化工学报 ›› 2019, Vol. 70 ›› Issue (7): 2540-2547.DOI: 10.11949/0438-1157.20190065
收稿日期:2019-01-21
修回日期:2019-04-04
出版日期:2019-07-05
发布日期:2019-07-05
通讯作者:
段学志
作者简介:宋楠(1983—),女,博士研究生,讲师,<email>cuiky@ecust.edu.cn</email>
基金资助:
Nan SONG( ),Minjian PAN,Bingxu CHEN,Gang QIAN,Xuezhi DUAN(
),Minjian PAN,Bingxu CHEN,Gang QIAN,Xuezhi DUAN( ),Xinggui ZHOU
),Xinggui ZHOU
Received:2019-01-21
Revised:2019-04-04
Online:2019-07-05
Published:2019-07-05
Contact:
Xuezhi DUAN
摘要:
Fe2C是低温Fe基费托催化剂的主要活性相,研究其上费托反应机理具有十分重要的意义。从原子尺度上通过密度泛函理论(DFT)计算研究了Fe2C稳定晶相η-Fe2C的(011)表面上甲烷形成和C—C耦合的反应机理。计算结果表明,η-Fe2C(011)表面上甲烷形成的有效能垒为1.03 eV,其低于CHi+CHj耦合反应的有效能垒(1.52~2.98 eV),且最可能的C—C耦合反应路径为C+CH3。进一步比较研究了η-Fe2C(011)表面与其他Fe基费托催化剂表面之间的CH4和C2+选择性差异,发现选择性高度敏感于Fe基催化剂的表面与体相结构,其中η-Fe2C(011)表面具有较高的甲烷选择性。
中图分类号:
宋楠, 潘敏建, 陈炳旭, 钱刚, 段学志, 周兴贵. 费托催化剂η-Fe2C (011)上CH4形成及C-C耦合机理研究[J]. 化工学报, 2019, 70(7): 2540-2547.
Nan SONG, Minjian PAN, Bingxu CHEN, Gang QIAN, Xuezhi DUAN, Xinggui ZHOU. CH4 formation and C—C coupling mechanism on (011) surface of η-Fe2C Fischer-Tropsch catalyst[J]. CIESC Journal, 2019, 70(7): 2540-2547.

图1 H(a)和CHx(b)在η-Fe2C(011)面上稳定吸附构型的俯视图和侧视图及该面上涉及甲烷化基元反应的过渡态构型的俯视图和侧视图(c)(蓝色:Fe;灰色:催化剂的表面C;绿色:涉及反应的表面C;白色:H;黄色:涉及反应的H)
Fig.1 Top and side views of favorable adsorption configurations of H (a) and CHx (b) on η-Fe2C(011) surface and corresponding top and side views of TSs of elementary steps involved in methanation (c)(blue: Fe atoms; grey: C atoms of iron carbide; green: C atoms involved in reactions; white: H atoms; yellow: H atoms involved in reactions)
| Site | rFe-H / nm | rC-H / nm | Eads /eV |
|---|---|---|---|
| 2F | 0.172,0.170 | -2.22 | |
| 3F | 0.170,0.178,0.184 | -2.27 | |
| 4F | 0.112 | -2.03 |
表1 H在完美η-Fe2C(011)面上稳定吸附构型的吸附能和对应结构参数
Table 1 Key energetics and structural parameters of identified H adsorption on perfect η-Fe2C(011) surface
| Site | rFe-H / nm | rC-H / nm | Eads /eV |
|---|---|---|---|
| 2F | 0.172,0.170 | -2.22 | |
| 3F | 0.170,0.178,0.184 | -2.27 | |
| 4F | 0.112 | -2.03 |
| Reaction | dC—H /nm | Ea /eV | ΔrE /eV |
|---|---|---|---|
| C+ H | 0.142 | 0.49 (0.44) | 0.15 (0.19) |
| CH + H | 0.147 | 0.93 (0.83) | 0.44 (0.50) |
| CH2 + H | 0.160 | 0.27 (0.25) | -0.41 (-0.31) |
| CH3+ H | 0.157 | 0.56 (0.56) | -0.18 (0.08) |
表2 η-Fe2C(011) 面上涉及甲烷形成基元步骤的C—H距离(dC—H)、反应能垒(Ea)和反应能(ΔrE)(括号内数据经ZPE校正)
Table 2 C—H distances (dC—H) at TSs, reaction barriers (Ea) and reaction energies (ΔrE) involved in CH4 formation on η-Fe2C(011) surface (values including ZPE in parentheses)
| Reaction | dC—H /nm | Ea /eV | ΔrE /eV |
|---|---|---|---|
| C+ H | 0.142 | 0.49 (0.44) | 0.15 (0.19) |
| CH + H | 0.147 | 0.93 (0.83) | 0.44 (0.50) |
| CH2 + H | 0.160 | 0.27 (0.25) | -0.41 (-0.31) |
| CH3+ H | 0.157 | 0.56 (0.56) | -0.18 (0.08) |
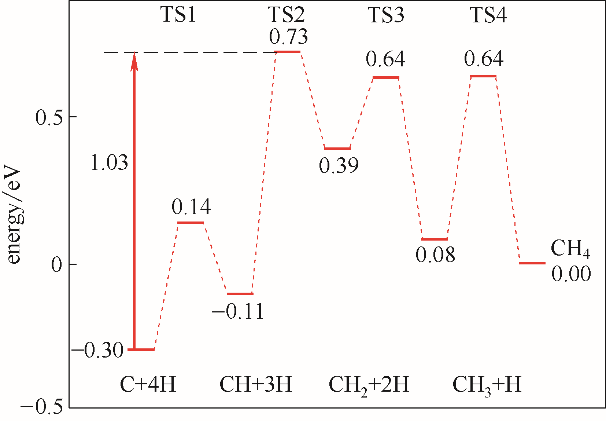
图2 η-Fe2C(011)面甲烷形成势能图(有效能垒在图中由红色箭头标出)
Fig.2 Energy profiles of CH4 formation on η-Fe2C(011) surface (corresponding effective barriers were also presented)
| Reaction | dC-C/nm | Ea/eV | ΔrE/eV | |
|---|---|---|---|---|
| C+C | 0.222 | 3.00 (2.98) | 1.15 (1.15) | 3.00 (2.98) |
| C+CH | 0.224 | 1.70 (1.74) | 0.56 (0.60) | 1.85 (1.93) |
| C+CH2 | 0.201 | 1.17 (1.18) | 0.33 (0.38) | 1.76 (1.87) |
| C+CH3 | 0.197 | 1.14 (1.14) | 0.54 (0.58) | 1.32 (1.52) |
| CH+CH | 0.192 | 1.89 (1.90) | 0.62 (0.68) | 2.18 (2.29) |
| CH+CH2 | 0.206 | 1.22 (1.19) | 0.54 (0.57) | 1.95 (2.08) |
| CH+CH3 | 0.203 | 1.60 (1.63) | 0.80 (0.84) | 1.92 (2.21) |
| CH2+CH2 | 0.215 | 0.96 (0.98) | -0.02 (0.09) | 2.13(2.36) |
| CH2+CH3 | 0.203 | 1.19 (1.27) | 0.42 (0.57) | 1.95 (2.34) |
表3 η-Fe2C(011)面上C1+C1耦合反应的能垒(Ea)、反应能(ΔEr)、C-C的距离(dTS)以及相应的有效能垒(Eeff,CHi+CHj)(括号内数据经ZPE校正)
Table 3 C-C distances (dC-C) at TSs and reaction barriers (Ea), reaction energies (ΔrE) and effective activation energies (Eeff,CHi-CHj) of C1+C1 coupling reactions on η-Fe2C(011) surface (values including ZPE in parentheses)
| Reaction | dC-C/nm | Ea/eV | ΔrE/eV | |
|---|---|---|---|---|
| C+C | 0.222 | 3.00 (2.98) | 1.15 (1.15) | 3.00 (2.98) |
| C+CH | 0.224 | 1.70 (1.74) | 0.56 (0.60) | 1.85 (1.93) |
| C+CH2 | 0.201 | 1.17 (1.18) | 0.33 (0.38) | 1.76 (1.87) |
| C+CH3 | 0.197 | 1.14 (1.14) | 0.54 (0.58) | 1.32 (1.52) |
| CH+CH | 0.192 | 1.89 (1.90) | 0.62 (0.68) | 2.18 (2.29) |
| CH+CH2 | 0.206 | 1.22 (1.19) | 0.54 (0.57) | 1.95 (2.08) |
| CH+CH3 | 0.203 | 1.60 (1.63) | 0.80 (0.84) | 1.92 (2.21) |
| CH2+CH2 | 0.215 | 0.96 (0.98) | -0.02 (0.09) | 2.13(2.36) |
| CH2+CH3 | 0.203 | 1.19 (1.27) | 0.42 (0.57) | 1.95 (2.34) |
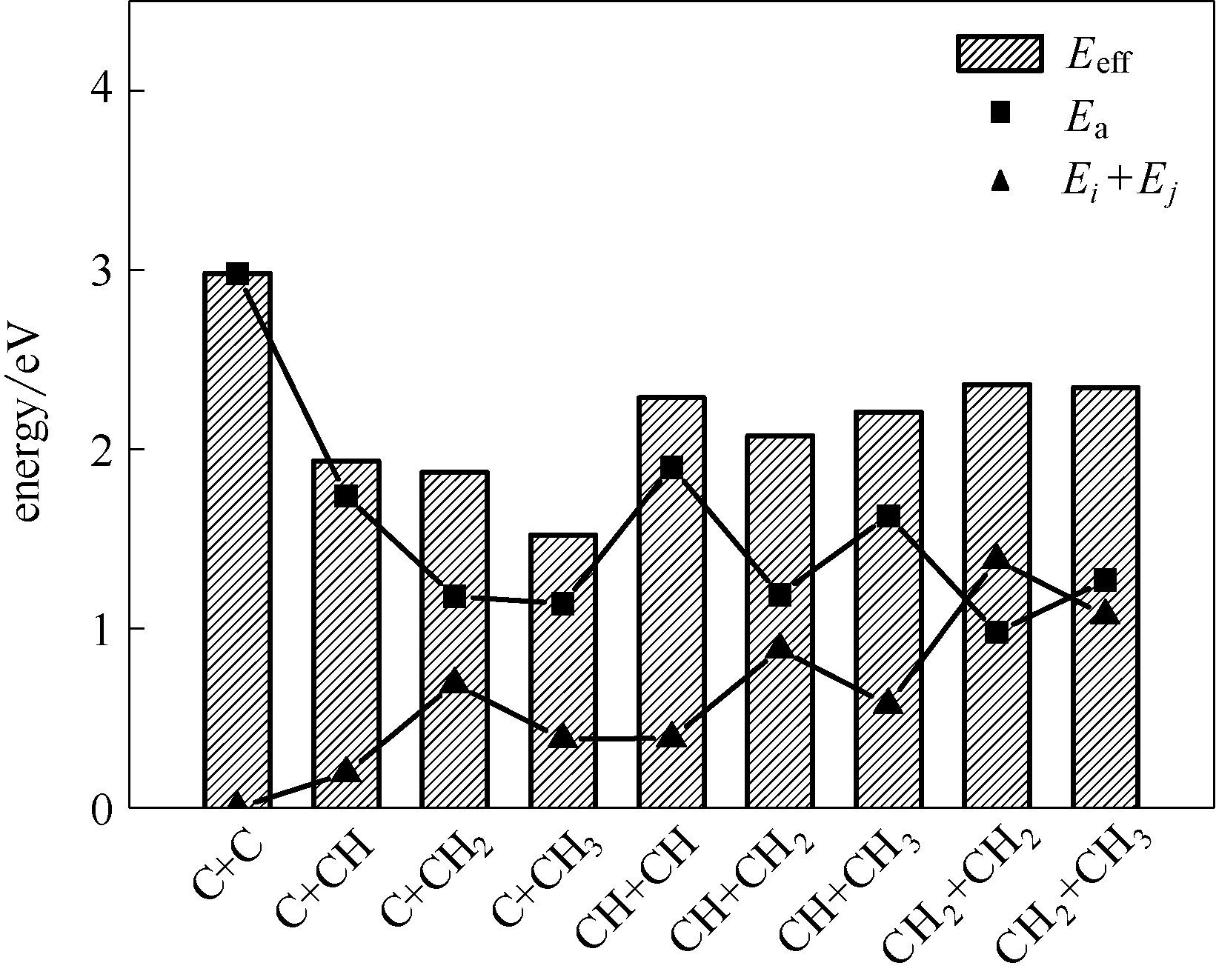
图4 η-Fe2C (011)面上CHi+CHj偶联反应的能垒(Ea)、反应物的相对能量(Ei+Ej)以及有效能垒(Eeff)
Fig.4 Barriers, relative energy of reactants and effective barriers of CHi+CHj coupling on η-Fe2C(011) surface
| Surface | The most possible C—C coupling route | Ea/eV | Ref. | |
|---|---|---|---|---|
Fe | (210) | C+CH3 | 1.10 | [38] |
(100) | C+CH2 C+CH3 | 0.73 | [31] [31] | |
| 0.49 | ||||
χ-Fe5C2 | (100) | C+CH3 | 1.02 | [26] |
| (001) | C+CO | 0.66 | [39] | |
(510) | C+CH CH+CH | 1.09 | [16] [16] | |
| 0.96 | ||||
θ-Fe3C | (031) | CH+CH CH2+CH2 | 0.76 | [17] [17] |
| 0.50 | ||||
η-Fe2C | (011) | C+CH3 CH2+CH2 | 1.14 | this work this work |
| 0.98 | ||||
表4 不同碳化铁表面上最可能的C1+C1耦合路径及其能垒
Table 4 Most possible routes and their activation barriers of C1+C1 coupling on different iron carbide surfaces
| Surface | The most possible C—C coupling route | Ea/eV | Ref. | |
|---|---|---|---|---|
Fe | (210) | C+CH3 | 1.10 | [38] |
(100) | C+CH2 C+CH3 | 0.73 | [31] [31] | |
| 0.49 | ||||
χ-Fe5C2 | (100) | C+CH3 | 1.02 | [26] |
| (001) | C+CO | 0.66 | [39] | |
(510) | C+CH CH+CH | 1.09 | [16] [16] | |
| 0.96 | ||||
θ-Fe3C | (031) | CH+CH CH2+CH2 | 0.76 | [17] [17] |
| 0.50 | ||||
η-Fe2C | (011) | C+CH3 CH2+CH2 | 1.14 | this work this work |
| 0.98 | ||||
| Surface | ΔEeff/eV | Site | Ref. | ||
|---|---|---|---|---|---|
| Fe(210) | 2.13 | 2.19 | -0.06 | step | [28] |
| Fe(100) | 2.13 | 1.92 | 0.21 | step | [24-45] |
| χ-Fe5C2(100) | 1.89 | 1.94 | -0.05 | step | [26] |
| χ-Fe5C2(510) | 2.39 | 1.66 | 0.73 | terrace | [16] |
| θ-Fe3C(031) | 2.29 | 0.99 | 1.30 | terrace | [17] |
| η-Fe2C(011) | 1.03 | 1.52 | -0.49 | step | this work |
表5 不同碳化铁表面上甲烷形成和C1+C1耦合的有效能垒及其差值
Table 5 Effective barriers of CH4 formation and C1+C1 coupling and their barrier differences on different iron carbide surfaces
| Surface | ΔEeff/eV | Site | Ref. | ||
|---|---|---|---|---|---|
| Fe(210) | 2.13 | 2.19 | -0.06 | step | [28] |
| Fe(100) | 2.13 | 1.92 | 0.21 | step | [24-45] |
| χ-Fe5C2(100) | 1.89 | 1.94 | -0.05 | step | [26] |
| χ-Fe5C2(510) | 2.39 | 1.66 | 0.73 | terrace | [16] |
| θ-Fe3C(031) | 2.29 | 0.99 | 1.30 | terrace | [17] |
| η-Fe2C(011) | 1.03 | 1.52 | -0.49 | step | this work |
| 1 | ZhangQ, KangJ, WangY. Development of novel catalysts for Fischer-Tropsch synthesis: tuning the product selectivity[J]. ChemCatChem, 2010, 2(9): 1030-1058. |
| 2 | 王野, 成康, 张庆红. 一氧化碳加氢制碳氢化合物反应选择性的调控[J]. 中国科学: 化学, 2012, 42(4): 263-375. |
| WangY, ChengK, ZhangQ H. Selectivity tuning for the hydrogenation of carbon monoxide into hydrocarbons[J]. Scientia Sinica Chimica, 2012, 42(4): 263-375. | |
| 3 | JagerB, EspinozaR. Advances in low temperature Fischer-Tropsch synthesis[J]. Catalysis Today, 1995, 23: 17-28. |
| 4 | XuJ, YangY, LiY W. Fischer-Tropsch synthesis process development: steps from fundamentals to industrial practices[J]. Current Opinion in Chemical Engineering, 2013, 2: 354-362. |
| 5 | JahangiriH, BennettJ, MahjoubiP, et al. A review of advanced catalyst development for Fischer-Tropsch synthesis of hydrocarbons from biomass derived syngas[J]. Catalysis Science & Technology, 2014, 4(8): 2210-2229. |
| 6 | SchulzH. Short history and present trends of Fischer-Tropsch synthesis[J]. Applied Catalysis A: General, 1999, 186: 3-12. |
| 7 | 温晓东, 杨勇, 李永旺, 等. 费托合成铁基催化剂的设计基础: 从理论走向实践[J]. 中国科学: 化学, 2017, (11): 72-85. |
| WenX D, YangY, LiY W, et al. The design principle of iron-based catalysts for Fischer-Tropsch synthesis: from theory to practice[J]. Scientia Sinica Chimica, 2017, (11): 72-85. | |
| 8 | de SmitE, CinquiniF, WeckhuysenB M, et al. Stability and reactivity of ϵ-χ-θ iron carbide catalyst phases in Fischer-Tropsch synthesis: controlling μC[J]. Journal of the American Chemical Society, 2010, 132: 14928-14941. |
| 9 | XuK, SunB, QiaoM H, et al. ε-Iron carbide as a low-temperature Fischer-Tropsch synthesis catalyst[J]. Nature Communication, 2014, 5: 5783-5790. |
| 10 | LiuX W, ZhaoS, MengY. Mössbauer spectroscopy of iron carbides: from prediction to experimental confirmation[J]. Scientific Reports, 2016, 6: 26184. |
| 11 | JoséG R C, MaartenK S , Marie-FrancoiseR. First principle study on the adsorption of hdrocarbon chains involved in Fischer-Tropsch synthesis over iron carbides[J]. The Journal of Physical Chemistry C, 2017, 121: 25052-25063. |
| 12 | Le CaerG, DuboisJ M, BussiereP, et al. Characterization by Mossbauer spectroscopy of iron carbides formed by Fischer-Tropsch synthesis[J]. The Journal of Physical Chemistry, 1982, 86(24): 4799-4808. |
| 13 | FangC M, SluiterM H F, van HuisM A, et al. Origin of predominance of cementite among iron carbides in steel at elevated temperature[J]. Physical Review Letters, 2010, 105(5): 055503. |
| 14 | BaoL L, HuoC F, LiY W, et al. Structure and stability of the crystal Fe2C and low index surfaces[J]. Journal of Fuel Chemistry and Technology, 2009, 37: 104-108. |
| 15 | ChenW, LinT J, SunY H, et al. Recent advances in the investigation of nanoeffects of Fischer-Tropsch catalysts[J]. Catalysis Today, 2018, 311: 8-22. |
| 16 | PhamT H, QiY, YangJ, et al. Insights into Hägg iron-carbide-catalyzed Fischer-Tropsch synthesis: suppression of CH4 formation and enhancement of C-C coupling on χ-Fe5C2 (510)[J]. ACS Catalysis, 2015, 5(4): 2203-2208. |
| 17 | WangY, LiY, HuangS, et al. Insight into CH4 formation and chain growth mechanism of Fischer-Tropsch synthesis on θ-Fe3C (031)[J]. Chemical Physics Letters, 2017, 682: 115-121. |
| 18 | PerdewJ P, YueW. Accurate and simple density functional for the electronic exchange energy: generalized gradient approximation[J]. Physical Review B, 1986, 33(12): 8800. |
| 19 | PerdewJ P, BurkeK, ErnzerhofM. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865. |
| 20 | KresseG, JoubertD. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758. |
| 21 | HenkelmanG, JónssonH. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives[J]. The Journal of Chemical Physics, 1999, 111(15): 7010-7022. |
| 22 | HuoC F, LiY W, WangJ, et al. Insight into CH4 formation in iron-catalyzed Fischer-Tropsch synthesis[J]. Journal of the American Chemical Society, 2009, 131(41): 14713-14721. |
| 23 | 宋楠, 段学志, 周兴贵, 等. 费托催化剂η-Fe2C(011)上CO吸附与活化行为[J]. 陕西师范大学学报(自然科学版), 2019, 47(1): 13-19. |
| SongN, DuanX Z, ZhouX G, et al. Adsorption and activation of CO on η-Fe2C(011) surface of Fischer-Tropsch synthesis catalyst[J]. Journal of Shaanxi Normal University (Natural Science Edition), 2019, 47(1): 13-19. | |
| 24 | LoJ M H, ZieglerT. Density functional theory and kinetic studies of methanation on iron surface[J]. The Journal of Physical Chemistry C, 2007, 111(29): 11012-11025. |
| 25 | GovenderA, CurullaF D, NiemantsverdrietJ W. A density functional theory study on the effect of zero-point energy corrections on the methanation profile on Fe (100)[J]. ChemPhysChem, 2012, 13(6): 1591-1596. |
| 26 | ChengJ, HuP, EllisP, et al. Density functional theory study of iron and cobalt carbides for Fischer-Tropsch synthesis[J]. The Journal of Physical Chemistry C, 2010, 114(2): 1085-1093. |
| 27 | FischerF, TropschH. The preparation of synthetic oil mixtures (synthol) from carbon monoxide and hydrogen[J]. Brennstoff-Chem, 1923, 4: 276-285. |
| 28 | PichlerH, SchultzH. New insights in the area of the synthesis of hydrocarbons from CO and H2[J]. Chem. Ing. Tech., 1970, 12(18): 1160-1174. |
| 29 | KummerJ T, EmmettP H. Fischer-Tropsch synthesis mechanism studies. The addition of radioactive alcohols to the synthesis gas[J]. Journal of the American Chemical Society, 1953, 75(21): 5177-5183. |
| 30 | Ciobı̂căI M, KramerG J, GeQ, et al. Mechanisms for chain growth in Fischer-Tropsch synthesis over Ru (0001)[J]. Journal of Catalysis, 2002, 212(2): 136-144. |
| 31 | LiuZ P, HuP. A new insight into Fischer-Tropsch synthesis[J]. Journal of the American Chemical Society, 2002, 124(39): 11568-11569. |
| 32 | LoJ M H, ZieglerT. Theoretical studies of the formation and reactivity of C2 hydrocarbon species on the Fe (100) surface[J]. The Journal of Physical Chemistry C, 2007, 111(35): 13149-13162. |
| 33 | ZhaoY H, SunK, MaX, et al. Carbon chain growth by formyl insertion on rhodium and cobalt catalysts in syngas conversion[J]. Angewandte Chemie International Edition, 2011, 50(23): 5335-5338. |
| 34 | MichaelidesA, HuP. Insight into microscopic reaction pathways in heterogeneous catalysis[J]. Journal of the American Chemical Society, 2000, 122(40): 9866-9867. |
| 35 | ChengJ, GongX Q, HuP, et al. A quantitative determination of reaction mechanisms from density functional theory calculations: Fischer-Tropsch synthesis on flat and stepped cobalt surfaces[J]. Journal of Catalysis, 2008, 254(2): 285-295. |
| 36 | ChengJ, HuP, EllisP, et al. An energy descriptor to quantify methane selectivity in Fischer-Tropsch synthesis: a density functional theory study[J]. The Journal of Physical Chemistry C, 2009, 113(20): 8858-8863. |
| 37 | PeterM M, ValerioZ. The role of electrophilic species in the Fischer-Tropsch reaction[J]. Chemical Communications, 2009, 40(27): 1619-1634. |
| 38 | ChengJ, HuP, EllisP, et al. Chain growth mechanism in Fischer-Tropsch synthesis: a DFT study of C—C coupling over Ru, Fe, Rh, and Re surfaces[J]. The Journal of Physical Chemistry C, 2008, 112: 6082-6086. |
| 39 | CaoD B, LiY W, JiaoH J, et al. Chain growth mechanism of Fischer-Tropsch synthesis on Fe5C2 (001)[J]. Journal of Molecular Catalysis A: Chemical, 2011, 346: 55-69. |
| 40 | ZhaoY H, SunK, LiW X, et al. Carbon chain growth by formyl insertion on rhodium and cobalt catalysts in syngas conversion[J]. Angewandte Chemie International Edition, 2011, 50: 5335-5338. |
| 41 | SorescuD C. First-principles calculations of the adsorption and hydrogenation reactions of CHx (x= 0, 4) species on a Fe (100) surface[J]. Physical Review B, 2006, 73(15): 155420. |
| 42 | CaoD B, LiY W, WangJ, et al. Adsorption and reaction of surface carbon species on Fe5C2 (001)[J]. The Journal of Physical Chemistry C, 2008, 112(38): 14883-14890. |
| 43 | ParkJ Y, LeeY J, KhannaP K, et al. Alumina-supported iron oxide nanoparticles as Fischer-Tropsch catalysts: effect of particle size of iron oxide[J]. Journal of Molecular Catalysis A: Chemical, 2010, 323(1/2): 84-90. |
| 44 | TorresG H M, BitterJ H, DavidianT, et al. Iron particle size effects for direct production of lower olefins from synthesis gas[J]. Journal of the American Chemical Society, 2012, 134(39): 16207-16215. |
| 45 | GovenderA, Curulla-FerréD, Pérez-JigatoM, et al. First-principles elucidation of the surface chemistry of the C2Hx (x= 0—6) adsorbate series on Fe (100)[J]. Molecules, 2013, 18(4): 3806-3824. |
| [1] | 刘鑫, 潘阳, 刘公平, 方静, 李春利, 李浩. 渗透汽化-隔壁塔精馏耦合初步分离费托合成水的过程研究[J]. 化工学报, 2022, 73(5): 2020-2030. |
| [2] | 金科, 王晨光, 马隆龙, 张琦. 核壳纳米材料制备及其在CO/CO2热催化加氢中的应用[J]. 化工学报, 2022, 73(3): 990-1007. |
| [3] | 陈康伟, 熊文婷, 符继乐, 陈秉辉. 合成气费托合成制重质烃Ru-Co/SiC催化剂的制备及性能[J]. 化工学报, 2021, 72(7): 3648-3657. |
| [4] | 刘文萱, 张嘉毅, 陆奇, 张皓晨. 基于机器学习的二氧化碳电化学还原制备甲酸盐研究[J]. 化工学报, 2021, 72(12): 6262-6273. |
| [5] | 应景涛, 李涛. 费托合成蛋壳型催化剂活性组分厚度的模拟计算[J]. 化工学报, 2019, 70(9): 3404-3411. |
| [6] | 刘意, 刘勇, 陈建峰, 张燚. 不同氧化锰载体对费托钴基催化剂合成低碳烯烃的影响[J]. 化工学报, 2015, 66(9): 3413-3420. |
| [7] | 王燕1,葛喜慧2,张敏卿1,朱怀工3,张子建2,王明1. 费托合成高温油相产品中正构烃的分离[J]. 化工进展, 2014, 33(11): 2894-2898. |
| [8] | 孙启文,吴建民,张宗森,庞利峰. 煤间接液化技术及其研究进展[J]. 化工进展, 2013, 32(01): 1-12. |
| [9] | 管国锋,王 磊,王锋娜. 氧化物助剂对费托合成钴基催化剂的促进作用[J]. 化工进展, 2012, 31(12): 2595-2602. |
| [10] | 岳晨, 史翊翔, 蔡宁生. 煤气化费托合成/电联产系统建模及热力学分析 [J]. 化工学报, 2011, 62(4): 1070-1076. |
| [11] | 侯朝鹏,夏国富,李明丰,聂 红,李大东. FT合成反应器的研究进展 [J]. CIESC Journal, 2011, 30(2): 251-. |
| [12] | 王 祥 云. 反应循环气中二氧化碳脱除技术的进展 [J]. CIESC Journal, 2011, 30(1): 52-. |
| [13] | 董立华, 郝栩, 曹立仁, 李永旺. 费托合成油品体系相关二元物系汽液平衡预测方法的评价 [J]. 化工学报, 2009, 60(6): 1367-1372. |
| [14] | 杨霞珍,刘化章,唐浩东,蔡丽萍,吴再国. Fe、Co基费托合成催化剂助剂研究进展 [J]. CIESC Journal, 2006, 25(8): 867-. |
| [15] | 陈建刚, 相宏伟, 李永旺, 孙予罕. 费托法合成液体燃料关键技术研究进展 [J]. 化工学报, 2003, 54(4): 516-523. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号