化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3084-3094.DOI: 10.11949/0438-1157.20201682
宋奕慧1( ),雷志轶2,范国利1(
),雷志轶2,范国利1( ),杨兰1,林彦军1,3(
),杨兰1,林彦军1,3( ),李峰1
),李峰1
收稿日期:2020-11-25
修回日期:2021-02-19
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
范国利,林彦军
作者简介:宋奕慧(1995—),女,硕士研究生,基金资助:
SONG Yihui1( ),LEI Zhiyi2,FAN Guoli1(
),LEI Zhiyi2,FAN Guoli1( ),YANG Lan1,LIN Yanjun1,3(
),YANG Lan1,LIN Yanjun1,3( ),LI Feng1
),LI Feng1
Received:2020-11-25
Revised:2021-02-19
Online:2021-06-05
Published:2021-06-05
Contact:
FAN Guoli,LIN Yanjun
摘要:
采用成核-晶化隔离法制备LiAl-CO3-LDHs晶核,在LDHs晶核晶化的过程中引入葡萄糖分子作为碳源,构筑组成和结构可调的LDHs/C型杂化复合前体。通过高温处理,实现前体的结构拓扑转变及无定形碳组分的去除,得到高比表面积的LiAl复合金属氧化物型固体碱催化剂。采用XRD、FT-IR、BET、TEM、SEM、CO2-TPD等表征手段对催化剂的组成、结构、织构性能、表面碱性进行了详细研究,并以苯甲醛和氰基乙酸乙酯间的Knoevenagel缩合反应为探针反应系统地研究了催化剂的碱催化性能。研究结果表明,LDHs/C杂化前体制备过程中葡萄糖与金属离子的摩尔比、水热晶化温度以及焙烧温度是影响催化剂活性的主要因素,晶化温度和焙烧温度的提升不利于碱性位的充分暴露。在150℃的水热晶化温度下,葡萄糖与Al3+的摩尔比为3时的杂化复合前体经500℃焙烧得到的LiAl-MMO-150-3-500固体催化剂比表面积高达229 m2·g-1,苯酚吸附测得催化剂的总碱量为855 μmol·g-1,对苯甲醛的转化率高达88.21%。
中图分类号:
宋奕慧, 雷志轶, 范国利, 杨兰, 林彦军, 李峰. 基于LiAl-LDH/C杂化前体制备高比表面固体碱催化剂及其催化性能研究[J]. 化工学报, 2021, 72(6): 3084-3094.
SONG Yihui, LEI Zhiyi, FAN Guoli, YANG Lan, LIN Yanjun, LI Feng. Preparation and property of high specific surface solid base catalyst based on LiAl-LDH /C hybrid precursor[J]. CIESC Journal, 2021, 72(6): 3084-3094.
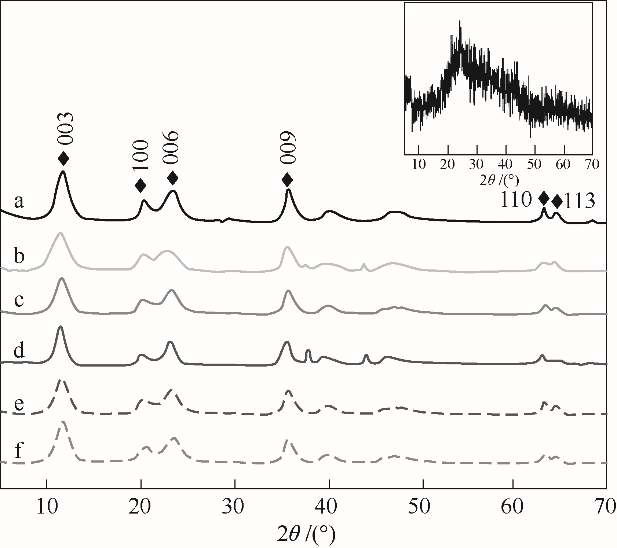
图1 不同条件下制备得到的LiAl-LDHs-x-y杂化复合前体的XRD谱图a— LiAl-LDHs-150-0; b—LiAl-LDHs-150-1; c—LiAl-LDHs-150-3; d—LiAl-LDHs-150-4; e—LiAl-LDHs-160-3; f—LiAl-LDHs-180-3
Fig.1 XRD patterns of LiAl-LDHs-x-y prepared at different conditions
| Samples | hkl | d / nm | a / nm | c / nm | Da / nm | Dc / nm |
|---|---|---|---|---|---|---|
| LiAl-LDHs-150-0 | (003) (110) | 0.7360 0.1466 | 0.2932 | 2.208 | 35.37 | 30.26 |
| LiAl-LDHs-150-1 | (003) (110) | 0.7725 0.1474 | 0.2948 | 2.318 | 14.41 | 6.994 |
| LiAl-LDHs-150-3 | (003) (110) | 0.7583 0.1471 | 0.2942 | 2.2749 | 12.82 | 5.567 |
| LiAl-LDHs-150-4 | (003) (110) | 0.7730 0.1475 | 0.2950 | 2.319 | 19.21 | 4.503 |
| LiAl-LDHs-160-3 | (003) (110) | 0.7814 0.1530 | 0.3060 | 2.344 | 13.39 | 5.932 |
| LiAl-LDHs-180-3 | (003) (110) | 0.7729 0.1521 | 0.3042 | 2.319 | 17.07 | 6.222 |
表1 不同条件下制备的LiAl-LDHs-x-y的XRD数据
Table 1 XRD parameters of LiAl-LDHs-x-y prepared at different conditions
| Samples | hkl | d / nm | a / nm | c / nm | Da / nm | Dc / nm |
|---|---|---|---|---|---|---|
| LiAl-LDHs-150-0 | (003) (110) | 0.7360 0.1466 | 0.2932 | 2.208 | 35.37 | 30.26 |
| LiAl-LDHs-150-1 | (003) (110) | 0.7725 0.1474 | 0.2948 | 2.318 | 14.41 | 6.994 |
| LiAl-LDHs-150-3 | (003) (110) | 0.7583 0.1471 | 0.2942 | 2.2749 | 12.82 | 5.567 |
| LiAl-LDHs-150-4 | (003) (110) | 0.7730 0.1475 | 0.2950 | 2.319 | 19.21 | 4.503 |
| LiAl-LDHs-160-3 | (003) (110) | 0.7814 0.1530 | 0.3060 | 2.344 | 13.39 | 5.932 |
| LiAl-LDHs-180-3 | (003) (110) | 0.7729 0.1521 | 0.3042 | 2.319 | 17.07 | 6.222 |
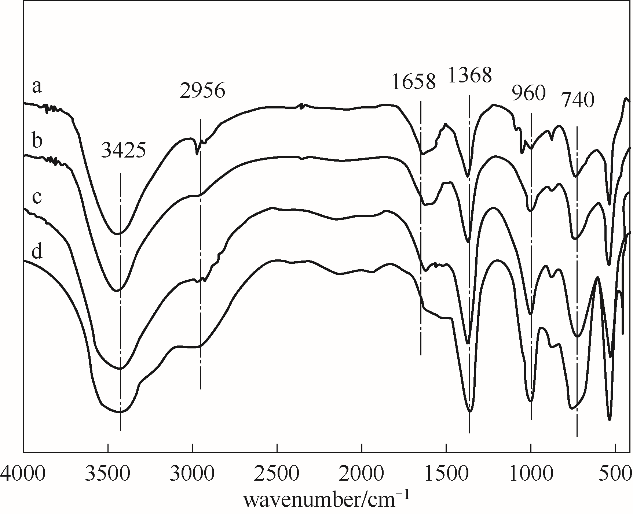
图2 系列LiAl-LDHs-150-y的红外谱图a—LiAl-LDHs-150-0; b—LiAl-LDHs-150-1; c—LiAl-LDHs-150-3; d—LiAl-LDHs-150-4
Fig.2 FT-IR spectra of series LiAl-LDHs-150-y prepared at different conditions
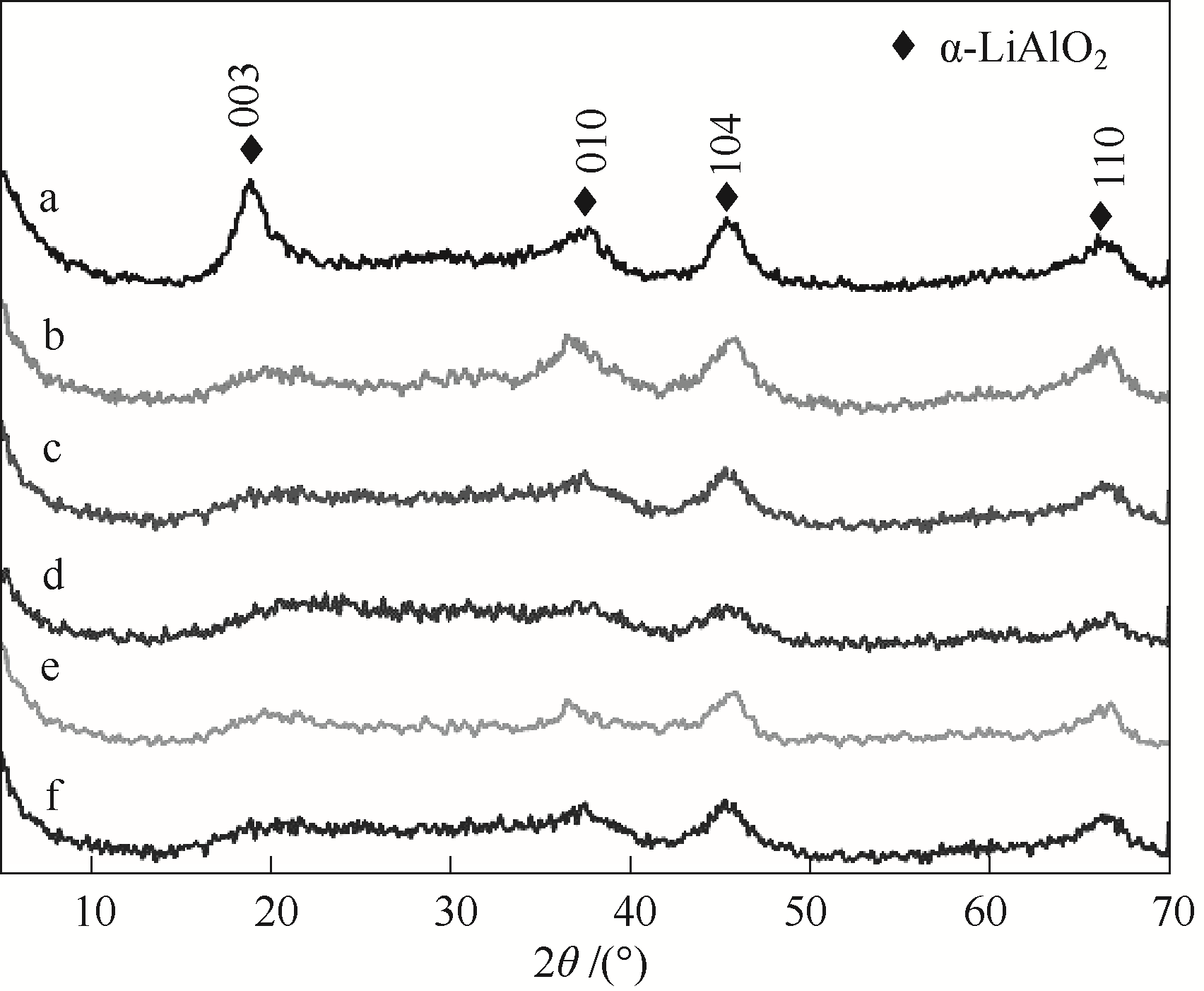
图3 不同条件下得到的系列LiAl-MMO-150-y-z的XRD谱图a—LiAl-MMO-150-0-500; b—LiAl-MMO-150-1-500; c—LiAl-MMO-150-3-500; d—LiAl-MMO-150-4-500; e—LiAl-MMO-150-3-400; f—LiAl-MMO-150-3-700
Fig.3 XRD patterns of LiAl-MMO-150-y-z prepared at different conditions
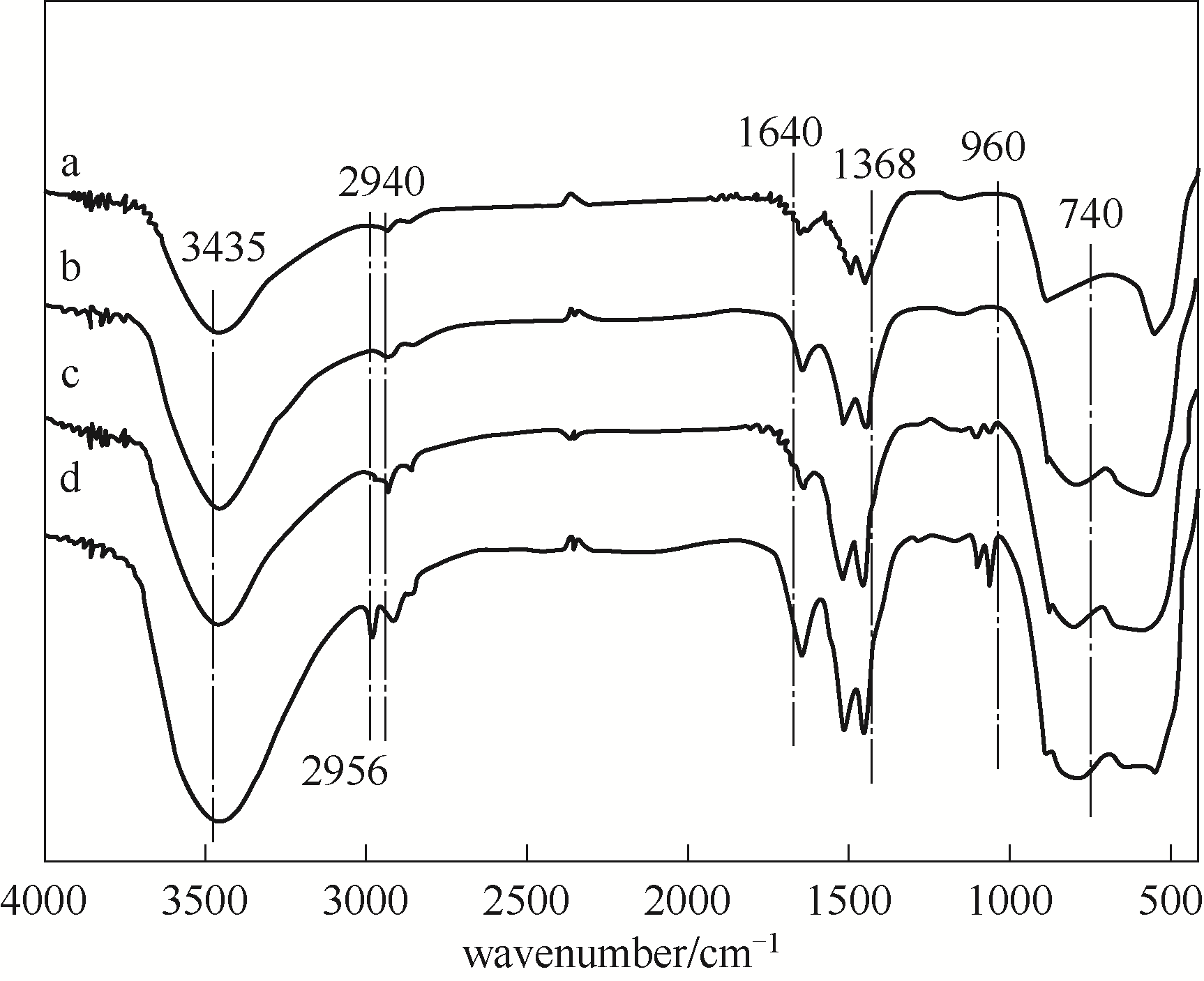
图4 系列LiAl-MMO-150-y-500样品的红外谱图a—LiAl-MMO-150-0-500; b—LiAl-MMO-150-1-500; c—LiAl-MMO-150-3-500; d—LiAl-MMO-150-4-500
Fig.4 FT-IR spectra of series LiAl-MMO-150-y-500 samples

图8 系列LiAl-MMO-x-y-z的N2吸-脱附等温线a—LiAl-MMO-150-0-500; b—LiAl-MMO-150-1-500; c—LiAl-MMO-150-3-500; d—LiAl-MMO-150-4-500; e—LiAl-MMO-180-3-500; f—LiAl-MMO-150-3-700; g—LiAl-MMO-160-3-500; h—LiAl-MMO-150-3-400
Fig.8 N2 adsorption-desorption isotherms of series LiAl-MMO-x-y-z
| Samples | Specific area/(m2·g-1) | Total volume/(ml·g-1) | Optimum pore size/nm |
|---|---|---|---|
| LiAl-MMO-150-0-500 | 123 | 0.2170 | 3.9 |
| LiAl-MMO-150-1-500 | 225 | 1.0168 | 14.0 |
| LiAl-MMO-150-3-500 | 229 | 0.8962 | 7.2 |
| LiAl-MMO-150-4-500 | 241 | 1.2230 | 12.0 |
| LiAl-MMO-160-3-500 | 206 | 0.5393 | 9.7 |
| LiAl-MMO-180-3-500 | 178 | 1.0440 | 9.5 |
| LiAl-MMO-150-3-400 | 232 | 0.9421 | 65 |
| LiAl-MMO-150-3-700 | 94 | 0.6095 | 20.1 |
表2 系列LiAl-MMO-x-y-z的比表面积和孔径分布参数
Table 2 Specific surface areas and pore distribution parameters of series of LiAl-MMO-x-y-z samples
| Samples | Specific area/(m2·g-1) | Total volume/(ml·g-1) | Optimum pore size/nm |
|---|---|---|---|
| LiAl-MMO-150-0-500 | 123 | 0.2170 | 3.9 |
| LiAl-MMO-150-1-500 | 225 | 1.0168 | 14.0 |
| LiAl-MMO-150-3-500 | 229 | 0.8962 | 7.2 |
| LiAl-MMO-150-4-500 | 241 | 1.2230 | 12.0 |
| LiAl-MMO-160-3-500 | 206 | 0.5393 | 9.7 |
| LiAl-MMO-180-3-500 | 178 | 1.0440 | 9.5 |
| LiAl-MMO-150-3-400 | 232 | 0.9421 | 65 |
| LiAl-MMO-150-3-700 | 94 | 0.6095 | 20.1 |

图9 系列LiAl-MMO-x-y-z的CO2-TPD曲线a—LiAl-MMO-150-0-500; b—LiAl-MMO-150-1-500; c—LiAl-MMO-150-3-500; d—LiAl-MMO-150-4-500; e—LiAl-MMO-180-3-500; f—LiAl-MMO-150-3-700; g—LiAl-MMO-160-3-500; h—LiAl-MMO-150-3-400
Fig.9 CO2-TPD curves of series LiAl-MMO-x-y-z
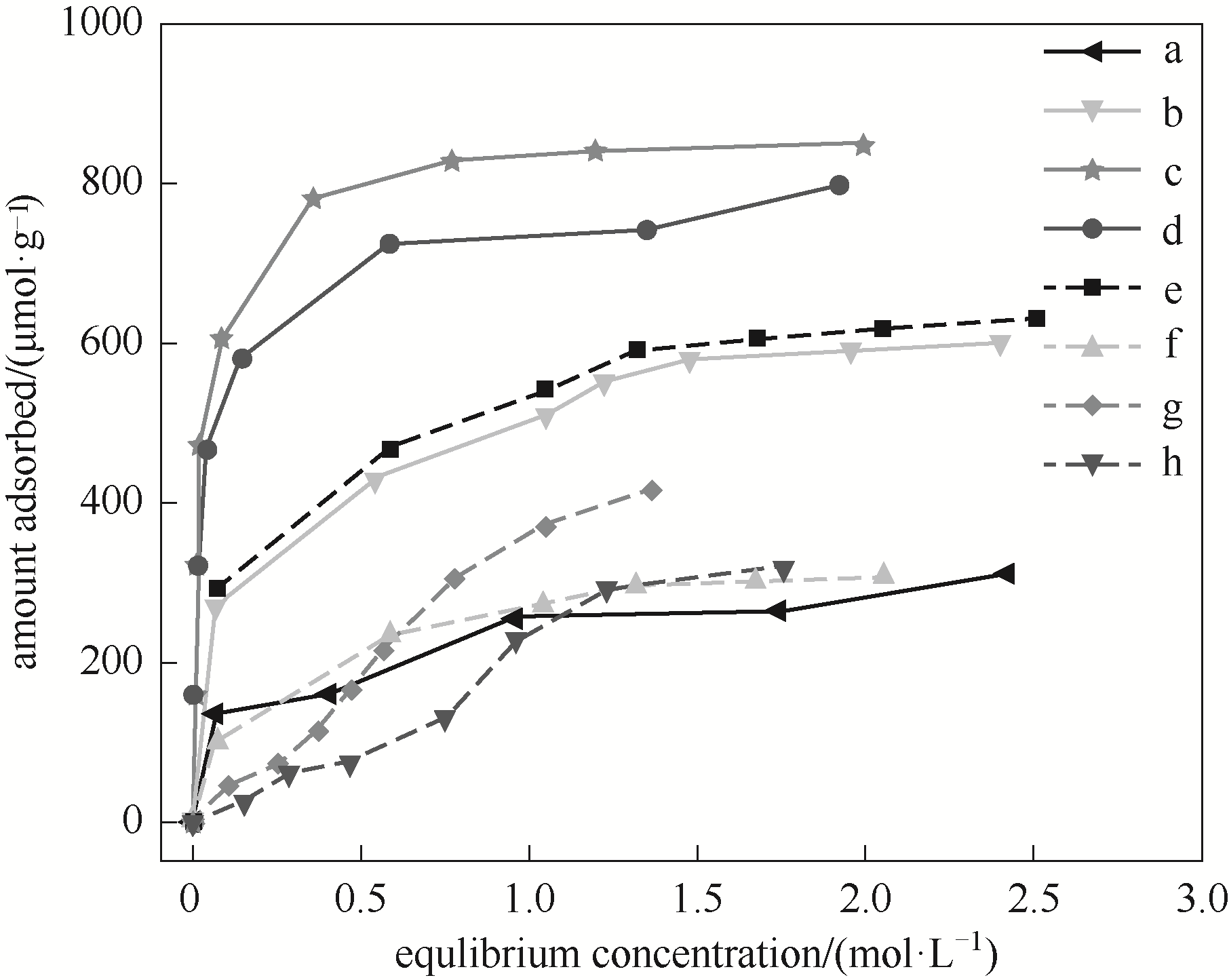
图10 系列LiAl-MMO-x-y-z的苯酚吸附等温线a—LiAl-MMO-150-0-500; b—LiAl-MMO-150-1-500; c—LiAl-MMO-150-3-500; d—LiAl-MMO-150-4-500; e—LiAl-MMO-180-3-500; f—LiAl-MMO-150-3-700; g—LiAl-MMO-160-3-500; h—LiAl-MMO-150-3-400
Fig.10 Phenol adsorption isotherms of series LiAl-MMO-x-y-z
| Samples | Number of base sites/(μmol of phenol·g-1) | Density of base sites/(μmol of phenol·m-2) |
|---|---|---|
| LiAl-MMO-150-0-500 | 306 | 2.49 |
| LiAl-MMO-150-1-500 | 635 | 2.82 |
| LiAl-MMO-150-3-500 | 855 | 3.74 |
| LiAl-MMO-150-4-500 | 800 | 3.32 |
| LiAl-MMO-160-3-500 | 730 | 3.54 |
| LiAl-MMO-180-3-500 | 635 | 3.57 |
| LiAl-MMO-150-3-400 | 582 | 2.51 |
| LiAl-MMO-150-3-700 | 308 | 3.28 |
表3 系列LiAl-MMO-x-y-z的碱性参数
Table 3 Basic sites data of series of LiAl-MMO-x-y-z
| Samples | Number of base sites/(μmol of phenol·g-1) | Density of base sites/(μmol of phenol·m-2) |
|---|---|---|
| LiAl-MMO-150-0-500 | 306 | 2.49 |
| LiAl-MMO-150-1-500 | 635 | 2.82 |
| LiAl-MMO-150-3-500 | 855 | 3.74 |
| LiAl-MMO-150-4-500 | 800 | 3.32 |
| LiAl-MMO-160-3-500 | 730 | 3.54 |
| LiAl-MMO-180-3-500 | 635 | 3.57 |
| LiAl-MMO-150-3-400 | 582 | 2.51 |
| LiAl-MMO-150-3-700 | 308 | 3.28 |
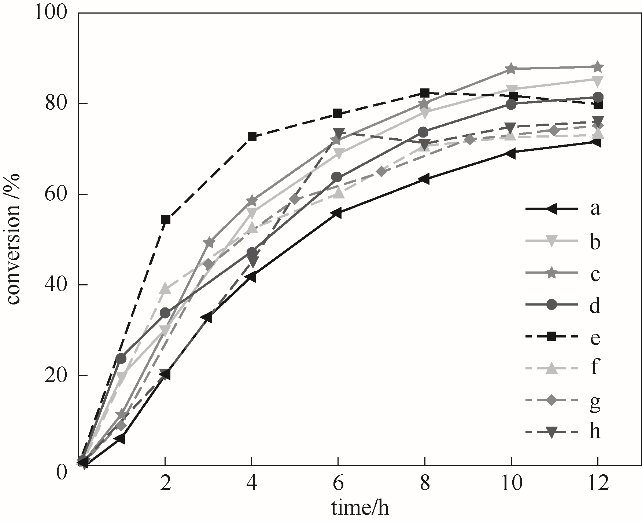
图11 系列LiAl-MMO-x-y-z的催化性能曲线a—LiAl-MMO-150-0-500; b—LiAl-MMO-150-1-500; c—LiAl-MMO-150-3-500; d—LiAl-MMO-150-4-500; e—LiAl-MMO-180-3-500; f—LiAl-MMO-150-3-700; g—LiAl-MMO-160-3-500; h—LiAl-MMO-150-3-400
Fig.11 Catalytic performance of series LiAl-MMO-x-y-z
| 1 | 乜贞, 伍倩, 卜令忠, 等. 国内外盐湖锂资源开发利用进展[J]. 中国盐业, 2015, (24): 45-50. |
| Nie Z, Wu Q, Bu L Z, et al. Development and utilization of salt lake lithium resources at home and abroad [J]. China Salt Industry, 2015, (24): 45-50. | |
| 2 | 宋彭生, 李武, 孙柏, 等. 盐湖资源开发利用进展[J]. 无机化学学报, 2011, 27(5): 801-815. |
| Song P S, Li W, Sun B, et al. Recent development on comprehensive utilization of salt lake resources[J]. Chinese Journal of Inorganic Chemistry, 2011, 27(5): 801-815. | |
| 3 | 王志国. 盐湖锂资源开发利用与研究进展[J]. 广州化工, 2011, 39(7): 23-24, 38. |
| Wang Z G. Research progress on development and utilization of lithium resource[J]. Guangzhou Chemical Industry, 2011, 39(7): 23-24, 38. | |
| 4 | 宋彭生. 盐湖及相关资源开发利用进展[J]. 盐湖研究, 2000, 8(1): 1-16. |
| Song P S. Comprehensive utilization of salt lake and related resources[J]. Journal of Salt Lake Research, 2000, 8(1): 1-16. | |
| 5 | 马钥德. 我国盐湖资源概况[C]//中国化工学会无机盐学术年会, 2003. |
| Ma Y D. Overview of salt lake resources in China[C]// Annual Meeting of Inorganic Salts of China Chemical Industry Association. 2003. | |
| 6 | 张苏江, 崔立伟, 孔令湖, 等. 国内外锂矿资源及其分布概述[J]. 有色金属工程, 2020, 10(10): 95-104. |
| Zhang S J, Cui L W, Kong L H, et al. Summarize on the lithium mineral resources and their distribution at home and abroad[J]. Nonferrous Metals Engineering, 2020, 10(10): 95-104. | |
| 7 | 柴文帅. 新冠疫情影响下的全球锂资源供需情况分析[J]. 新材料产业, 2020, (4): 46-50. |
| Chai W S. Analysis of global lithium resource supply and demand under the influence of COVID-19 [J]. Advanced Materials Industry, 2020, (4): 46-50. | |
| 8 | 王翘楚, 孙鑫, 郝瀚, 等. 锂的城市矿产利用: 前景、挑战及政策建议[J]. 科技导报, 2020, 38(15): 6-15. |
| Wang Q C, Sun X, Hao H, et al. Urban mining of lithium: prospects, challenges and policy recommendations[J]. Science & Technology Review, 2020, 38(15): 6-15. | |
| 9 | 张苏江, 张彦文, 张立伟, 等. 中国锂矿资源现状及其可持续发展策略[J]. 无机盐工业, 2020, 52(7): 1-7. |
| Zhang S J, Zhang Y W, Zhang L W, et al. Present situation and sustainable development strategy of China's lithium resources[J]. Inorganic Chemicals Industry, 2020, 52(7): 1-7. | |
| 10 | 张润泽. 锂——战略性新兴资源的关键[J]. 世界有色金属, 2020(11): 259-260. |
| Zhang R Z. Lithium—key to strategic emerging resources[J]. World Nonferrous Metals, 2020(11): 259-260. | |
| 11 | 汪明泉. 柴达木盆地一里坪盐湖富锂卤水成因研究[D]. 北京: 中国地质大学(北京), 2020. |
| Wang M Q. Origin of lithium-rich brine in Yiliping salt lake, Qaidam basin[D]. Beijing: ChinaUniversity of Geosciences, 2020. | |
| 12 | 陈玉明, 陈喜峰, 赵宏军, 等. 南美“锂三角”战略地位日益凸显[N]. 中国矿业报, 2020-4-29(1). |
| Chen Y M, Chen X F, Zhao H J, et al. The strategic position of “Lithium Triangle” in South America is increasingly prominent[N]. China Mining News, 2020-4-29(1). | |
| 13 | Brindley G W, Kikkawa S. Thermal behavior of hydrotalcite and of anion-exchanged forms of hydrotalcite[J]. Clays and Clay Minerals, 1980, 28(2): 87-91. |
| 14 | Reichle W T. Catalytic reactions by thermally activated, synthetic, anionic clay minerals[J]. Journal of Catalysis, 1985, 94(2): 547-557. |
| 15 | Cavani F, Trifirò F, Vaccari A. Hydrotalcite-type anionic clays: preparation, properties and applications[J]. Catalysis Today, 1991, 11(2): 173-301. |
| 16 | 魏彤, 王谋华, 魏伟, 等. 固体碱催化剂[J]. 化学通报, 2002, 65(9): 594-600. |
| Wei T, Wang M H, Wei W, et al. The investigation status and development trend of solid base catalysts[J]. Chemistry, 2002, 65(9): 594-600. | |
| 17 | Sideris P J, Nielsen U G, Gan Z H, et al. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy[J]. Science, 2008, 321(5885): 113-117. |
| 18 | Wang Q, O'Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets[J]. Chemical Reviews, 2012, 112(7): 4124-4155. |
| 19 | 沈家骢. 超分子层状结构: 组装与功能[M]. 北京: 科学出版社, 2005. |
| Shen J C, et al. Supramolecular Layer Structure: Assembly and Function[M]. Beijing: Science Press, 2005. | |
| 20 | Miyata S, Kumura T, Hattori H, et al. Physico-chemical properties and structure of magnesia-alumina[J]. Nippon Kagaku Zassi, 1971, 92(6): 514-519. |
| 21 | Taylor H F W. Crystal structures of some double hydroxide minerals[J]. Mineralogical Magazine, 1973, 39(304): 377-389. |
| 22 | Zou L, Xiang X, Fan J, et al. Single-source precursor to complex metal oxide monoliths with tunable microstructures and properties: the case of Mg-containing materials[J]. Chemistry of Materials, 2007, 19(26): 6518-6527. |
| 23 | Xiang X, Hima H I, Wang H, et al. Facile synthesis and catalytic properties of nickel-based mixed-metal oxides with mesopore networks from a novel hybrid composite precursor[J]. Chemistry of Materials, 2008, 20(3): 1173-1182. |
| 24 | 张毅. 镁基水滑石紫外阻隔材料的制备及在聚丙烯中的应用[D]. 北京: 中国科学院大学(中国科学院青海盐湖研究所), 2019. |
| Zhang Y. Preparation of manganese-based layered double hydroxides UV shielding material and its application in polypropylene[D]. Beijing: University of Chinese Academy of Sciences, 2019. | |
| 25 | 阿旦春, 肖学英, 文静, 等. 氢氧化镁煅烧工艺和原料配比对氯氧镁水泥抗压强度的影响[J]. 盐湖研究, 2020, 28(3): 85-92. |
| A D C, Xiao X Y, Wen J, et al. Influence of calcining process of magnesium hydroxide and raw material ratio on compressive strength of MOC based on orthogonal experiment[J]. Journal of Salt Lake Research, 2020, 28(3): 85-92. | |
| 26 | 绪连萧. 混合离子溶液中镁、锂分离与提取的反应-分离耦合方法的研究[D]. 北京: 北京化工大学, 2016. |
| Xu L X. Research on separation and extraction Mg/Li from mixed ionic solutions with reaction-separation coupling technique[D]. Beijing: Beijing University of Chemical Technology, 2016. | |
| 27 | 雷志轶. 新型高比表面固体碱催化剂制备、结构及其性能研究[D]. 北京: 北京化工大学, 2009. |
| Lei Z Y. Investigations on synthesis, structure and properties of new-style high-surface-area solid base catalyst[D]. Beijing: Beijing University of Chemical Technology, 2009. | |
| 28 | 高志. 金属加氢催化剂活性位的精细调控及其催化生物质平台分子转化性能[D]. 北京: 北京化工大学, 2018. |
| Gao Z. Fine control of active sites of metal hydrogenation catalysts and their performance for catalytic transformation of biomass platform molecules[D]. Beijing: Beijing University of Chemical Technology, 2018. | |
| 29 | Gao Z, Yang L, Fan G L, et al. Promotional role of surface defects on carbon-supported ruthenium-based catalysts in the transfer hydrogenation of furfural[J]. ChemCatChem, 2016, 8(24): 3769-3779. |
| 30 | Titulaer M K, Jansen J B H, Geus J W. The quantity of reduced nickel in synthetic takovite: effects of preparation conditions and calcination temperature[J]. Clays Clay Miner., 1994, 42: 249-258. |
| 31 | Marezio M, Remeika J P. Polymorphism of LiMO2 compounds and high‐pressure single‐crystal synthesis of LiBO2 [J]. J. Chem. Phys., 1966, 44(19): 3143-3144. |
| 32 | 段雪, 张法智. 插层组装与功能材料[M]. 北京: 化学工业出版社, 2007. |
| Duan X, Zhang F Z. Intercalation Assembly and Functional Materials [M]. Beijing: Chemical Industry Press, 2007. | |
| 33 | Gregg S J, Sing K S W. Adsorption, Surface Area and Porosity[M]. 2nd ed. New York: Academic Press, 1982. |
| 34 | 田部浩三. 新固体酸和碱及其催化作用[M]. 郑禄彬, 译. 北京: 化学工业出版社, 1992. |
| Tanabe Kozo. New Solid Acids and Bases and Their Catalytic Properties[M]. Zheng L B, trans. Beijing: Chemical Industry Press, 1992. | |
| 35 | 雷经新, 石秋杰. 固体碱催化剂在有机合成中的应用及进展[J]. 化工时刊, 2005, 19(2): 49-53. |
| Lei J X, Shi Q J. Application and progress of solid base catalysts in organicsynthesis[J]. Chemical Industry Times, 2005, 19(2): 49-53. | |
| 36 | 辛勤. 固体催化剂研究方法[M]. 北京: 科学出版社, 2004. |
| Xin Q. Solid Catalyst Research Methods [M]. Beijing: Science Press, 2004. | |
| 37 | Zhang M, Zhao Y, Liu Q, et al. A La-doped Mg-Al mixed metal oxide supported copper catalyst with enhanced catalytic performance in transfer dehydrogenation of 1-decanol[J]. Dalton Transactions (Cambridge, England), 2016, 45(3): 1093-1102. |
| [1] | 茹绍青, 武亚飞, 车黎明. 低过冷度石蜡Pickering乳液的制备和表征[J]. 化工学报, 2021, 72(4): 2309-2316. |
| [2] | 赵杰,郭月,沈桢,杨立军,吴强,王喜章,胡征. 高倍率容量层状双金属氢氧化物超级电容材料的研究进展[J]. 化工学报, 2020, 71(11): 4851-4872. |
| [3] | 王瑞瑞, 赵有璟, 邵明飞, 项顼, 段雪. 层状双金属氢氧化物用于催化水氧化的研究进展[J]. 化工学报, 2016, 67(1): 54-72. |
| [4] | 王俊格, 梁金花, 孙守飞, 张文飞, 任晓乾, 姜岷. 超声辅助制备酸碱双功能CaO/HMCM-22分子筛催化剂[J]. 化工学报, 2014, 65(10): 3924-3930. |
| [5] | 李亚丹, 王玉军, 张卫东, 骆广生. 膜分散微反应器沉淀法制备高孔容SiO2材料[J]. 化工学报, 2013, 64(6): 2276-2284. |
| [6] | 孙金陆1,2,甄卫军1,2,李 进1,2. LDHs材料的结构、性质及其应用研究进展[J]. 化工进展, 2013, 32(03): 610-616. |
| [7] | 牛怀成,李利春,李 瑛,韩文峰,唐浩东,刘化章. 高比表面积氟化镁的合成及其在催化中的应用研究进展[J]. 化工进展, 2012, 31(07): 1484-1492. |
| [8] | 柳 杨1,2,衣怀峰3,陈 宇1,2,吴玉龙2,陈 曾1,杨明德2,童军茂1. SBA-15的改性及催化文冠果油制备生物柴油 [J]. CIESC Journal, 2011, 30(6): 1247-. |
| [9] | 方建辉,姚伯元,韩福顺. 氢氧化钾活化石油焦制备高比表面积活性炭[J]. CIESC Journal, 2011, 30(10): 2258-. |
| [10] | 樊欣梅,张德虎,黄 彪,王华杰,韦藤幼,童张法. 负载型膨润土固体碱催化剂的制备及在生物柴油合成中的应用 [J]. CIESC Journal, 2009, 28(11): 1951-. |
| [11] | 文利柏,王 运,关燕萍,胡圣扬,韩鹤友. 制备生物柴油的负载型催化剂KF/CaO/陶土的研究 [J]. CIESC Journal, 2009, 28(11): 1946-. |
| [12] | 程文萍;王雯娟;赵月昌;刘玲;邵丽丽;杨建国 . ZnMgAl复合氧化物五催化合成丙二醇苯醚 [J]. CIESC Journal, 2007, 58(12): 3072-3076. |
| [13] | 颜姝丽, 鲁厚芳, 姜利寒, 梁斌. 固体碱催化剂用于油脂甲醇酯交换反应制备生物柴油 [J]. 化工学报, 2007, 58(10): 2506-2512. |
| [14] | 姚伯元;黄广民;窦智峰;林白云 . 海南椰壳与椰壳渣制备高比表面积活性炭原料脱灰工艺 [J]. CIESC Journal, 2006, 57(6): 1458-1463. |
| [15] | 李为民, 郑晓林, 徐春明, 徐鸽, 邬国英. 固体碱法制备生物柴油及其性能 [J]. 化工学报, 2005, 56(4): 711-716. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号