CIESC Journal ›› 2023, Vol. 74 ›› Issue (8): 3353-3365.DOI: 10.11949/0438-1157.20230499
• Catalysis, kinetics and reactors • Previous Articles Next Articles
Rubin ZENG1,2( ), Zhongjie SHEN1,2(
), Zhongjie SHEN1,2( ), Qinfeng LIANG1,2, Jianliang XU1,2, Zhenghua DAI1,2,3, Haifeng LIU1,2
), Qinfeng LIANG1,2, Jianliang XU1,2, Zhenghua DAI1,2,3, Haifeng LIU1,2
Received:2023-05-23
Revised:2023-07-25
Online:2023-10-18
Published:2023-08-25
Contact:
Zhongjie SHEN
曾如宾1,2( ), 沈中杰1,2(
), 沈中杰1,2( ), 梁钦锋1,2, 许建良1,2, 代正华1,2,3, 刘海峰1,2
), 梁钦锋1,2, 许建良1,2, 代正华1,2,3, 刘海峰1,2
通讯作者:
沈中杰
作者简介:曾如宾(1999—),男,硕士研究生,y82210127@mail.ecust.edu.cn
基金资助:CLC Number:
Rubin ZENG, Zhongjie SHEN, Qinfeng LIANG, Jianliang XU, Zhenghua DAI, Haifeng LIU. Study of the sintering mechanism of Fe2O3 nanoparticles based on molecular dynamics simulation[J]. CIESC Journal, 2023, 74(8): 3353-3365.
曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365.
Add to citation manager EndNote|Ris|BibTeX
| 原子对 | Aij /eV | Bij /Å | Cij /(eV/Å6) |
|---|---|---|---|
| Charge | 采用固定电荷值为 | ||
| Fe-O | 62775.704 | 0.165 | 32.055 |
| O-O | 3834.644 | 0.305 | 123.029 |
| Fe-Fe | 2500.943 | 0.029 | 6.383 |
Table 1 Potential parameters of the Buckingham-Coulomb potential in this study[26]
| 原子对 | Aij /eV | Bij /Å | Cij /(eV/Å6) |
|---|---|---|---|
| Charge | 采用固定电荷值为 | ||
| Fe-O | 62775.704 | 0.165 | 32.055 |
| O-O | 3834.644 | 0.305 | 123.029 |
| Fe-Fe | 2500.943 | 0.029 | 6.383 |
| 模拟编号 | 粒径D0/nm | 温度T/K | 空位浓度Cv/% | 原子数 |
|---|---|---|---|---|
| 1~6 | 4 | 300、900、1000、1100、1200、1300 | 0 | 6660 |
| 7~11 | 4 | 900、1000、1100、1200、1300 | 10.0 | 5980 |
| 12 | 4 | 1300 | 7.5 | 6150 |
| 13 | 4 | 1300 | 5.0 | 6310 |
| 14 | 4 | 1300 | 2.5 | 6480 |
| 15 | 3 | 1300 | 0 | 2950 |
| 16 | 5 | 1300 | 0 | 13060 |
Table 2 Configuration of initial structural parameters for all models
| 模拟编号 | 粒径D0/nm | 温度T/K | 空位浓度Cv/% | 原子数 |
|---|---|---|---|---|
| 1~6 | 4 | 300、900、1000、1100、1200、1300 | 0 | 6660 |
| 7~11 | 4 | 900、1000、1100、1200、1300 | 10.0 | 5980 |
| 12 | 4 | 1300 | 7.5 | 6150 |
| 13 | 4 | 1300 | 5.0 | 6310 |
| 14 | 4 | 1300 | 2.5 | 6480 |
| 15 | 3 | 1300 | 0 | 2950 |
| 16 | 5 | 1300 | 0 | 13060 |

Fig.7 Sintering process of nanoparticles under different temperatures(a) shrinkage rate; (b) gyration radius; (c) neck width; (d) number of atoms in the neck
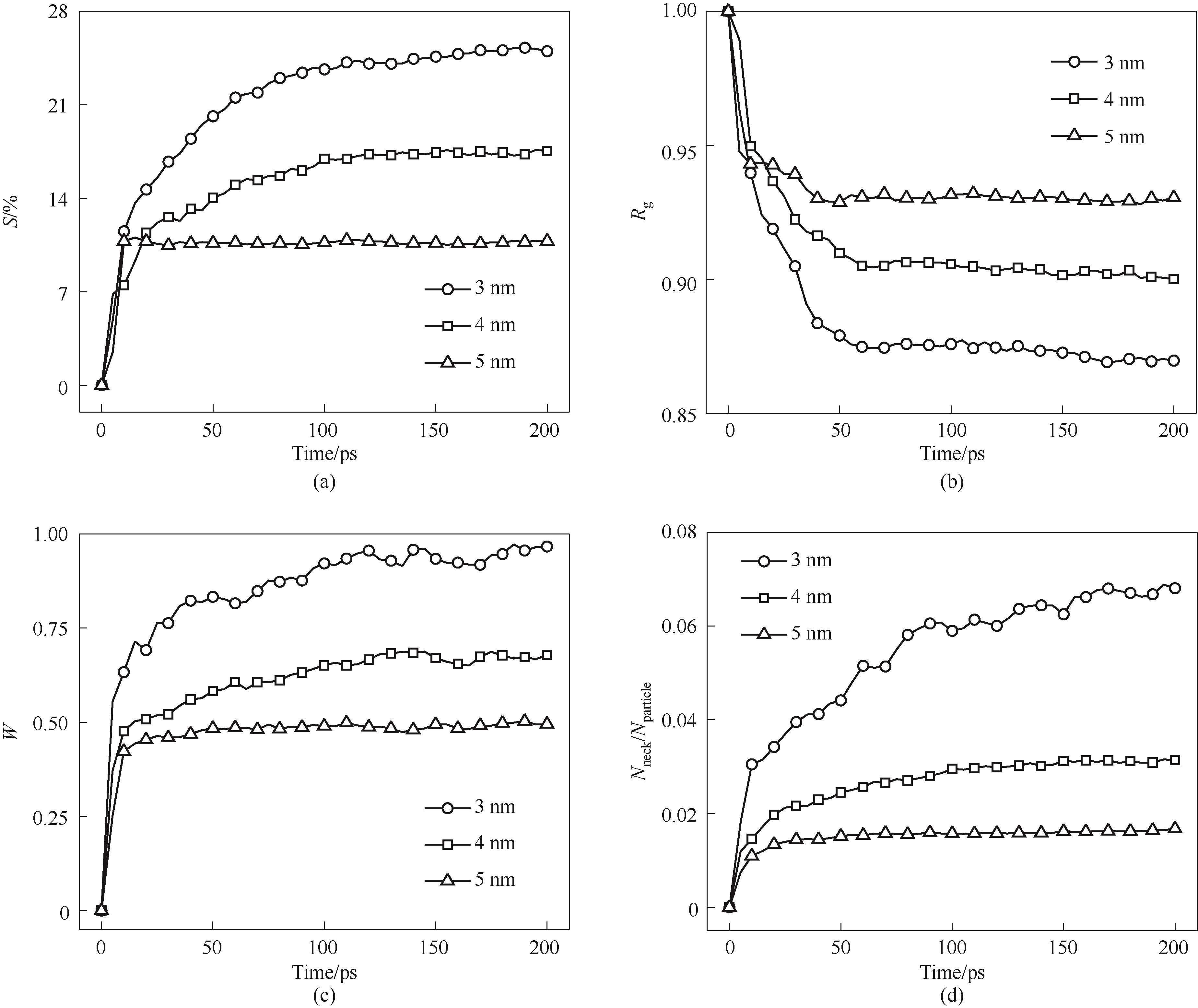
Fig.9 Sintering process of nanoparticles with different particle sizes(a) shrinkage rate; (b) normalized gyration radius; (c) normalized neck width; (d) number of atoms in the neck/total number of atoms
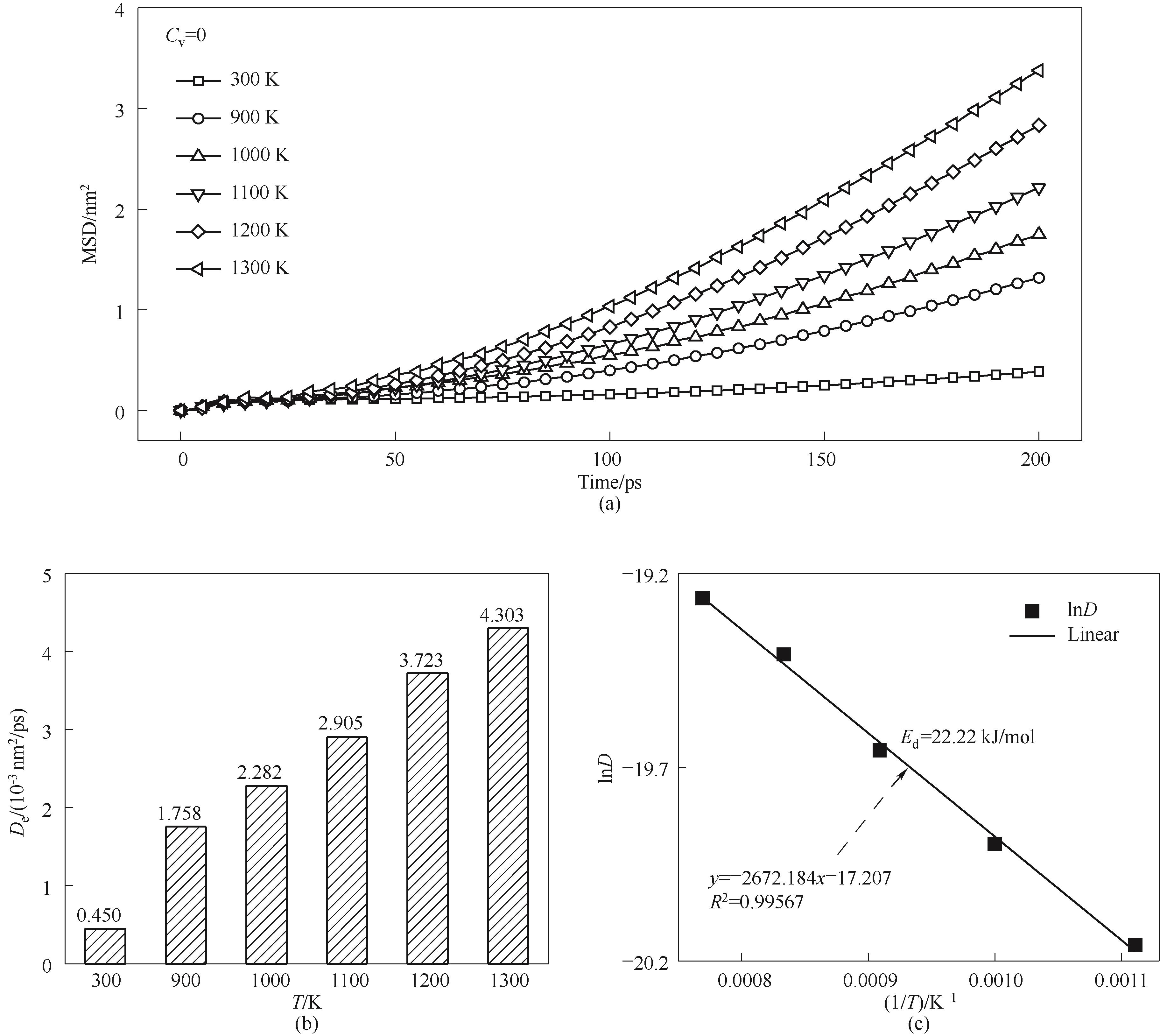
Fig.10 Atomic diffusion properties of sintering simulations under different temperatures(a) MSD curves; (b) diffusion coefficient; (c) fitted diffusion activation energy
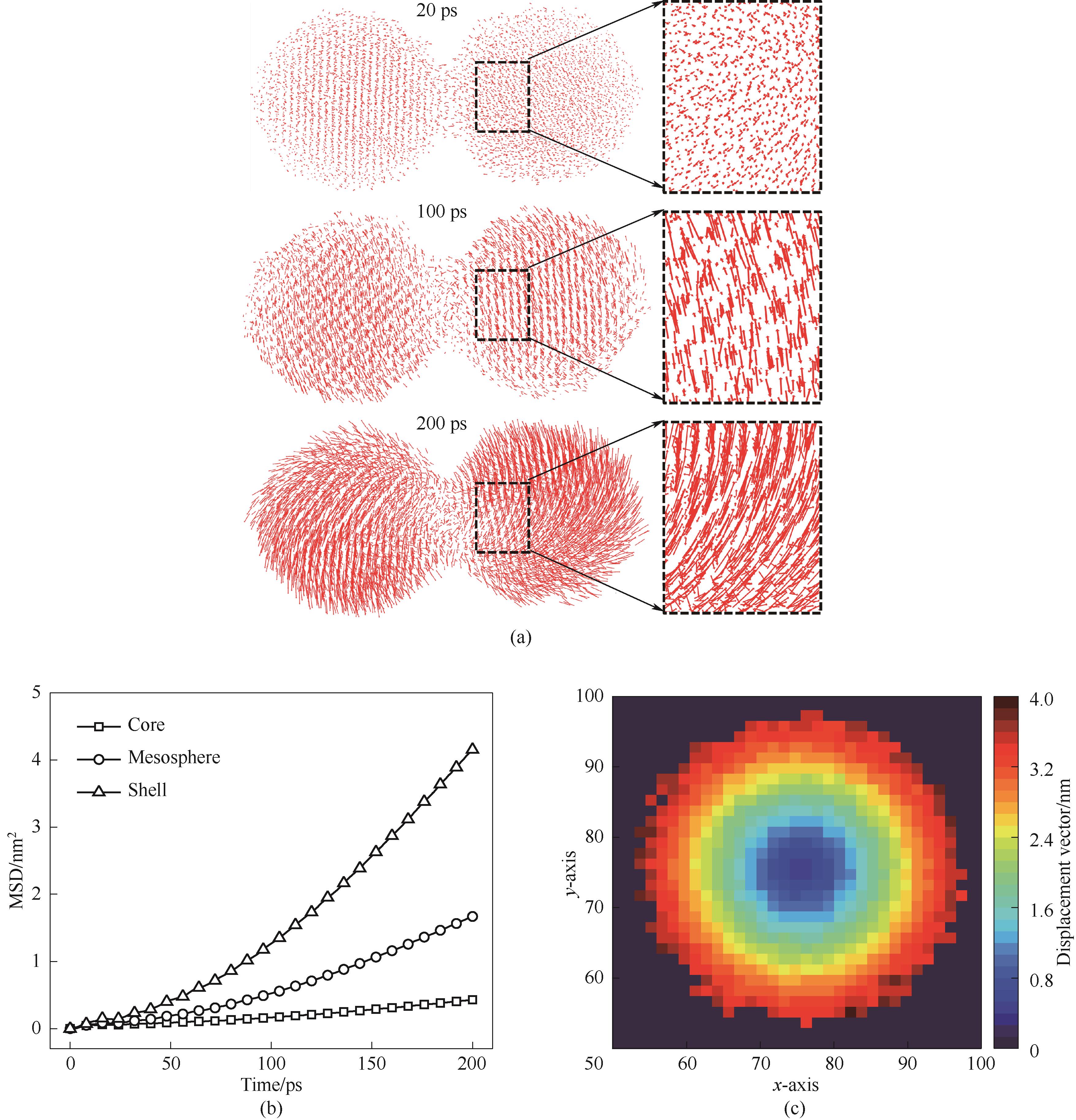
Fig.11 Atomic migration properties in 1300 K sintering simulations(a) atomic displacement vectors; (b) MSD of different layers of atoms; (c) single particle atomic displacement

Fig.13 Crystal structure evolution of sintered nanoparticles at 1300 K simulation(a) changes in crystal structure (blue — BCC, red — HCP, yellow — amorphous); (b) changes in proportions of different structures
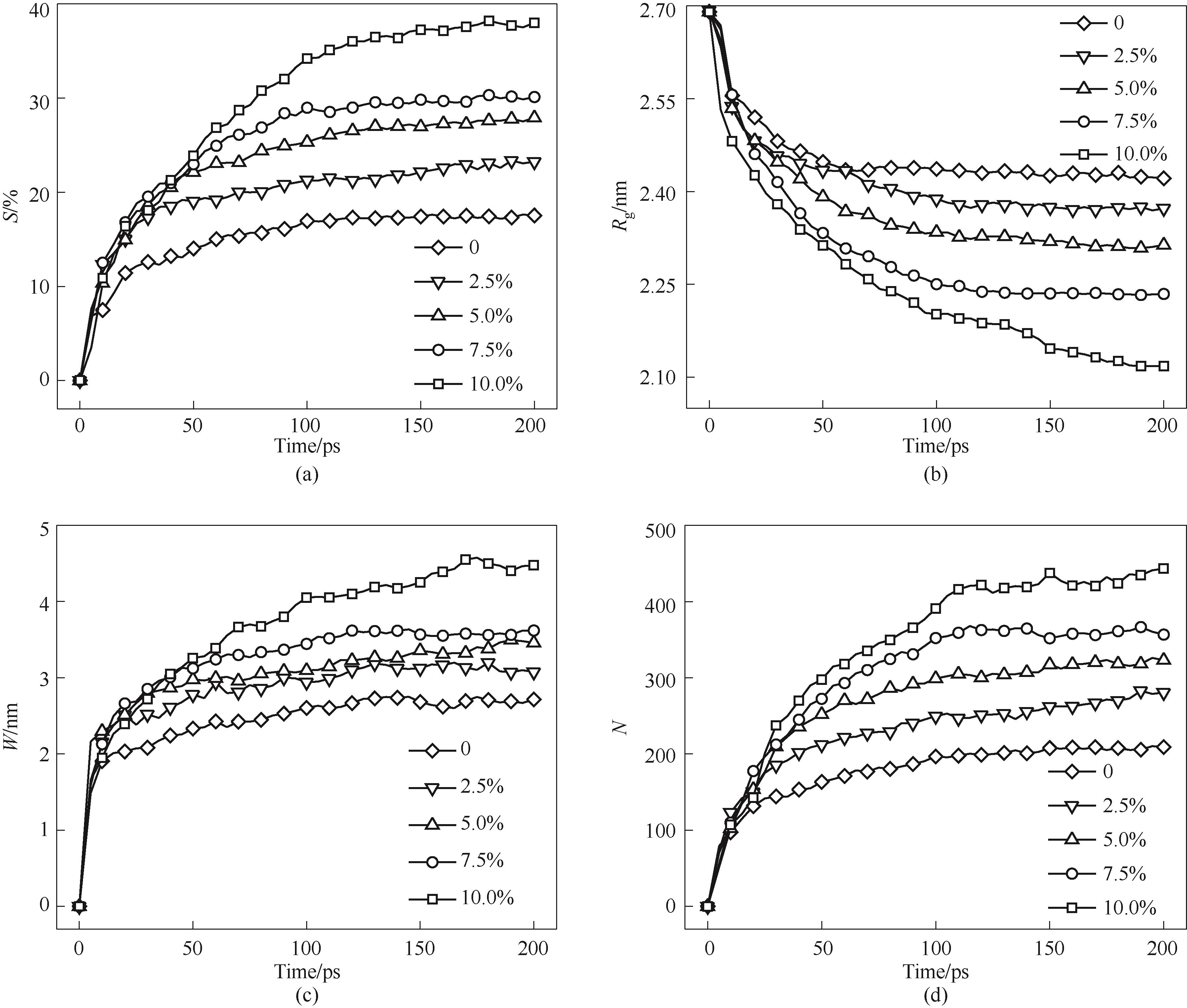
Fig.15 Sintering process of nanoparticles with different vacancy concentrations(a) shrinkage rate; (b) gyration radius; (c) neck width; (d) number of atoms in the neck
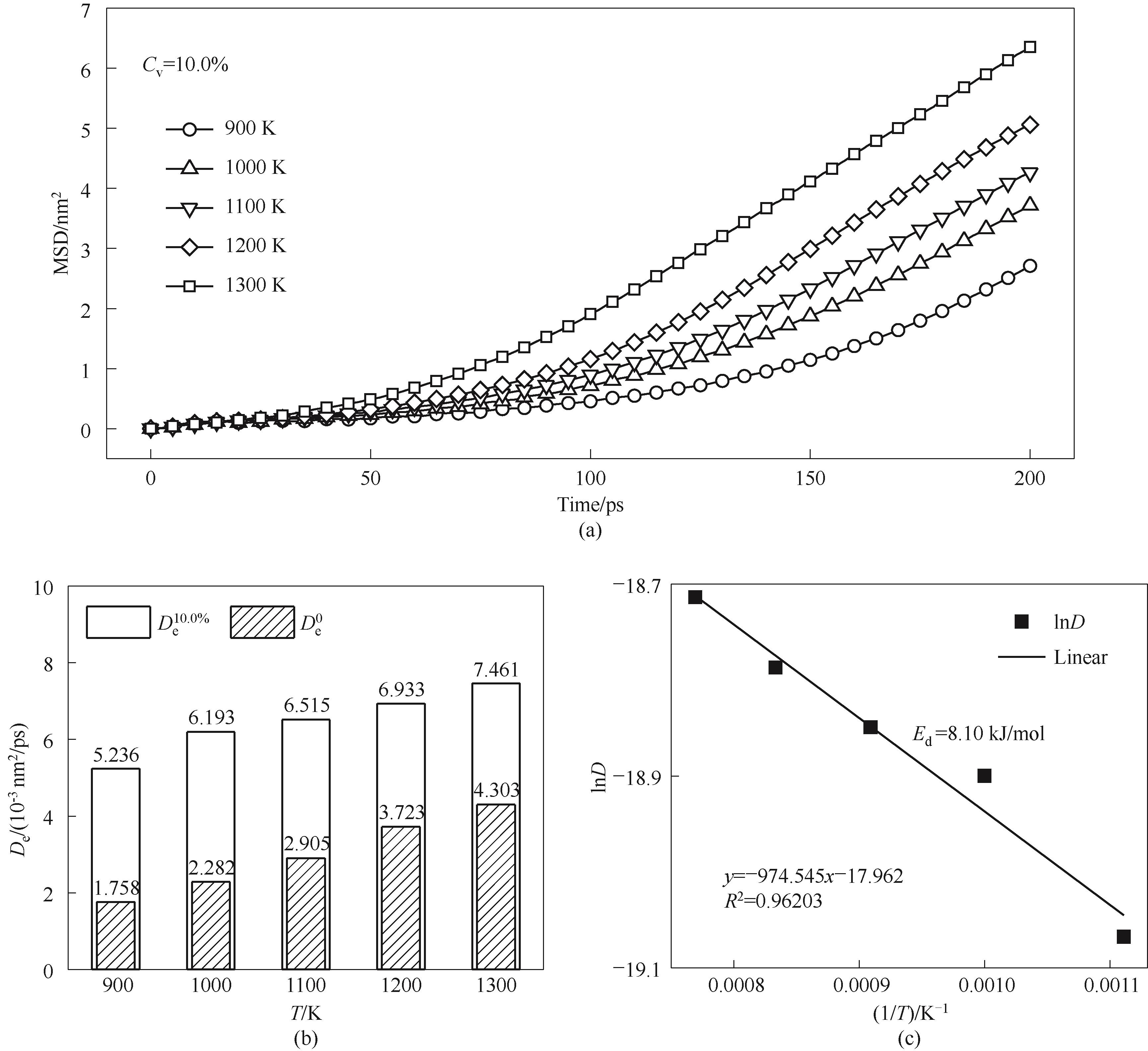
Fig.16 Atomic diffusion characteristics of Cv=10.0% nanoparticles sintered under different temperatures(a) MSD curves; (b) diffusion coefficients; (c) fitted diffusion activation energy
| 1 | Mishra M, Chun D M. α-Fe2O3 as a photocatalytic material: a review[J]. Applied Catalysis A: General, 2015, 498: 126-141. |
| 2 | Pourmadadi M, Rahmani E, Shamsabadipour A, et al. Role of iron oxide (Fe2O3) nanocomposites in advanced biomedical applications: a state-of-the-art review[J]. Nanomaterials, 2022, 12(21): 3873. |
| 3 | Kumar S, Kumar M, Singh A. Synthesis and characterization of iron oxide nanoparticles (Fe2O3, Fe3O4): a brief review[J]. Contemporary Physics, 2021, 62(3): 144-164. |
| 4 | Zeng Y X, Yu M H, Meng Y E, et al. Iron-based supercapacitor electrodes: advances and challenges[J]. Advanced Energy Materials, 2016, 6(24): 1601053. |
| 5 | Qasim M, Ayoub M, Ghazali N A, et al. Recent advances and development of various oxygen carriers for the chemical looping combustion process: a review[J]. Industrial & Engineering Chemistry Research, 2021, 60(24): 8621-8641. |
| 6 | Fan J J, Qiu G Z, Jiang T, et al. Roasting properties of pellets with iron concentrate of complex mineral composition[J]. Journal of Iron and Steel Research, International, 2011, 18(7): 1-7. |
| 7 | Forsmo S P E, Forsmo S E, Samskog P O, et al. Mechanisms in oxidation and sintering of magnetite iron ore green pellets[J]. Powder Technology, 2008, 183(2): 247-259. |
| 8 | Lyngfelt A. Chemical looping combustion: status and development challenges[J]. Energy & Fuels, 2020, 34(8): 9077-9093. |
| 9 | Wang W, Chen X H, Xu R S, et al. Research progress on multiscale structural characteristics and characterization methods of iron ore sinter[J]. Journal of Iron and Steel Research International, 2020, 27(4): 367-379. |
| 10 | Yu Z L, Li C Y, Jing X L, et al. Effects of CO2 atmosphere and K2CO3 addition on the reduction reactivity, oxygen transport capacity, and sintering of CuO and Fe2O3 oxygen carriers in coal direct chemical looping combustion[J]. Energy & Fuels, 2013, 27(5): 2703-2711. |
| 11 | Tian M, Wang C J, Li L, et al. High performance of La-promoted Fe2O3/α-Al2O3 oxygen carrier for chemical looping combustion[J]. AIChE Journal, 2017, 63(7): 2827-2838. |
| 12 | Ma S W, Chen S Y, Zhu M, et al. Enhanced sintering resistance of Fe2O3/CeO2 oxygen carrier for chemical looping hydrogen generation using core-shell structure[J]. International Journal of Hydrogen Energy, 2019, 44(13): 6491-6504. |
| 13 | 孔令菲, 陈延佩, 王维. 气固流态化中颗粒介尺度结构的动力学研究[J]. 化工学报, 2022, 73(6): 2486-2495. |
| Kong L F, Chen Y P, Wang W. Dynamic study of mesoscale structures of particles in gas-solid fluidization[J]. CIESC Journal, 2022, 73(6): 2486-2495. | |
| 14 | 崔文政, 沈照杰, 毛东旭, 等. 纳米流体中纳米颗粒微运动的分子动力学模拟[J]. 化工学报, 2017, 68(S1): 48-53. |
| Cui W Z, Shen Z J, Mao D X, et al. Micro-movements of nanoparticles in nanofluids: molecular dynamics simulation[J]. CIESC Journal, 2017, 68(S1): 48-53. | |
| 15 | Zhao H B, Gui J F, Cao J E, et al. Molecular dynamics simulation of the microscopic sintering process of CuO nanograins inside an oxygen carrier particle[J]. The Journal of Physical Chemistry C, 2018, 122(44): 25595-25605. |
| 16 | Gu M F, Liu T F, Xiao X Z, et al. Simulation and experimental study of the multisized silver nanoparticles sintering process based on molecular dynamics[J]. Nanomaterials, 2022, 12(6): 1030. |
| 17 | Yang H Z, Sun B Y, Zhu Y F, et al. Critical role of surficial activity in the sintering process of TiO2 nanoparticles by molecular dynamics simulation[J]. Powder Technology, 2022, 398: 117071. |
| 18 | Dieckmann R. Point defects and transport in haematite (Fe2O3- ε )[J]. Philosophical Magazine A, 1993, 68(4): 725-745. |
| 19 | Liu Z J, Cheng Q, Li K J, et al. The interaction of nanoparticulate Fe2O3 in the sintering process: a molecular dynamics simulation[J]. Powder Technology, 2020, 367: 97-104. |
| 20 | Liu Z J, Cheng Q, Wang Y Z, et al. Three-body aggregation of Fe2O3 nanoparticles: a molecular dynamics simulation[J]. Chemical Physics Letters, 2020, 760: 137901. |
| 21 | Khanh B T H L, Hoang V V, Zung H. Structural properties of amorphous Fe2O3 nanoparticles[J]. The European Physical Journal D, 2008, 49(3): 325-332. |
| 22 | Eom N, Messing M E, Johansson J, et al. Sintering mechanism of Core@Shell Metal@Metal oxide nanoparticles[J]. The Journal of Physical Chemistry C, 2021, 125(29): 16220-16227. |
| 23 | Roy S, Prakash A, Sandfeld S. Sintering of alumina nanoparticles: comparison of interatomic potentials, molecular dynamics simulations, and data analysis[J]. Modelling and Simulation in Materials Science and Engineering, 2022, 30(6): 065009. |
| 24 | Lewis G V, Catlow C A. Potential models for ionic oxides[J]. Journal of Physics C: Solid State Physics, 1985, 18(6): 1149-1161. |
| 25 | Buesser B, Gröhn A J, Pratsinis S E. Sintering rate and mechanism of TiO2 nanoparticles by molecular dynamics[J]. The Journal of Physical Chemistry C, Nanomaterials and Interfaces, 2011, 115(22): 11030-11035. |
| 26 | Erlebach A, Kurland H D, Grabow J, et al. Structure evolution of nanoparticulate Fe2O3 [J]. Nanoscale, 2015, 7(7): 2960-2969. |
| 27 | Hirel P. Atomsk: a tool for manipulating and converting atomic data files[J]. Computer Physics Communications, 2015, 197: 212-219. |
| 28 | Plimpton S. Fast parallel algorithms for short-range molecular dynamics[J]. Journal of Computational Physics, 1995, 117(1): 1-19. |
| 29 | Stukowski A. Visualization and analysis of atomistic simulation data with OVITO—the open visualization tool[J]. Modelling and Simulation in Materials Science and Engineering, 2010, 18(1): 015012. |
| 30 | Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics[J]. Journal of Molecular Graphics, 1996, 14(1): 33-38. |
| 31 | Tian X K, Lin S C, Yan J, et al. Sintering mechanism of calcium oxide/calcium carbonate during thermochemical heat storage process[J]. Chemical Engineering Journal, 2022, 428: 131229. |
| 32 | Stukowski A. Structure identification methods for atomistic simulations of crystalline materials[J]. Modelling and Simulation in Materials Science and Engineering, 2012, 20(4): 045021. |
| 33 | Vollath D, Fischer F D, Holec D. Surface energy of nanoparticles-influence of particle size and structure[J]. Beilstein Journal of Nanotechnology, 2018, 9: 2265-2276. |
| 34 | Babalola B J, Ayodele O O, Olubambi P A. Sintering of nanocrystalline materials: sintering parameters[J]. Heliyon, 2023, 9(3): e14070. |
| [1] | Yongquan ZHANG, Weiwei XUAN. Mechanism of alkali metal/(FeO+CaO+MgO) influence on the structure and viscosity of silicate ash slag [J]. CIESC Journal, 2023, 74(4): 1764-1771. |
| [2] | Yugong CHEN, Hao CHEN, Yaosong HUANG. Study on pyrolysis mechanism of hexamethyldisiloxane using reactive molecular dynamics simulations [J]. CIESC Journal, 2022, 73(7): 2844-2857. |
| [3] | Ming LIU, Zhe XU. Phonon heat conduction and quantum correction of methane hydrate [J]. CIESC Journal, 2020, 71(4): 1424-1431. |
| [4] | Wanqiang LIU,Fan YANG,Hua YUAN,Yuanda ZHANG,Pinggui YI,Hu ZHOU. Molecular dynamics simulation and mechanism study on thermal conductivity of alcohols [J]. CIESC Journal, 2020, 71(11): 5159-5168. |
| [5] | He ZHENG, Shengjiang YANG, Yongchao ZHENG, Yan CUI, Xuan GUO, Jinyi ZHONG, Jian ZHOU. Molecular dynamics simulation of denaturation of DhaA induced by urea and dimethyl sulfoxide [J]. CIESC Journal, 2019, 70(11): 4337-4345. |
| [6] | QI Chang, LU Diannan, LIU Yongmin. Prediction of thermodynamic properties of n-alkanes based on temperature-corrected force field [J]. CIESC Journal, 2018, 69(8): 3338-3347. |
| [7] | XU Shang, ZHAO Lingling, CAI Zhuangli, CHEN Chao. Modeling study on thermal conductivity of two-dimensional hexagonal aluminum nitride [J]. CIESC Journal, 2017, 68(9): 3321-3327. |
| [8] | NAN Yiling, KONG Xian, LI Jipeng, LU Diannan. Non-equilibrium molecular dynamics simulation of water flow inside nano-slit [J]. CIESC Journal, 2017, 68(5): 1786-1793. |
| [9] | FENG Zhiming, LI Weiwei, LI Xue, ZHAO Yang, XIE Xiaofeng, CHAI Chunpeng, LUO Yunjun. Molecular dynamics simulation on effect of different carboxylic acid group contents on norbornene derivatives proton exchange membranes bearing bifunctional groups [J]. CIESC Journal, 2016, 67(S1): 253-259. |
| [10] | SHAN Chenxu, CAO Xulong, ZHU Yangwen, LIU Kun, QU Guangmiao, LÜ Pengfei, XUE Chunlong, DING Wei. Molecular dynamics simulation for interface behavior of octylphenol polyoxyethylene ether sulfonate [J]. CIESC Journal, 2016, 67(4): 1416-1423. |
| [11] | GAO Ning, WANG Yichao, LIU Yuhong. Molecular dynamics simulations of thermal pyrolysis of novel dipropargyl ether of bisphenol A based boron-containing polymer [J]. CIESC Journal, 2015, 66(4): 1557-1564. |
| [12] | WANG Wei, DONG Hanqiong, WANG Biao, ZHAO Yunchao, WANG Kai. Sintering mechanism of ceramics prepared from BOF slag [J]. CIESC Journal, 2014, 65(9): 0-0. |
| [13] | WANG Wei, DONG Hanqiong, WANG Biao, ZHAO Yunchao, WANG Kai. Sintering mechanism of ceramics prepared from BOF slag [J]. CIESC Journal, 2014, 65(9): 3732-3737. |
| [14] | XU Yao, LIU Yuelong, LIU Gousheng. Molecular dynamics simulation of water molecules adsorbed at muscovite (001) surface [J]. CIESC Journal, 2014, 65(12): 4814-4822. |
| [15] | LIU Lin, PAN Xiaona, ZHANG Qiang, QIAN Jianhua. Corrosion inhibition and olecular structure of thiadiazole derivatives in sulfur-ethanol system [J]. CIESC Journal, 2014, 65(10): 4039-4048. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||