化工学报 ›› 2020, Vol. 71 ›› Issue (11): 4851-4872.DOI: 10.11949/0438-1157.20201296
赵杰1( ),郭月1,沈桢1,杨立军1,吴强1(
),郭月1,沈桢1,杨立军1,吴强1( ),王喜章1,胡征1,2(
),王喜章1,胡征1,2( )
)
收稿日期:2020-09-09
修回日期:2020-09-17
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
吴强,胡征
作者简介:赵杰(1987—),男,博士研究生,基金资助:
Jie ZHAO1( ),Yue GUO1,Zhen SHEN1,Lijun YANG1,Qiang WU1(
),Yue GUO1,Zhen SHEN1,Lijun YANG1,Qiang WU1( ),Xizhang WANG1,Zheng HU1,2(
),Xizhang WANG1,Zheng HU1,2( )
)
Received:2020-09-09
Revised:2020-09-17
Online:2020-11-05
Published:2020-11-05
Contact:
Qiang WU,Zheng HU
摘要:
层状双金属氢氧化物(LDHs)是由带正电荷的金属氢氧化物层板、层间带负电荷的阴离子和水分子组成的二维层状材料,可通过氢氧化物与羟基氧化物之间的可逆氧化还原反应存储与释放电荷,具有理论容量高、形貌与组分可调、成本低、易宏量制备等优点,成为近年来备受关注的超级电容器电极材料。超级电容材料在大电流密度下的比容量与其应用潜力密切相关,研究者们通过材料设计及电极工程,探索了多种提升LDHs倍率容量(即不同电流密度下的容量)的方法与技术,但至今LDHs的实际储能性能仍然远低于预期。简述了LDHs的结构、储能机理与面临的挑战,从增加反应活性、促进电荷传输动力学的角度归纳总结了提升LDHs倍率容量的研究进展,探讨了通过匹配电子传输和离子输运能力进一步提升LDHs倍率容量的新思路。
中图分类号:
赵杰,郭月,沈桢,杨立军,吴强,王喜章,胡征. 高倍率容量层状双金属氢氧化物超级电容材料的研究进展[J]. 化工学报, 2020, 71(11): 4851-4872.
Jie ZHAO,Yue GUO,Zhen SHEN,Lijun YANG,Qiang WU,Xizhang WANG,Zheng HU. Research progress of high-rate capacity layered double hydroxide supercapacitor materials[J]. CIESC Journal, 2020, 71(11): 4851-4872.

图4 各类电能存储器件的Ragone图[126](文献[33-34,46,93,97,106,109,120-123,125]中的器件性能供对比参考。器件的时间常数标于图中)
Fig.4 Ragone plot for various electrical energy storage devices[126](performances of supercapacitors in Ref. [33-34,46,93,97,106,109,120-123,125] are also provided for comparison, the time constants of the devices are marked in the figure)

图5 超薄NiTi-LDH纳米片的结构表征[36]:透射电镜照片(a);高分辨透射电镜照片(b);原子力显微镜照片(c);纳米片的厚度(d)(图(d)中的1~3对应于图(c)中1~3纳米片)
Fig.5 Characterization of the monolayer NiTi-LDH nanosheets[36]: TEM image (a); HRTEM image (b); AFM image(c) and the corresponding height profiles (d) (profiles of 1—3 in Fig.(d) correspond to the nanosheets of 1—3 in Fig. (c))

图6 分级结构NiAl-LDH样品及其倍率容量[39]:制备示意图(a); 核-壳结构[(b)、(c)]; 蛋黄-壳结构[(d)、(e)];空心结构[(f)、(g)];纳米颗粒(h);倍率容量(i)
Fig.6 Hierarchical NiAl-LDH and rate capacities[39]: schematic preparation (a), core-shell structure[(b),(c)], yolk-shell structure[(d),(e)], hollow structure[(f),(g)], nanoparticles (h), rate capacities (i)

图7 分级结构对CoAl-LDH纳米片阵列倍率容量的影响[43]:P-CO3-LDH与H-CO3-LDH的结构示意图(a);倍率容量(b)(图(b)中H-OH-LDH样品的性能供参考)
Fig.7 Influence of hierarchical structure on rate capacity of the CoAl-LDH nanosheet array[43]: schematic diagram of P-CO3-LDH and H-CO3-LDH (a), rate capacities (b) (performance of H-OH-LDH in Fig. (b) is provided for reference)

图8 CoAl-LDH与CoAl-LDH/CNTs复合材料的电化学性能[46]:CoAl-LDH/CNTs复合材料的制备示意图(a); 电化学阻抗谱(b)(等效电路插图中,Rs为欧姆电阻,Rct为电荷转移电阻);倍率容量(c)(图(b)、(c)中CNTs的数据供参考)
Fig.8 Electrochemical performances of the CoAl-LDH and the CoAl-LDH/CNTs composite[46]: schematic preparation of the CoAl-LDH/CNTs composite (a), electrochemical impedance spectra (b) (Rs is the intrinsic Ohmic resistance, Rct is the charge transfer resistance), rate capacities(c) ( in Fig. (b),(c) , the data of CNTs are also presented for reference)

图9 NiAl-LDH与NiAl-LDH/rGO复合材料的电化学性能[58]: NiAl-LDH/rGO复合材料的制备示意图(a);电化学阻抗谱(b);3.57~17.86 A·g-1范围内的倍率容量(c)
Fig.9 Electrochemical performances of the NiAl-LDH and the NiAl-LDH/rGO composite[58]: schematic preparation of the NiAl-LDH/rGO composite (a), electrochemical impedance spectra (b), rate capacities at 3.57—17.86 A·g-1 (c)

图10 CC-LDH与CC-NC-LDH电极的电化学性能[93]:CC-NC-LDH电极的制备示意图(a); 电化学阻抗谱(b); 倍率容量(c)(图(c)中NiCo-LDH样品的性能供对比参考)
Fig.10 Electrochemical performances of CC-LDH and CC-NC-LDH electrodes[93]: schematic preparation of the CC-NC-LDH electrode (a), electrochemical impedance spectra (b), rate capacities (c) (the performance of NiCo-LDH in Fig.(c) is also presented for reference)

图12 层间距对CoAl-LDH倍率容量的影响[109]:CoAl-LDH的层间距调控示意图(a);电化学阻抗谱(b);1~32 A·g-1范围内的倍率容量(c)(文献[109]中DS-为十二烷基硫酸根阴离子)
Fig.12 Influence of interlayer distance on rate capacity of CoAl-LDH[109]: schematic regulation of the interlayer distance (a), electrochemical impedance spectra (b), rate capacities at 1—32 A·g-1(c) ( DS- is dodecyl sulfate anion in Ref.[109])

图13 剥离-自组装策略制备LDHs基复合材料示意图:CoAl-LDH/rGO薄膜(a)[120];CoNi-LDH/PEDOT:PSS复合材料(b) [122]
Fig.13 Schematic preparation of the LDHs-based composites by exfoliation-self-assembly method: CoAl-LDH/rGO film (a) [120],CoNi-LDH/PEDOT:PSS composite (b) [122]
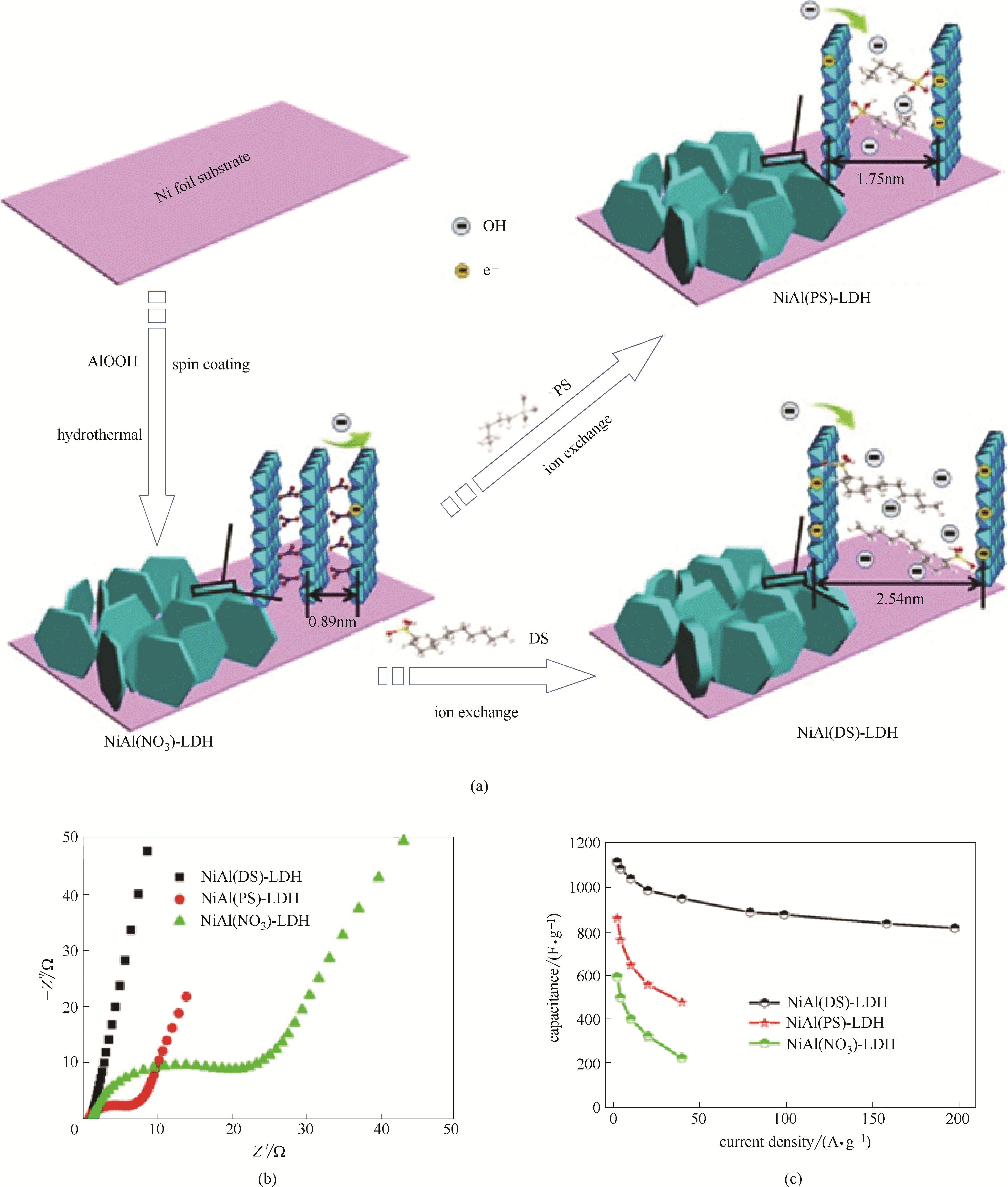
图14 NiAl-LDH阵列的层间距调控与电化学性能[124]:NiAl-LDH阵列的层间距调控示意图(a); 电化学阻抗谱(b);倍率容量(c)(图(c)中DS为十二烷基磺酸根阴离子,PS为戊烷基磺酸根阴离子)
Fig.14 The regulation of interlayer distance of NiAl-LDH nanosheet arrays and the related electrochemical performances[124]: schematic regulation of the interlayer distance(a), electrochemical impedance spectra(b), rate capacities(c)(in Fig.(c), DS is dodecanesulfonate anion. PS is 1-pentanesulfonate anion)

图15 层间距对NiCo-LDH的等效串联电阻及倍率容量的影响[125]:层间距调控示意图(a);直链构型羧基阴离子插层LDHs的RESR(b)和倍率容量(c); 共轭构型羧基阴离子插层LDHs的RESR(d)和倍率容量(e)S4—[C2H4(COO)2]2-; S6—[C4H8(COO)2]2-; S10—[C8H16(COO)2]2-; CBT—[C6H3(COO)3]3-; CND—[C10H6(COO)2]2-; CBD—[C6H4(COO)2]2-; CPT—[C20H8(COO)4]4-
Fig.15 Influences of the interlayer distance on RESR and rate capacity in NiCo-LDH[125]: schematic regulation of interlayer distance(a), RESR(b) and rate capacities(c) of the straight-chain anions intercalated LDHs, RESR (d) and rate capacities(e) of the conjugated-plane anions intercalated LDHs
| Sample | Capacitance/(F·g-1) | Ref. | Sample | Capacitance/(F·g-1) | Ref. | ||
|---|---|---|---|---|---|---|---|
at low current (≤ 50 A·g-1) | at high current (> 50 A·g-1) | at low current (≤ 50 A·g-1) | at high current (> 50 A·g-1) | ||||
| regulating compositions | depositing/growing on conductive substrates | ||||||
| NiCoAl-LDH | 2062 (1) 553 (20) | ― | [ | CoAl-LDH/CC | 616.9 (1) 454.4 (20) | ― | [ |
| CoNi-LDH | 2614 (5) | ― | [ | NiCo-LDH/ N-doped CC | 1817 (1) | 1092 (100) | [ |
| CoⅡCoⅢ-LDH | 715 (0.5) 130 (50) | ― | [ | CoMn-LDH/CC | 1079 (2.1) 891 (42) | ― | [ |
| amorphizating | NiCo-LDH/CC | 1927 (2) 1546 (30) | ― | [ | |||
| NiCoMn-LDHs | 1440 (1) 1104 (50) | ― | [ | NiCo-LDH/CC | 2105 (2) 1191.3 (20) | ― | [ |
| nanostructuring | NiCo-LDH/NF | 2682 (3) 1706 (20) | ― | [ | |||
| NiTi-LDH | 2310 (1.5) 1206 (30) | ― | [ | MnCo-LDHs@ Ni(OH)2/NF | 2320 (3) 1308 (30) | ― | [ |
| constructing hierarchical structures | NiMn-LDH/NF | 1511 (2.5) 1210 (48) | ― | [ | |||
| NiAl-LDH | 735 (2) 548 (25) | ― | [ | NiCo-LDH/NF | 2184 (1) 1494 (20) | ― | [ |
| NiCo-LDH | 2275.5 (1) 1007.8 (25) | ― | [ | NiCo-LDH/SS | 2104 (1) | ― | [ |
| NiCo-LDH | 1887.5 (1) 1187.5 (10) | ― | [ | expanding interlayer distance | |||
| NiFe-LDH | 1061 (1) 598 (10) | ― | [ | CoFe-LDH | 456 (2) 337 (20) | ― | [ |
| CoAl-LDH | 1031 (1) 763 (40) | 680 (100) | [ | CoAl-LDH | 1481.7 (1) 856.7 (32) | ― | [ |
| compositing with carbon | NiCo-LDH | 1646 (3) 680 (10) | ― | [ | |||
| NiCo-LDH/CNTs | 1843 (0.5) 1231 (10) | ― | [ | CoAl-LDH | 1100 (1) 750 (30) | ― | [ |
| NiAl-LDH/CNTs | 2034 (1) 1729 (10) | ― | [ | CoⅡCoⅢ-LDH | 1055 (1) 300 (15) | ― | [ |
| NiMn-LDH/CNTs | 2960 (1.5) 2353 (30) | ― | [ | NiMn-LDH | 1881 (1) 649 (10) | ― | [ |
| NiCo-LDH/CNTs | 1896 (1) 1479 (40) | ― | [ | NiCo-LDH | 1580 (10) | ― | [ |
| CoAl-LDH/CNTs | 1949.5 (1) 1066.4 (10) | ― | [ | CoⅡCoⅢ-LDH | 590 (10) | ― | [ |
| NiCoAl-LDH/rGO | 1866 (1) 1360 (10) | ― | [ | selective etching | |||
| NiAl-LDH/rGO | 2712.7 (1) 1174 (50) | ― | [ | NiCoAl-LDH | 1289 (1) 738 (30) | ― | [ |
| NiCo-LDH/rGO | 1911.1 (2) 1469.8 (20) | ― | [ | exfoliation-self-assembly | |||
| MgAl-LDH/rGO | 1334 (1) | ― | [ | CoAl-LDH/rGO | 1043 (1) 912 (20) | ― | [ |
| CoMn-LDH/rGO | 1635 (1) 1161 (10) | ― | [ | CoNi-LDH/ PEDOT:PSS | 960 (2) 804 (30) | ― | [ |
| NiCoAl-LDH/rGO | 1544 (1) 1081 (40) | ― | [ | depositing/growing on conductive substrates+ expanding interlayer distance | |||
| NiAl-LDH/NF | 1125 (1) | 819 (200) | [ | ||||
| NiFe-LDH/rGO | 1196 (1) 861 (10) | ― | [ | sub-nanometer-scale fine regulation of interlayer distance | |||
| NiCo-LDH/C | 2558 (1) 1916 (20) | ― | [ | NiCo-LDH | 2115 (1) 949 (50) | 626 (100) 410 (150) | [ |
表1 LDHs基电极材料的调控策略及其倍率容量(三电极测试体系)
Table 1 Rate capacities of LDHs-based electrode materials in each strategy (three-electrode test system)
| Sample | Capacitance/(F·g-1) | Ref. | Sample | Capacitance/(F·g-1) | Ref. | ||
|---|---|---|---|---|---|---|---|
at low current (≤ 50 A·g-1) | at high current (> 50 A·g-1) | at low current (≤ 50 A·g-1) | at high current (> 50 A·g-1) | ||||
| regulating compositions | depositing/growing on conductive substrates | ||||||
| NiCoAl-LDH | 2062 (1) 553 (20) | ― | [ | CoAl-LDH/CC | 616.9 (1) 454.4 (20) | ― | [ |
| CoNi-LDH | 2614 (5) | ― | [ | NiCo-LDH/ N-doped CC | 1817 (1) | 1092 (100) | [ |
| CoⅡCoⅢ-LDH | 715 (0.5) 130 (50) | ― | [ | CoMn-LDH/CC | 1079 (2.1) 891 (42) | ― | [ |
| amorphizating | NiCo-LDH/CC | 1927 (2) 1546 (30) | ― | [ | |||
| NiCoMn-LDHs | 1440 (1) 1104 (50) | ― | [ | NiCo-LDH/CC | 2105 (2) 1191.3 (20) | ― | [ |
| nanostructuring | NiCo-LDH/NF | 2682 (3) 1706 (20) | ― | [ | |||
| NiTi-LDH | 2310 (1.5) 1206 (30) | ― | [ | MnCo-LDHs@ Ni(OH)2/NF | 2320 (3) 1308 (30) | ― | [ |
| constructing hierarchical structures | NiMn-LDH/NF | 1511 (2.5) 1210 (48) | ― | [ | |||
| NiAl-LDH | 735 (2) 548 (25) | ― | [ | NiCo-LDH/NF | 2184 (1) 1494 (20) | ― | [ |
| NiCo-LDH | 2275.5 (1) 1007.8 (25) | ― | [ | NiCo-LDH/SS | 2104 (1) | ― | [ |
| NiCo-LDH | 1887.5 (1) 1187.5 (10) | ― | [ | expanding interlayer distance | |||
| NiFe-LDH | 1061 (1) 598 (10) | ― | [ | CoFe-LDH | 456 (2) 337 (20) | ― | [ |
| CoAl-LDH | 1031 (1) 763 (40) | 680 (100) | [ | CoAl-LDH | 1481.7 (1) 856.7 (32) | ― | [ |
| compositing with carbon | NiCo-LDH | 1646 (3) 680 (10) | ― | [ | |||
| NiCo-LDH/CNTs | 1843 (0.5) 1231 (10) | ― | [ | CoAl-LDH | 1100 (1) 750 (30) | ― | [ |
| NiAl-LDH/CNTs | 2034 (1) 1729 (10) | ― | [ | CoⅡCoⅢ-LDH | 1055 (1) 300 (15) | ― | [ |
| NiMn-LDH/CNTs | 2960 (1.5) 2353 (30) | ― | [ | NiMn-LDH | 1881 (1) 649 (10) | ― | [ |
| NiCo-LDH/CNTs | 1896 (1) 1479 (40) | ― | [ | NiCo-LDH | 1580 (10) | ― | [ |
| CoAl-LDH/CNTs | 1949.5 (1) 1066.4 (10) | ― | [ | CoⅡCoⅢ-LDH | 590 (10) | ― | [ |
| NiCoAl-LDH/rGO | 1866 (1) 1360 (10) | ― | [ | selective etching | |||
| NiAl-LDH/rGO | 2712.7 (1) 1174 (50) | ― | [ | NiCoAl-LDH | 1289 (1) 738 (30) | ― | [ |
| NiCo-LDH/rGO | 1911.1 (2) 1469.8 (20) | ― | [ | exfoliation-self-assembly | |||
| MgAl-LDH/rGO | 1334 (1) | ― | [ | CoAl-LDH/rGO | 1043 (1) 912 (20) | ― | [ |
| CoMn-LDH/rGO | 1635 (1) 1161 (10) | ― | [ | CoNi-LDH/ PEDOT:PSS | 960 (2) 804 (30) | ― | [ |
| NiCoAl-LDH/rGO | 1544 (1) 1081 (40) | ― | [ | depositing/growing on conductive substrates+ expanding interlayer distance | |||
| NiAl-LDH/NF | 1125 (1) | 819 (200) | [ | ||||
| NiFe-LDH/rGO | 1196 (1) 861 (10) | ― | [ | sub-nanometer-scale fine regulation of interlayer distance | |||
| NiCo-LDH/C | 2558 (1) 1916 (20) | ― | [ | NiCo-LDH | 2115 (1) 949 (50) | 626 (100) 410 (150) | [ |
| 33 | Chen H C, Qin Y, Cao H, et al. Synthesis of amorphous nickel-cobalt-manganese hydroxides for supercapacitor-battery hybrid energy storage system[J]. Energy Storage Mater., 2019, 17: 194-203. |
| 34 | Li H B, Yu M H, Wang F X, et al. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials[J]. Nat. Commun., 2013, 4: 1894. |
| 35 | Li S, Zhang Y, Liu N, et al. Operando revealing dynamic reconstruction of NiCo carbonate hydroxide for high-rate energy storage[J]. Joule, 2020, 4: 1-15. |
| 36 | Zhao Y, Wang Q, Bian T, et al. Ni3+ doped monolayer layered double hydroxide nanosheets as efficient electrodes for supercapacitors[J]. Nanoscale, 2015, 7(16): 7168-7173. |
| 37 | Yang J, Yu C, Hu C, et al. Surface-confined fabrication of ultrathin nickel cobalt-layered double hydroxide nanosheets for high-performance supercapacitors[J]. Adv. Funct. Mater., 2018, 28(44): 1803272. |
| 38 | Gu Z, Atherton J J, Xu Z P. Hierarchical layered double hydroxide nanocomposites: structure, synthesis and applications[J]. Chem. Commun., 2015, 51(15): 3024-3036. |
| 39 | Shao M, Ning F, Zhao Y, et al. Core-shell layered double hydroxide microspheres with tunable interior architecture for supercapacitors[J]. Chem. Mater., 2012, 24(6): 1192-1197. |
| 40 | Yan T, Li Z, Li R, et al. Nickel-cobalt double hydroxides microspheres with hollow interior and hedgehog-like exterior structures for supercapacitors[J]. J. Mater. Chem., 2012, 22(44): 23587-23592. |
| 41 | Yan T, Zhu H, Li R, et al. Microwave synthesis of nickel/cobalt double hydroxide ultrathin flowerclusters with three-dimensional structures for high-performance supercapacitors[J]. Electrochim. Acta, 2013, 111: 71-79. |
| 42 | Li X, Zai J, Liu Y, et al. Atomically thin layered NiFe double hydroxides assembled 3D microspheres with promoted electrochemical performances[J]. J. Power Sources, 2016, 325: 675-681. |
| 43 | Liu X, Zhou A, Pan T, et al. Ultrahigh-rate-capability of a layered double hydroxide supercapacitor based on a self-generated electrolyte reservoir[J]. J. Mater. Chem. A, 2016, 4(21): 8421-8427. |
| 44 | Yang J, Yu C, Fan X, et al. 3D architecture materials made of NiCoAl-LDH nanoplates coupled with NiCo-carbonate hydroxide nanowires grown on flexible graphite paper for asymmetric supercapacitors[J]. Adv. Energy Mater., 2014, 4(18): 1400761. |
| 1 | Miller J R, Simon P. Electrochemical capacitors for energy management[J]. Science, 2008, 321(5889): 651-652. |
| 2 | Wang Y, Song Y, Xia Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications[J]. Chem. Soc. Rev., 2016, 45(21): 5925-5950. |
| 3 | Zhu Q, Zhao D, Cheng M, et al. A new view of supercapacitors: integrated supercapacitors[J]. Adv. Energy Mater., 2019, 9(36): 1901081. |
| 4 | Gao Q, Demarconnay L, Raymundo-Piñero E, et al. Exploring the large voltage range of carbon/carbon supercapacitors in aqueous lithium sulfate electrolyte[J]. Energy Environ. Sci., 2012, 5(11): 9611-9617. |
| 5 | Wu T H, Hsu C T, Hu C C, et al. Important parameters affecting the cell voltage of aqueous electrical double-layer capacitors[J]. J. Power Sources, 2013, 242: 289-298. |
| 6 | Xia J, Chen F, Li J, et al. Measurement of the quantum capacitance of graphene[J]. Nat. Nanotechnol., 2009, 4(8): 505-509. |
| 7 | Xiang K, Xu Z, Qu T, et al. Two dimensional oxygen-vacancy-rich Co3O4 nanosheets with excellent supercapacitor performances[J]. Chem. Commun., 2017, 53(92): 12410-12413. |
| 8 | Ma Q, Yao Y, Yan M, et al. Effective enhancement of electrochemical energy storage of cobalt-based nanocrystals by hybridization with nitrogen-doped carbon nanocages[J]. Sci. China Mater., 2019, 62(10): 1393-1402. |
| 9 | Lai H, Shang L, Wu Q, et al. Spinel nickel cobaltite mesostructures assembled from ultrathin nanosheets for high-performance electrochemical energy storage[J]. ACS Appl. Energy Mater., 2018, 1(2): 684-691. |
| 10 | Lai H, Wu Q, Zhao J, et al. Mesostructured NiO/Ni composites for high-performance electrochemical energy storage[J]. Energy Environ. Sci., 2016, 9(6): 2053-2060. |
| 11 | Song W, Wu J, Wang G, et al. Rich-mixed-valence NixCo3-xPy porous nanowires interwelded junction-free 3D network architectures for ultrahigh areal energy density supercapacitors[J]. Adv. Funct. Mater., 2018, 28(46): 1804620. |
| 12 | Chen L Y, Hou Y, Kang J L, et al. Toward the theoretical capacitance of RuO2 reinforced by highly conductive nanoporous gold[J]. Adv. Energy Mater., 2013, 3(7): 851-856. |
| 13 | Zhao M, Zhao Q, Li B, et al. Recent progress in layered double hydroxide based materials for electrochemical capacitors: design, synthesis and performance[J]. Nanoscale, 2017, 9(40): 15206-15225. |
| 14 | Zhou G, Xu L, Hu G, et al. Nanowires for electrochemical energy storage[J]. Chem. Rev., 2019, 119: 11042-11109. |
| 15 | Shao Y, El-Kady M F, Sun J, et al. Design and mechanisms of asymmetric supercapacitors[J]. Chem. Rev., 2018, 118: 9233-9280. |
| 16 | Yin H, Tang Z. Ultrathin two-dimensional layered metal hydroxides: an emerging platform for advanced catalysis, energy conversion and storage[J]. Chem. Soc. Rev., 2016, 45(18): 4873-4891. |
| 17 | Gao X, Wang P, Pan Z, et al. Recent progress in two-dimensional layered double hydroxides and their derivatives for supercapacitors[J]. ChemSusChem, 2020, 13(6): 1226-1254. |
| 18 | 孟格, 刘军枫, 孙晓明, 等. 水滑石纳米阵列电极在能量储存和转化中的应用[J]. 中国科学: 化学, 2017, 47(4): 408-419. |
| Meng G, Liu J F, Sun X M, et al. Layered double hydroxide nanoarrays toward electrochemical energy storage and conversion[J]. Sci. Sin. Chim., 2017, 47(4): 408-419. | |
| 19 | 安哲, 何静, 段雪. 基于层状前体制备活性位高分散催化材料[J]. 催化学报, 2013, 34(1): 225-234. |
| An Z, He J, Duan X. Catalysts with catalytic sites highly dispersed from layered double hydroxide as precursors[J]. Chin. J. Catal., 2013, 34(1): 225-234. | |
| 20 | 闫东鹏, 陆军, 段雪. 层状复合金属氢氧化物: 主客体结构研究进展[J]. 中国科学: 化学, 2013, 43(1): 1-14. |
| Yan D P, Lu J, Duan X. Layered double hydroxide: research progress of host-guest structure[J]. Sci. Sin. Chim., 2013, 43(1): 1-14. | |
| 21 | 陈艳, 王丽秋, 王晨晔, 等. 以钢渣为原料合成层状双氢氧化物及其结构表征[J]. 化工学报, 2015, 66(12): 5149-5156. |
| Chen Y, Wang L Q, Wang C Y, et al. Synthesis and structural characterization of layered double hydroxide using steel slag as raw material[J]. CIESC Journal, 2015, 66(12): 5149-5156. | |
| 22 | 王瑞瑞, 赵有璟, 邵明飞, 等. 层状双金属氢氧化物用于催化水氧化的研究进展[J]. 化工学报, 2016, 67(1): 54-72. |
| Wang R R, Zhao Y J, Shao M F, et al. Recent progresses in water oxidation over layered double hydroxide catalysts[J]. CIESC Journal, 2016, 67(1): 54-72. | |
| 23 | Li X, Du D, Zhang Y, et al. Layered double hydroxides toward high-performance supercapacitors[J]. J. Mater. Chem. A, 2017, 5(30): 15460-15485. |
| 24 | Yu J, Wang Q, O’Hare D, et al. Preparation of two dimensional layered double hydroxide nanosheets and their applications[J]. Chem. Soc. Rev., 2017, 46(19): 5950-5974. |
| 25 | Wang Q, O’Hare D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets[J]. Chem. Rev., 2012, 112(7): 4124-4155. |
| 26 | Chen J, Wang X, Wang J, et al. Sulfidation of NiMn-layered double hydroxides/graphene oxide composites toward supercapacitor electrodes with enhanced performance[J]. Adv. Energy Mater., 2016, 6(5): 1501745. |
| 27 | Quan W, Tang Z, Hong Y, et al. Hydroxyl compensation effects on the cycle stability of nickel-cobalt layered double hydroxides synthesized via solvothermal method[J]. Electrochim. Acta, 2015, 182: 445-451. |
| 28 | Wang X, Lin Y, Su Y, et al. Design and synthesis of ternary-component layered double hydroxides for high-performance supercapacitors: understanding the role of trivalent metal ions[J]. Electrochim. Acta, 2017, 225: 263-271. |
| 29 | Li Z, Duan H, Shao M, et al. Ordered-vacancy-induced cation intercalation into layered double hydroxides: a general approach for high-performance supercapacitors[J]. Chem, 2018, 4(9): 2168-2179. |
| 30 | Xie L, Hu Z, Lv C, et al. CoxNi1-x double hydroxide nanoparticles with ultrahigh specific capacitances as supercapacitor electrode materials[J]. Electrochim. Acta, 2012, 78: 205-211. |
| 31 | Vialat P, Mousty C, Taviot-Gueho C, et al. High-performing monometallic cobalt layered double hydroxide supercapacitor with defined local structure[J]. Adv. Funct. Mater., 2014, 24(30): 4831-4842. |
| 32 | Vialat P, Rabu P, Mousty C, et al. Insight of an easy topochemical oxidative reaction in obtaining high performance electrochemical capacitor based on CoⅡCoⅢ monometallic cobalt layered double hydroxide[J]. J. Power Sources, 2015, 293: 1-10. |
| 45 | Li Z, Shao M, Zhou L, et al. A flexible all-solid-state micro-supercapacitor based on hierarchical CuO@layered double hydroxide core–shell nanoarrays[J]. Nano Energy, 2016, 20: 294-304. |
| 46 | Yu L, Shi N, Liu Q, et al. Facile synthesis of exfoliated Co-Al LDH-carbon nanotube composites with high performance as supercapacitor electrodes[J]. Phys. Chem. Chem. Phys., 2014, 16(33): 17936-17942. |
| 47 | Chen H, Cai F, Kang Y, et al. Facile assembly of Ni-Co hydroxide nanoflakes on carbon nanotube network with highly electrochemical capacitive performance[J]. ACS Appl. Mater. Interfaces, 2014, 6(22): 19630-19637. |
| 48 | Wang Y, Chen Z, Li H, et al. The synthesis and electrochemical performance of core-shell structured Ni-Al layered double hydroxide/carbon nanotubes composites[J]. Electrochim. Acta, 2016, 222: 185-193. |
| 49 | Zhao J, Chen J, Xu S, et al. Hierarchical NiMn layered double hydroxide/carbon nanotubes architecture with superb energy density for flexible supercapacitors[J]. Adv. Funct. Mater., 2014, 24(20): 2938-2946. |
| 50 | Liu C G, Lee Y S, Kim Y J, et al. Electrochemical characteristics of hydrothermally deposited nickel hydroxide on multi-walled carbon nanotube for supercapacitor electrode[J]. Synthetic Met., 2009, 159: 2009-2012. |
| 51 | Yang W, Gao Z, Wang J, et al. Solvothermal one-step synthesis of Ni-Al layered double hydroxide/carbon nanotube/reduced graphene oxide sheet ternary nanocomposite with ultrahigh capacitance for supercapacitors[J]. ACS Appl. Mater. Interfaces, 2013, 5(12): 5443-5454. |
| 52 | Zhou Q, Fan T, Li Y, et al. Hollow-structure NiCo hydroxide/carbon nanotube composite for high-performance supercapacitors[J]. J. Power Sources, 2019, 426: 111-115. |
| 53 | Yu C, Yang J, Zhao C, et al. Nanohybrids from NiCoAl-LDH coupled with carbon for pseudocapacitors: understanding the role of nano-structured carbon[J]. Nanoscale, 2014, 6(6): 3097-3104. |
| 54 | Bai C, Sun S, Xu Y, et al. Facile one-step synthesis of nanocomposite based on carbon nanotubes and Nickel-Aluminum layered double hydroxides with high cycling stability for supercapacitors[J]. J. Colloid Interf. Sci., 2016, 480: 57-62. |
| 55 | Li M, Liu F, Zhang X B, et al. A comparative study of Ni-Mn layered double hydroxide/carbon composites with different morphologies for supercapacitors[J]. Phys. Chem. Chem. Phys., 2016, 18(43): 30068-30078. |
| 56 | Su L, Zhang X, Yuan C, et al. Symmetric self-hybrid supercapacitor consisting of multiwall carbon nanotubes and Co-Al layered double hydroxides[J]. J. Electrochem. Soc., 2008, 155(2): A110-A114. |
| 57 | Lai F, Miao Y E, Zuo L, et al. Biomass-derived nitrogen-doped carbon nanofiber network: a facile template for decoration of ultrathin nickel-cobalt layered double hydroxide nanosheets as high-performance asymmetric supercapacitor electrode[J]. Small, 2016, 12(24): 3235-3244. |
| 58 | Xu J, Gai S, He F, et al. A sandwich-type three-dimensional layered double hydroxide nanosheet array/graphene composite: fabrication and high supercapacitor performance[J]. J. Mater. Chem. A, 2014, 2(4): 1022-1031. |
| 59 | Gao Z, Wang J, Li Z, et al. Graphene nanosheet/Ni2+/Al3+ layered double-hydroxide composite as a novel electrode for a supercapacitor[J]. Chem. Mater., 2011, 23(15): 3509-3516. |
| 60 | Zhang L, Wang J, Zhu J, et al. 3D porous layered double hydroxides grown on graphene as advanced electrochemical pseudocapacitor materials[J]. J. Mater. Chem. A, 2013, 1(32): 9046-9053. |
| 61 | Wimalasiri Y, Fan R, Zhao X S, et al. Assembly of Ni-Al layered double hydroxide and graphene electrodes for supercapacitors[J]. Electrochim. Acta, 2014, 134: 127-135. |
| 62 | Yan L, Li R, Li Z, et al. Three-dimensional activated reduced graphene oxide nanocup/nickel aluminum layered double hydroxides composite with super high electrochemical and capacitance performances[J]. Electrochim. Acta, 2013, 95: 146-154. |
| 63 | Huang P, Cao C, Sun Y, et al. One-pot synthesis of sandwich-like reduced graphene oxide@CoNiAl layered double hydroxide with excellent pseudocapacitive properties[J]. J. Mater. Chem. A, 2015, 3(20): 10858-10863. |
| 64 | Cai X, Shen X, Ma L, et al. Solvothermal synthesis of NiCo-layered double hydroxide nanosheets decorated on RGO sheets for high performance supercapacitor[J]. Chem. Eng. J., 2015, 268: 251-259. |
| 65 | Zhong Y, Liao Y, Gao A, et al. Supercapacitive behavior of electrostatic self-assembly reduced graphene oxide/CoAl-layered double hydroxides nanocomposites[J]. J. Alloy. Compd., 2016, 669: 146-155. |
| 66 | Fang J, Li M, Li Q, et al. Microwave-assisted synthesis of CoAl-layered double hydroxide/graphene oxide composite and its application in supercapacitors[J]. Electrochim. Acta, 2012, 85: 248-255. |
| 67 | Zhang L, Zhang X, Shen L, et al. Enhanced high-current capacitive behavior of graphene/CoAl-layered double hydroxide composites as electrode material for supercapacitors[J]. J. Power Sources, 2012, 199: 395-401. |
| 68 | Dong X, Wang L, Wang D, et al. Layer-by-layer engineered Co-Al hydroxide nanosheets/graphene multilayer films as flexible electrode for supercapacitor[J]. Langmuir, 2012, 28(1): 293-298. |
| 69 | Zhang W, Ma C, Fang J, et al. Asymmetric electrochemical capacitors with high energy and power density based on graphene/CoAl-LDH and activated carbon electrodes[J]. RSC Adv., 2013, 3(7): 2483-2490. |
| 70 | Yan T, Li R, Li Z. Nickel-cobalt layered double hydroxide ultrathin nanoflakes decorated on graphene sheets with a 3D nanonetwork structure as supercapacitive materials[J]. Mater. Res. Bull., 2014, 51: 97-104. |
| 71 | Wan H, Liu J, Ruan Y, et al. Hierarchical configuration of NiCo2S4 nanotube@Ni-Mn layered double hydroxide arrays/three-dimensional graphene sponge as electrode materials for high-capacitance supercapacitors[J]. ACS Appl. Mater. Interfaces, 2015, 7(29): 15840-15847. |
| 72 | Li M, Cheng J P, Liu F, et al. 3D-Architectured nickel-cobalt-manganese layered double hydroxide/reduced graphene oxide composite for high-performance supercapacitor[J]. Chem. Phys. Lett., 2015, 640: 5-10. |
| 73 | Zhang L, Hui K N, Hui K S, et al. Facile synthesis of porous CoAl-layered double hydroxide/graphene composite with enhanced capacitive performance for supercapacitors[J]. Electrochim. Acta, 2015, 186: 522-529. |
| 74 | Hatui G, Nayak G C, Udayabhanu G. One pot solvothermal synthesis of sandwich-like Mg Al layered double hydroxide anchored reduced graphene oxide: an excellent electrode material for supercapacitor[J]. Electrochim. Acta, 2016, 219: 214-226. |
| 75 | Li M, Cheng J P, Wang J, et al. The growth of nickel-manganese and cobalt-manganese layered double hydroxides on reduced graphene oxide for supercapacitor[J]. Electrochim. Acta, 2016, 206: 108-115. |
| 76 | Zheng C H, Yao T, Xu T R, et al. Growth of ultrathin Ni-Co-Al layered double hydroxide on reduced graphene oxide and superb supercapacitive performance of the resulting composite[J]. J. Alloy. Compd., 2016, 678: 93-101. |
| 77 | 严琳, 孔惠, 李在均. 3D石墨烯/镍铝层状双金属氢氧化物的制备及超级电容性能[J]. 化学学报, 2013, 71(5): 822-828. |
| Yan L, Kong H, Li Z J. Synthesis and supercapacitor property of three-dimensional graphene/Ni-Al layered double hydroxide composite[J]. Acta Chim. Sinica, 2013, 71(5): 822-828. | |
| 78 | Gao X, Lv H, Li Z, et al. Low-cost and high-performance of a vertically grown 3D Ni-Fe layered double hydroxide/graphene aerogel supercapacitor electrode material[J]. RSC Adv., 2016, 6(109): 107278-107285. |
| 79 | Peng W, Li H, Song S. Synthesis of fluorinated graphene/CoAl-layered double hydroxide composites as electrode materials for supercapacitors[J]. ACS Appl. Mater. Interfaces, 2017, 9(6): 5204-5212. |
| 80 | Daud M, Kamal M S, Shehzad F, et al. Graphene/layered double hydroxides nanocomposites: a review of recent progress in synthesis and applications[J]. Carbon, 2016, 104: 241-252. |
| 81 | Gu T H, Gunjakar J L, Kim I Y, et al. Porous hybrid network of graphene and metal oxide nanosheets as useful matrix for improving the electrode performance of layered double hydroxides[J]. Small, 2015, 11(32): 3921-3931. |
| 82 | Wei Y, Zhang X, Wu X, et al. Carbon quantum dots/Ni-Al layered double hydroxide composite for high-performance supercapacitors[J]. RSC Adv., 2016, 6(45): 39317-39322. |
| 83 | Xiong G, He P, Wang D, et al. Hierarchical Ni-Co hydroxide petals on mechanically robust graphene petal foam for high-energy asymmetric supercapacitors[J]. Adv. Funct. Mater., 2016, 26(30): 5460-5470. |
| 84 | Malak-Polaczyk A, Vix-Guterl C, Frackowiak E. Carbon/layered double hydroxide (LDH) composites for supercapacitor application[J]. Energy Fuels, 2010, 24(6): 3346-3351. |
| 85 | Yang Q, Li Z, Zhang R, et al. Carbon modified transition metal oxides/hydroxides nanoarrays toward high-performance flexible all-solid-state supercapacitors[J]. Nano Energy, 2017, 41: 408-416. |
| 86 | Wei J, Wang J, Song Y, et al. Synthesis of self-assembled layered double hydroxides/carbon composites by in situ solvothermal method and their application in capacitors[J]. J. Solid State Chem., 2012, 196: 175-181. |
| 87 | Zhang L, Ou M, Yao H, et al. Enhanced supercapacitive performance of graphite-like C3N4 assembled with NiAl-layered double hydroxide[J]. Electrochim. Acta, 2015, 186: 292-301. |
| 88 | Xu J, Ma C, Cao J, et al. Facile synthesis of core-shell nanostructured hollow carbon nanospheres@nickel cobalt double hydroxide as high-performance electrode materials for supercapacitors[J]. Dalton Trans., 2017, 46(10): 3276-3283. |
| 89 | Wang Y, Dou H, Wang J, et al. Three-dimensional porous MXene/layered double hydroxide composite for high performance supercapacitors[J]. J. Power Sources, 2016, 327: 221-228. |
| 90 | Zhang D, Cao J, Zhang X, et al. NiMn layered double hydroxide nanosheets in-situ anchored on Ti3C2 MXene via chemical bonds for superior supercapacitors[J]. ACS Appl. Energy Mater., 2020, 3(6): 5949-5964. |
| 91 | Li S, Cheng P, Luo J, et al. High-performance flexible asymmetric supercapacitor based on CoAl-LDH and rGO electrodes[J]. Nano-Micro Lett., 2017, 9(3): 31. |
| 92 | Shi L, Sun P, Du L, et al. Flexible honeycomb-like NiMn layered double hydroxide/carbon cloth architecture for electrochemical energy storage[J]. Mater. Lett., 2016, 175: 275-278. |
| 93 | Li S, Yu C, Yang J, et al. A superhydrophilic “nanoglue” for stabilizing metal hydroxides onto carbon materials for high-energy and ultralong-life asymmetric supercapacitors[J]. Energy Environ. Sci., 2017, 10(9): 1958-1965. |
| 94 | Zhao J, Chen J, Xu S, et al. CoMn-layered double hydroxide nanowalls supported on carbon fibers for high-performance flexible energy storage devices[J]. J. Mater. Chem. A, 2013, 1(31): 8836-8843. |
| 95 | Shakir I, Shahid M, Rana U A, et al. Nickel-cobalt layered double hydroxide anchored zinc oxide nanowires grown on carbon fiber cloth for high-performance flexible pseudocapacitive energy storage devices[J]. Electrochim. Acta, 2014, 129: 28-32. |
| 96 | Nagaraju G, Raju G S R, Ko Y H, et al. Hierarchical Ni-Co layered double hydroxide nanosheets entrapped on conductive textile fibers: a cost-effective and flexible electrode for high-performance pseudocapacitors[J]. Nanoscale, 2016, 8(2): 812-825. |
| 97 | Chen H, Hu L, Chen M, et al. Nickel-cobalt layered double hydroxide nanosheets for high-performance supercapacitor electrode materials[J]. Adv. Funct. Mater., 2014, 24(7): 934-942. |
| 98 | Liu S, Lee S C, Patil U, et al. Hierarchical MnCo-layered double hydroxides@Ni(OH)2 core-shell heterostructures as advanced electrodes for supercapacitors[J]. J. Mater. Chem. A, 2017, 5(3): 1043-1049. |
| 99 | Gu Y, Lu Z, Chang Z, et al. NiTi layered double hydroxide thin films for advanced pseudocapacitor electrodes[J]. J. Mater. Chem. A, 2013, 1(36): 10655-10661. |
| 100 | Guo X L, Liu X Y, Hao X D, et al. Nickel-manganese layered double hydroxide nanosheets supported on nickel foam for high-performance supercapacitor electrode materials[J]. Electrochim. Acta, 2016, 194: 179-186. |
| 101 | Zheng X, Gu Z, Hu Q, et al. Ultrathin porous nickel-cobalt hydroxide nanosheets for high-performance supercapacitor electrodes[J]. RSC Adv., 2015, 5(22): 17007-17013. |
| 102 | Han J, Dou Y, Zhao J, et al. Flexible CoAl LDH@PEDOT core/shell nanoplatelet array for high-performance energy storage[J]. Small, 2013, 9(1): 98-106. |
| 103 | Abushrenta N, Wu X, Wang J, et al. Hierarchical Co-based porous layered double hydroxide arrays derived via alkali etching for highperformance supercapacitors[J]. Sci. Rep., 2015, 5: 13082. |
| 104 | Adhikari S P, Awasthi G P, Kim K S, et al. Synthesis of three-dimensional mesoporous Cu-Al layered double hydroxide/g-C3N4 nanocomposites on Ni-foam for enhanced supercapacitors with excellent long-term cycling stability[J]. Dalton Trans., 2018, 47(13): 4455-4466. |
| 105 | Wang J, Song Y, Li Z, et al. In situ Ni/Al layered double hydroxide and its electrochemical capacitance performance[J]. Energy Fuels, 2010, 24(12): 6463-6467. |
| 106 | Guo W, Yu C, Li S, et al. High-stacking-density, superior-roughness LDH bridged with vertically aligned graphene for high-performance asymmetric supercapacitors[J]. Small, 2017, 13(37): 1701288. |
| 107 | Gupta V, Gupta S, Miura N. Potentiostatically deposited nanostructured CoxNi1-x layered double hydroxides as electrode materials for redox-supercapacitors[J]. J. Power Sources, 2008, 175(1): 680-685. |
| 108 | Ge X, Gu C D, Wang X L, et al. Ionothermal synthesis of cobalt iron layered double hydroxides (LDHs) with expanded interlayer spacing as advanced electrochemical materials[J]. J. Mater. Chem. A, 2014, 2(40): 17066-17076. |
| 109 | Xiao Y, Su D, Wang X, et al. Layered double hydroxides with larger interlayer distance for enhanced pseudocapacitance[J]. Sci. China Mater., 2018, 61(2): 263-272. |
| 110 | Lin Y, Xie X, Wang X, et al. Understanding the enhancement of electrochemical properties of NiCo layered double hydroxides via functional pillared effect: an insight into dual charge storage mechanisms[J]. Electrochim. Acta, 2017, 246: 406-414. |
| 111 | Zhang H, Tahir M U, Yan X, et al. Ni-Al layered double hydroxide with regulated interlayer spacing as electrode for aqueous asymmetric supercapacitor[J]. Chem. Eng. J., 2019, 368: 905-913. |
| 112 | Silva A S, Sanchis-Gual R, Carrasco J A, et al. Boosting the supercapacitive behavior of CoAl layered double hydroxides via tuning the metal composition and interlayer space[J]. Batter. Supercaps, 2020, 3(6): 499-509. |
| 113 | Wang L, Dong Z H, Wang Z G, et al. Layered α-Co(OH)2 nanocones as electrode materials for pseudocapacitors: understanding the effect of interlayer space on electrochemical activity[J]. Adv. Funct. Mater., 2013, 23(21): 2758-2764. |
| 114 | Wang X, Zhang J, Yang S, et al. Interlayer space regulating of NiMn layered double hydroxides for supercapacitors by controlling hydrothermal reaction time[J]. Electrochim. Acta, 2019, 295: 1-6. |
| 115 | Liu X, Ma R, Bando Y, et al. A general strategy to layered transition-metal hydroxide nanocones: tuning the composition for high electrochemical performance[J]. Adv. Mater., 2012, 24(16): 2148-2153. |
| 116 | Liu X, Ma R, Bando Y, et al. High-yield preparation, versatile structural modification, and properties of layered cobalt hydroxide nanocones[J]. Adv. Funct. Mater., 2014, 24(27): 4292-4302. |
| 117 | Wang X, Yan C, Sumboja A, et al. Achieving high rate performance in layered hydroxide supercapacitor electrodes[J]. Adv. Energy Mater., 2014, 4(6): 1301240. |
| 118 | Ge X, Gu C, Yin Z, et al. Periodic stacking of 2D charged sheets: self-assembled superlattice of Ni-Al layered double hydroxide (LDH) and reduced graphene oxide[J]. Nano Energy, 2016, 20: 185-193. |
| 119 | Ma R, Liu X, Liang J, et al. Molecular-scale heteroassembly of redoxable hydroxide nanosheets and conductive graphene into superlattice composites for high-performance supercapacitors[J]. Adv. Mater., 2014, 26(24): 4173-4178. |
| 120 | Zhang R, An H, Li Z, et al. Mesoporous graphene-layered double hydroxides free-standing films for enhanced flexible supercapacitors[J]. Chem. Eng. J., 2016, 289: 85-92. |
| 121 | Wu X, Jiang L, Long C, et al. Dual support system ensuring porous Co-Al hydroxide nanosheets with ultrahigh rate performance and high energy density for supercapacitors[J]. Adv. Funct. Mater., 2015, 25(11): 1648-1655. |
| 122 | Zhao J, Xu S, Tschulik K, et al. Molecular-scale hybridization of clay monolayers and conducting polymer for thin-film supercapacitors[J]. Adv. Funct. Mater., 2015, 25(18): 2745-2753. |
| 123 | Lan Y, Li M, Fan W, et al. Functional molecules regulated and intercalated nickel-cobalt LDH nano-sheets on carbon fiber cloths as an advanced free-standing electrode for high-performance asymmetric supercapacitors[J]. Electrochim. Acta, 2019, 321: 134708. |
| 124 | Yin Q, Zhang J, Liu X, et al. Pillaring-effect induced ultrahigh-rate pseudocapacitive energy storage based on layered double hydroxide nanoplate arrays[J]. Ind. Eng. Chem. Res., 2019, 58(27): 11954-11963. |
| 125 | Zhao J, Ge C, Zhao Z, et al. Sub-nanometer-scale fine regulation of interlayer distance in Ni-Co layered double hydroxides leading to high-rate supercapacitors[J]. Nano Energy, 2020, 76: 105026. |
| 126 | Simon P, Gogotsi Y. Perspectives for electrochemical capacitors and related devices [J]. Nat. Mater., 2020, . |
| [1] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [2] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [5] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [8] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [9] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [10] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [11] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [12] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [13] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [14] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [15] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号