced intracellular delivery and antibacterial efficacy of enrofloxacin-loaded docosanoic acid solid lipid nanoparticles against intracellular Salmonella [J]. Scientific Reports, 2017, 7: 41104. |
| 31 | Doktorovová S, Kovačević A B, Garcia M L, et al. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2016, 108: 235-252. |
| 32 | 邬欣, 曾利胜, 王剑龙, 等. 无机纳米颗粒在癌症治疗中的研究进展[J]. 材料导报, 2021, 35(S1): 87-93. |
| Wu X, Zeng L S, Wang J L, et al. Research progress of inorganic nanoparticles in cancer therapy[J]. Materials Reports, 2021, 35(S1): 87-93. | |
| 33 | Eleraky N E, Allam A, Hassan S B, et al. Nanomedicine fight against antibacterial resistance: an overview of the recent pharmaceutical innovations[J]. Pharmaceutics, 2020, 12(2): 142. |
| 34 | Malaekeh-Nikouei B, Fazly Bazzaz B S, Mirhadi E, et al. The role of nanotechnology in combating biofilm-based antibiotic resistance[J]. Journal of Drug Delivery Science and Technology, 2020, 60: 101880. |
| 35 | Spirescu V A, Chircov C, Grumezescu A M, et al. Polymeric nanoparticles for antimicrobial therapies: an up-to-date overview[J]. Polymers, 2021, 13(5): 724. |
| 36 | Wang L L, Hu C, Shao L Q. The antimicrobial activity of nanoparticles: present situation and prospects for the future[J]. International Journal of Nanomedicine, 2017, 12: 1227-1249. |
| 37 | Varier K M, Gudeppu M, Chinnasamy A, et al. Nanoparticles: antimicrobial applications and its prospects[M]//Advanced Nanostructured Materials for Environmental Remediation. Cham: Springer, 2019: 321-355. |
| 38 | Lee N Y, Ko W C, Hsueh P R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms[J]. Frontiers in Pharmacology, 2019, 10: 1153. |
| 39 | Ahlawat J, Hooda R, Sharma M, et al. Nanoparticles in biomedical applications[M]//Patra J, Fraceto L, Das G, et al. Green Nanoparticles. Cham: Springer, 2020: 227-250. |
| 40 | Anderson S D, Gwenin V V, Gwenin C D. Magnetic functionalized nanoparticles for biomedical, drug delivery and imaging applications[J]. Nanoscale Research Letters, 2019, 14: 188. |
| 41 | Kurtjak M, Aničić N, Vukomanovicć M. Inorganic nanoparticles: innovative tools for antimicrobial agents[M]//Antibacterial Agents. Londen: InTech, 2017. |
| 42 | Lai C Y, Trewyn B G, Jeftinija D M, et al. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules[J]. Journal of the American Chemical Society, 2003, 125(15): 4451-4459. |
| 43 | Shen D K, Yang J P, Li X M, et al. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres[J]. Nano Letters, 2014, 14(2): 923-932. |
| 44 | Argyo C, Weiss V, Bräuchle C, et al. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery[J]. Chemistry of Materials, 2014, 26(1): 435-451. |
| 45 | Clemens D L, Lee B Y, Xue M, et al. Targeted intracellular delivery of antituberculosis drugs to Mycobacterium tuberculosis-infected macrophages via functionalized mesoporous silica nanoparticles[J]. Antimicrobial Agents and Chemotherapy, 2012, 56(5): 2535-2545. |
| 46 | Kang J, Dietz M J, Hughes K, et al. Silver nanoparticles present high intracellular and extracellular killing against Staphylococcus aureus [J]. Journal of Antimicrobial Chemotherapy, 2019, 74(6): 1578-1585. |
| 47 | Ichimaru H, Harada A, Yoshimoto S, et al. Gold coating of silver nanoplates for enhanced dispersion stability and efficient antimicrobial activity against intracellular bacteria[J]. Langmuir, 2018, 34(35): 10413-10418. |
| 48 | Lacoma A, Usón L, Mendoza G, et al. Novel intracellular antibiotic delivery system against Staphylococcus aureus: cloxacillin-loaded poly(d,l-lactide-co-glycolide) acid nanoparticles [J]. Nanomedicine, 2020, 15(12): 1189-1203. |
| 49 | Zaki N M, Hafez M M. Enhanced antibacterial effect of ceftriaxone sodium-loaded chitosan nanoparticles against intracellular Salmonella typhimurium [J]. AAPS PharmSciTech, 2012, 13: 411-421. |
| 50 | Nguyen T K, Lam S J, Ho K K K, et al. Rational design of single-chain polymeric nanoparticles that kill planktonic and biofilm bacteria[J]. ACS Infectious Diseases, 2017, 3(3): 237-248. |
| 51 | Lenoir S, Pagnoulle C, Detrembleur C, et al. New antibacterial cationic surfactants prepared by atom transfer radical polymerization[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2006, 44(3): 1214-1224. |
| 52 | Qiao Y, Yang C, Coady D J, et al. Highly dynamic biodegradable micelles capable of lysing Gram-positive and Gram-negative bacterial membrane[J]. Biomaterials, 2012, 33(4): 1146-1153. |
| 53 | Qu S Q, Han Y M, Liu Y, et al. Milk exosomes facilitate oral delivery of drugs against intestinal bacterial infections[J]. Journal of Agricultural and Food Chemistry, 2022, 70(51): 16069-16079. |
| 54 | Sun D M, Zhuang X Y, Xiang X Y, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes[J]. Molecular Therapy, 2010, 18(9): 1606-1614. |
| 55 | Natsaridis E, Gkartziou F, Mourtas S, et al. Moxifloxacin liposomes: effect of liposome preparation method on physicochemical properties and antimicrobial activity against Staphylococcus epidermidis [J]. Pharmaceutics, 2022, 14(2): 370. |
| 56 | Karimi N, Ghanbarzadeh B, Hamishehkar H, et al. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC)[J]. Colloid and Interface Science Communications, 2018, 22: 18-24. |
| 57 | Subramaniam S, Thomas N, Gustafsson H, et al. Rifampicin-loaded mesoporous silica nanoparticles for the treatment of intracellular infections[J]. Antibiotics, 2019, 8(2): 39. |
| 58 | Aguilar-Colomer A, Colilla M, Izquierdo-Barba I, et al. Impact of the antibiotic-cargo from MSNs on Gram-positive and Gram-negative bacterial biofilms[J]. Microporous and Mesoporous Materials, 2021, 311: 110681. |
| 59 | Xie S Y, Tao Y F, Pan Y H, et al. Biodegradable nanoparticles for intracellular delivery of antimicrobial agents[J]. Journal of Controlled Release, 2014, 187: 101-117. |
| 60 | Wang C, Yang Y, Cao Y Y, et al. Nanocarriers for the delivery of antibiotics into cells against intracellular bacterial infection[J]. Biomaterials Science, 2023, 11(2): 432-444. |
| 61 | Gustafson H H, Holt-Casper D, Grainger D W, et al. Nanoparticle uptake: the phagocyte problem[J]. Nano Today, 2015, 10(4): 487-510. |
| 62 | Blanco E, Shen H F, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery[J]. Nature Biotechnology, 2015, 33: 941-951. |
| 63 | Vonarbourg A, Passirani C, Saulnier P, et al. Parameters influencing the stealthiness of colloidal drug delivery systems[J]. Biomaterials, 2006, 27(24): 4356-4373. |
| 64 | Zhao J C, Stenzel M H. Entry of nanoparticles into cells: the importance of nanoparticle properties[J]. Polymer Chemistry, 2018, 9(3): 259-272. |
| 65 | Cheng X J, Tian X, Wu A Q, et al. Protein corona influences cellular uptake of gold nanoparticles by phagocytic and nonphagocytic cells in a size-dependent manner[J]. ACS Applied Materials & Interfaces, 2015, 7(37): 20568-20575. |
| 66 | Hadji H, Bouchemal K. Effect of micro- and nanoparticle shape on biological processes[J]. Journal of Controlled Release, 2022, 342: 93-110. |
| 67 | Chen R Y, Pu X Q, Liu R R, et al. Biocompatible snowman-like dimer nanoparticles for improved cellular uptake in intrahepatic cholangiocarcinoma[J]. Pharmaceutics, 2023, 15(8): 2132. |
| 68 | Yue Z G, Wei W, You Z X, et al. Iron oxide nanotubes for magnetically guided delivery and pH-activated release of insoluble anticancer drugs[J]. Advanced Functional Materials, 2011, 21(18): 3446-3453. |
| 69 | Lagarrigue P, Moncalvo F, Cellesi F. Non-spherical polymeric nanocarriers for therapeutics: the effect of shape on biological systems and drug delivery properties[J]. Pharmaceutics, 2022, 15(1): 32. |
| 70 | Ahsan F, Rivas I P, Khan M A, et al. Targeting to macrophages: role of physicochemical properties of particulate carriers-liposomes and microspheres-on the phagocytosis by macrophages[J]. Journal of Controlled Release, 2002, 79(1/2/3): 29-40. |
| 71 | Fröhlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles[J]. International Journal of Nanomedicine, 2012, 7: 5577-5591. |
| 72 | 王好平, 罗根祥, 刘春生, 等. 球型胶体颗粒的表面电位和表面电荷密度的关系[J]. 高等学校化学学报, 2005, 26(4): 754-756. |
| Wang H P, Luo G X, Liu C S, et al. Relationship between surface charge density and surface potential of spherical colloidal particle[J]. Chemical Journal of Chinese Universities, 2005, 26(4): 754-756. | |
| 73 | He C B, Hu Y P, Yin L C, et al. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles[J]. Biomaterials, 2010, 31(13): 3657-3666. |
| 74 | Maghrebi S, Jambhrunkar M, Joyce P, et al. Engineering PLGA-lipid hybrid microparticles for enhanced macrophage uptake[J]. ACS Applied Bio Materials, 2020, 3(7): 4159-4167. |
| 75 | Saha K, Rahimi M, Yazdani M, et al. Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona[J]. ACS Nano, 2016, 10(4): 4421-4430. |
| 76 | Sanchez L, Yi Y, Yu Y. Effect of partial PEGylation on particle uptake by macrophages[J]. Nanoscale, 2017, 9(1): 288-297. |
| 77 | Tian H, Luo Z Y, Liu L L, et al. Cancer cell membrane-biomimetic oxygen nanocarrier for breaking hypoxia-induced chemoresistance[J]. Advanced Functional Materials, 2017, 27(38): 1703197. |
| 78 | Sun H P, Su J H, Meng Q S, et al. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer[J]. Advanced Functional Materials, 2017, 27(3): 1604300. |
| 79 | Rao L, Cai B, Bu L L, et al. Microfluidic electroporation-facilitated synthesis of erythrocyte membrane-coated magnetic nanoparticles for enhanced imaging-guided cancer therapy[J]. ACS Nano, 2017, 11(4): 3496-3505. |
| 80 | Silva A K, Di Corato R, Pellegrino T, et al. Cell-derived vesicles as a bioplatform for the encapsulation of theranostic nanomaterials[J]. Nanoscale, 2013, 5(23): 11374-11384. |
| 81 | Copp J A, Fang R H, Luk B T, et al. Clearance of pathological antibodies using biomimetic nanoparticles[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(37): 13481-13486. |
| 82 | 赵静, 刘艳, 解军波, 等. 中药纳米递药系统的研究进展[J]. 中华中医药学刊, 2022, 40(5): 134-137, 276. |
| Zhao J, Liu Y, Xie J B, et al. Research progress of nano-drug delivery system of traditional Chinese medicine[J]. Chinese Archives of Traditional Chinese Medicine, 2022, 40(5): 134-137, 276. | |
| 83 | Gao F, Xu L L, Yang B Q, et al. Kill the real with the fake: eliminate intracellular Staphylococcus aureus using nanoparticle coated with its extracellular vesicle membrane as active-targeting drug carrier[J]. ACS Infectious Diseases, 2019, 5(2): 218-227. |
| 84 | Pumerantz A, Muppidi K, Agnihotri S, et al. Preparation of liposomal vancomycin and intracellular killing of meticillin-resistant Staphylococcus aureus (MRSA)[J]. International Journal of Antimicrobial Agents, 2011, 37(2): 140-144. |
| 85 | Zhang C X, Zhao W Y, Bian C, et al. Antibiotic-derived lipid nanoparticles to treat intracellular Staphylococcus aureus [J]. ACS Applied Bio Materials, 2019, 2(3): 1270-1277. |
| 86 | Kumari S, Mg S, Mayor S. Endocytosis unplugged: multiple ways to enter the cell[J]. Cell Research, 2010, 20(3): 256-275. |
| 87 | Nishimura Y, Takeda K, Ezawa R, et al. A display of pH-sensitive fusogenic GALA peptide facilitates endosomal escape from a bio-nanocapsule via an endocytic uptake pathway[J]. Journal of Nanobiotechnology, 2014, 12: 11. |
| 88 | Allolio C, Magarkar A, Jurkiewicz P, et al. Arginine-rich cell-penetrating peptides induce membrane multilamellarity and subsequently enter via formation of a fusion pore[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(47): 11923-11928. |
| 89 | 徐柳, 钱晨, 朱辰奇, 等. 基于多肽的纳米药物递送系统的研究[J]. 化学进展, 2018, 30(9): 1341-1348. |
| Xu L, Qian C, Zhu C Q, et al. The study of peptides nanomedicine for drug delivery systems[J]. Progress in Chemistry, 2018, 30(9): 1341-1348 | |
| 90 | Ding X, Yang C, Lim T P, et al. Antibacterial and antifouling catheter coatings using surface grafted PEG-b-cationic polycarbonate diblock copolymers[J]. Biomaterials, 2012, 33(28): 6593-6603. |
| 91 | Kullberg M, Owens J L, Mann K. Listeriolysin O enhances cytoplasmic delivery by Her-2 targeting liposomes[J]. Journal of Drug Targeting, 2010, 18(4): 313-320. |
| 92 | Ding L, Yao C J, Yin X F, et al. Size, shape, and protein corona determine cellular uptake and removal mechanisms of gold nanoparticles[J]. Small, 2018, 14(42): 1801451. |
| 93 | Feng W L, Li G F, Kang X X, et al. Cascade-targeting poly(amino acid) nanoparticles eliminate intracellular bacteria via on-site antibiotic delivery[J]. Advanced Materials, 2022, 34(12): 2109789. |
| 94 | Lehar S M, Pillow T, Xu M, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus [J]. Nature, 2015, 527: 323-328. |
| 95 | Mudakavi R J, Vanamali S, Chakravortty D, et al. Development of arginine based nanocarriers for targeting and treatment of intracellular Salmonella [J]. RSC Advances, 2017, 7(12): 7022-7032. |
| 96 | Muhammad F, Guo M Y, Qi W X, et al. pH-triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids[J]. Journal of the American Chemical Society, 2011, 133(23): 8778-8781. |
| 97 | Frasconi M, Liu Z C, Lei J Y, et al. Photoexpulsion of surface-grafted ruthenium complexes and subsequent release of cytotoxic cargos to cancer cells from mesoporous silica nanoparticles[J]. Journal of the American Chemical Society, 2013, 135(31): 11603-11613. |
| 98 | Ruiz-Hernández E, Baeza A, Vallet-Regí M. Smart drug delivery through DNA/magnetic nanoparticle gates[J]. ACS Nano, 2011, 5(2): 1259-1266. |
| 99 | Menina S, Labouta H I, Geyer R, et al. Invasin-functionalized liposome nanocarriers improve the intracellular delivery of anti-infective drugs[J]. RSC Advances, 2016, 6(47): 41622-41629. |
| 100 | Manganiello M J, Cheng C, Convertine A J, et al. Diblock copolymers with tunable pH transitions for gene delivery[J]. Biomaterials, 2012, 33(7): 2301-2309. |
| 101 | Wei T, Yu Q, Zhan W J, et al. A smart antibacterial surface for the on-demand killing and releasing of bacteria[J]. Advanced Healthcare Materials, 2016, 5(4): 449-456. |
| 102 | Fenaroli F, Robertson J D, Scarpa E, et al. Polymersomes eradicating intracellular bacteria[J]. ACS Nano, 2020, 14(7): 8287-8298. |
| 103 | Pei Y H, Mohamed M F, Seleem M N, et al. Particle engineering for intracellular delivery of vancomycin to methicillin-resistant Staphylococcus aureus (MRSA)-infected macrophages[J]. Journal of Controlled Release, 2017, 267: 133-143. |
| 104 | Obuobi S, Julin K, Fredheim E G A, et al. Liposomal delivery of antibiotic loaded nucleic acid nanogels with enhanced drug loading and synergistic anti-inflammatory activity against S. aureus intracellular infections[J]. Journal of Controlled Release, 2020, 324: 620-632. |
| 105 | Yu Y J, Yan J H, Chen Q W, et al. Polymeric nano-system for macrophage reprogramming and intracellular MRSA eradication[J]. Journal of Controlled Release, 2023, 353: 591-610. |
| 106 | Liu J Y, Pang Y, Zhu Z Y, et al. Therapeutic nanocarriers with hydrogen peroxide-triggered drug release for cancer treatment[J]. Biomacromolecules, 2013, 14(5): 1627-1636. |
| 107 | Wang Z Y, Sheng L N, Yang X X, et al. Natural biomass-derived carbon dots as potent antimicrobial agents against multidrug-resistant bacteria and their biofilms[J]. Sustainable Materials and Technologies, 2023, 36: e00584. |
| 108 | Kang X X, Bu F Q, Feng W L, et al. Dual-cascade responsive nanoparticles enhance pancreatic cancer therapy by eliminating tumor-resident intracellular bacteria[J]. Advanced Materials, 2022, 34(49): 2206765. |
| 109 | Wang Y L, Li Z X, Yang D J, et al. Microwave-mediated fabrication of silver nanoparticles incorporated lignin-based composites with enhanced antibacterial activity via electrostatic capture effect[J]. Journal of Colloid and Interface Science, 2021, 583: 80-88. |
| 110 | Liu Y, Shi L Q, Su L Z, et al. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control[J]. Chemical Society Reviews, 2019, 48(2): 428-446. |
| 1 | Mak T W, Saunders M E. The Mmmune Response: Basic and Clinical Principles[M]. NY, US: Academic Press, 2005. |
| 2 | Zou J, Shankar N. The opportunistic pathogen Enterococcus faecalis resists phagosome acidification and autophagy to promote intracellular survival in macrophages[J]. Cellular Microbiology, 2016, 18(6): 831-843. |
| 3 | Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria[J]. Science, 2020, 368(6494): 973-980. |
| 111 | Yan L X, Chen L J, Zhao X, et al. pH switchable nanoplatform for in vivo persistent luminescence imaging and precise photothermal therapy of bacterial infection[J]. Advanced Functional Materials, 2020, 30(14): 1909042. |
| [1] | Lu DENG, Xiaojie JU, Wenjie ZHANG, Rui XIE, Wei WANG, Zhuang LIU, Dawei PAN, Liangyin CHU. Controllable preparation of radioactive chitosan embolic microspheres by microfluidic method [J]. CIESC Journal, 2023, 74(4): 1781-1794. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||