化工学报 ›› 2021, Vol. 72 ›› Issue (12): 6262-6273.DOI: 10.11949/0438-1157.20211258
收稿日期:2021-08-31
修回日期:2021-10-28
出版日期:2021-12-05
发布日期:2021-12-22
通讯作者:
张皓晨
作者简介:刘文萱(1999—),女,博士研究生,基金资助:
Wenxuan LIU( ),Jiayi ZHANG,Qi LU,Haochen ZHANG(
),Jiayi ZHANG,Qi LU,Haochen ZHANG( )
)
Received:2021-08-31
Revised:2021-10-28
Online:2021-12-05
Published:2021-12-22
Contact:
Haochen ZHANG
摘要:
系统研究了不同双金属中心催化剂催化二氧化碳电化学还原制备甲酸盐。借助机器学习,确定了反应中心金属原子序数、电负性和电离能等特征对双金属中心催化剂表面二氧化碳还原具有主要的影响。基于这些特征,通过高通量机器学习快速预测了105种双金属中心催化剂二氧化碳电还原制甲酸盐及其主要竞争反应的Gibbs自由能变,筛选出29种双金属中心催化剂更倾向于二氧化碳还原得到甲酸盐,是潜在的转化二氧化碳为甲酸盐的高性能催化材料。运用类似的方法预测了105种双金属中心催化剂表面二氧化碳还原中间体的结构,发现中间体吸附能与其吸附构型具有显著的相关关系。
中图分类号:
刘文萱, 张嘉毅, 陆奇, 张皓晨. 基于机器学习的二氧化碳电化学还原制备甲酸盐研究[J]. 化工学报, 2021, 72(12): 6262-6273.
Wenxuan LIU, Jiayi ZHANG, Qi LU, Haochen ZHANG. Investigation of electroreduction of carbon dioxide into formate based on machine learning[J]. CIESC Journal, 2021, 72(12): 6262-6273.

图1 石墨烯-N6-M1-M2双金属中心催化剂模型[灰色球为碳原子,淡蓝色球为氮原子,深蓝色球为金属原子(M1和M2)]
Fig.1 The structure of the graphene-N6-M1-M2 model[where grey, light blue, and dark blue spheres represent C, N, and transition-metal atoms (M1 and M2), respectively]
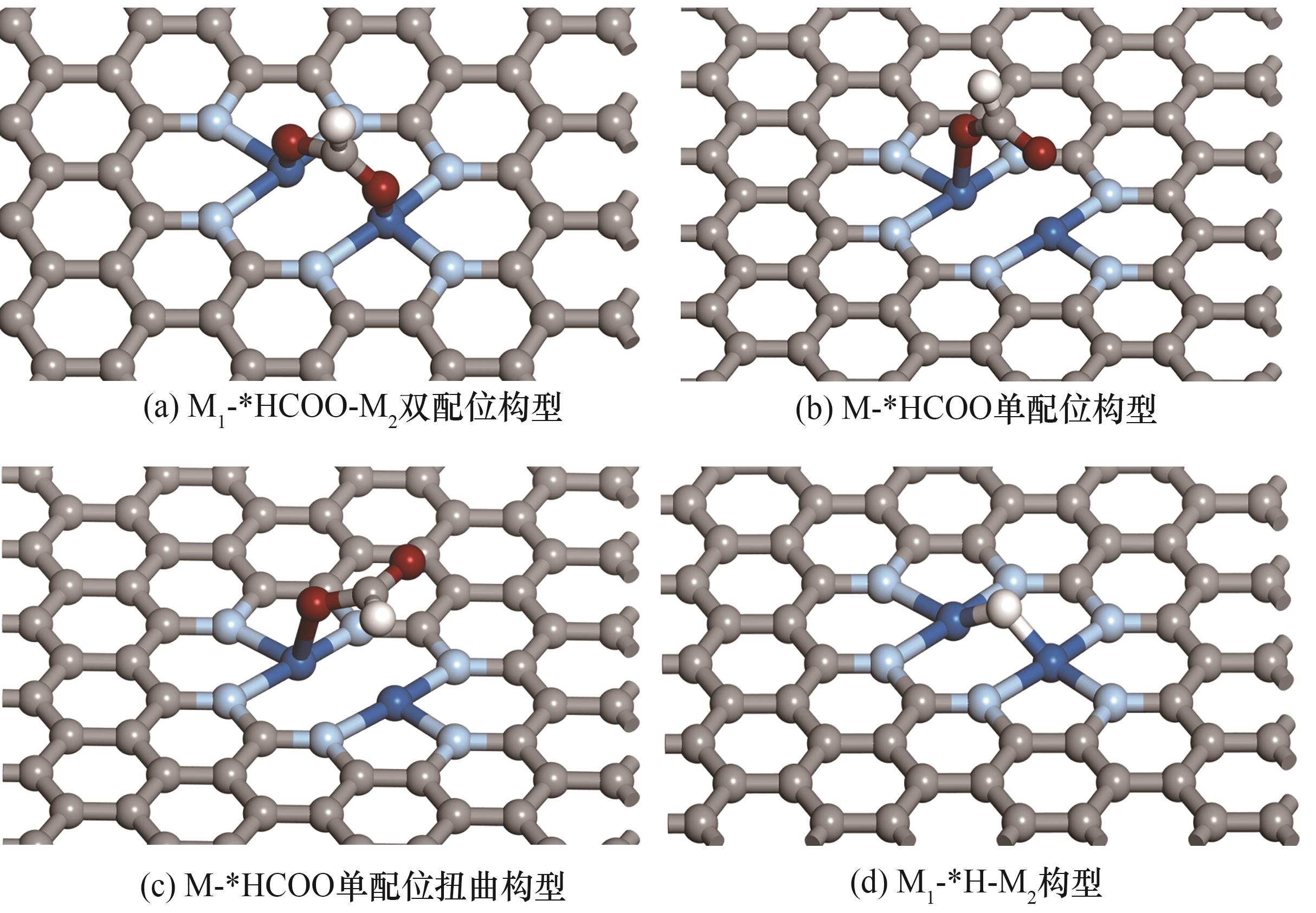
图2 CO2ER和HER关键反应中间体的不同吸附构型[灰色球为碳原子,淡蓝色球为氮原子,白色球为氢原子,红色球为氧原子,深蓝色球为金属原子(M1和M2)]
Fig.2 Different adsorption configurations of key intermediates of CO2ER and HER[where grey, light blue, white, red and dark blue spheres represent C, N, H, O and transition-metal atoms (M1 and M2), respectively]
| 序号 | M1 | M2 | *HCOO吸附构型 | ΔG*H/eV | ΔG*HCOO/eV |
|---|---|---|---|---|---|
| 1 | Cu | Ni | M1-*HCOO-M2双配位型 | 0.43 | 0.89 |
| 2 | Co | Pd | M-*HCOO单配位扭曲型 | -0.76 | 0.26 |
| 3 | Rh | Ir | M-*HCOO单配位扭曲型 | -0.50 | 0.51 |
| 4 | Rh | Pt | M-*HCOO单配位扭曲型 | -0.61 | 0.54 |
| 5 | Ir | Cr | M-*HCOO单配位型 | -0.32 | -0.43 |
| 6 | Ir | Mn | M-*HCOO单配位型 | -0.32 | 0.00 |
| 7 | Zn | Fe | M1-*HCOO-M2双配位型 | -0.45 | -0.85 |
| 8 | Ru | Fe | M1-*HCOO-M2双配位型 | -0.05 | 0.12 |
| 9 | Cr | Co | M1-*HCOO-M2双配位型 | -0.29 | -0.48 |
| 10 | Mn | Ag | M-*HCOO单配位型 | -0.66 | -0.62 |
| 11 | Ag | Co | M1-*HCOO-M2双配位型 | 0.08 | 0.27 |
| 12 | Cr | Pd | M-*HCOO单配位型 | -0.43 | -0.77 |
| 13 | Ni | Pt | M-*HCOO单配位扭曲型 | -0.34 | 1.31 |
| 14 | Ag | Au | M-*HCOO单配位型 | -0.24 | 0.92 |
| 15 | Mn | Pt | M-*HCOO单配位型 | -0.61 | -0.53 |
| 16 | Zn | Ni | M1-*HCOO-M2双配位型 | -0.31 | -0.25 |
| 17 | Ru | Cu | M1-*HCOO-M2双配位型 | -0.68 | -0.13 |
| 18 | Cu | Ag | M1-*HCOO-M2双配位型 | 0.13 | 0.13 |
| 19 | Ni | Co | M1-*HCOO-M2双配位型 | -0.59 | 0.53 |
| 20 | Ru | Co | M1-*HCOO-M2双配位型 | -0.24 | 0.34 |
| 21 | Cr | Mn | M1-*HCOO-M2双配位型 | -0.18 | -1.21 |
| 22 | Rh | Ag | M-*HCOO单配位型 | -0.02 | 0.96 |
| 23 | Pd | Zn | M-*HCOO单配位型 | -0.72 | -0.38 |
表1 随机抽取的23种DMSCs的ΔG*H、ΔG*HCOO和*HCOO吸附构型
Table 1 ΔG*H, ΔG*HCOO and *HCOO configurations of 23 randomly selected DMSCs
| 序号 | M1 | M2 | *HCOO吸附构型 | ΔG*H/eV | ΔG*HCOO/eV |
|---|---|---|---|---|---|
| 1 | Cu | Ni | M1-*HCOO-M2双配位型 | 0.43 | 0.89 |
| 2 | Co | Pd | M-*HCOO单配位扭曲型 | -0.76 | 0.26 |
| 3 | Rh | Ir | M-*HCOO单配位扭曲型 | -0.50 | 0.51 |
| 4 | Rh | Pt | M-*HCOO单配位扭曲型 | -0.61 | 0.54 |
| 5 | Ir | Cr | M-*HCOO单配位型 | -0.32 | -0.43 |
| 6 | Ir | Mn | M-*HCOO单配位型 | -0.32 | 0.00 |
| 7 | Zn | Fe | M1-*HCOO-M2双配位型 | -0.45 | -0.85 |
| 8 | Ru | Fe | M1-*HCOO-M2双配位型 | -0.05 | 0.12 |
| 9 | Cr | Co | M1-*HCOO-M2双配位型 | -0.29 | -0.48 |
| 10 | Mn | Ag | M-*HCOO单配位型 | -0.66 | -0.62 |
| 11 | Ag | Co | M1-*HCOO-M2双配位型 | 0.08 | 0.27 |
| 12 | Cr | Pd | M-*HCOO单配位型 | -0.43 | -0.77 |
| 13 | Ni | Pt | M-*HCOO单配位扭曲型 | -0.34 | 1.31 |
| 14 | Ag | Au | M-*HCOO单配位型 | -0.24 | 0.92 |
| 15 | Mn | Pt | M-*HCOO单配位型 | -0.61 | -0.53 |
| 16 | Zn | Ni | M1-*HCOO-M2双配位型 | -0.31 | -0.25 |
| 17 | Ru | Cu | M1-*HCOO-M2双配位型 | -0.68 | -0.13 |
| 18 | Cu | Ag | M1-*HCOO-M2双配位型 | 0.13 | 0.13 |
| 19 | Ni | Co | M1-*HCOO-M2双配位型 | -0.59 | 0.53 |
| 20 | Ru | Co | M1-*HCOO-M2双配位型 | -0.24 | 0.34 |
| 21 | Cr | Mn | M1-*HCOO-M2双配位型 | -0.18 | -1.21 |
| 22 | Rh | Ag | M-*HCOO单配位型 | -0.02 | 0.96 |
| 23 | Pd | Zn | M-*HCOO单配位型 | -0.72 | -0.38 |
| 特征 | 定义 | 特征 | 定义 |
|---|---|---|---|
| Z1 、Z2 | M1和M2的原子序数 | (Z1+Z2)/2 | 平均原子序数 |
| N1 、N2 | M1和M2的价电子数 | (N1+N2)/2 | 平均价电子数 |
| PE1 、PE2 | M1和M2的电负性[ | (PE1+PE2)/2 | 平均电负性 |
| IE1 、IE2 | M1和M2的第一电离能[ | (IE1+IE2)/2 | 平均第一电离能 |
| EA1 、EA2 | M1和M2的电子亲和能[ | (EA1+EA2)/2 | 平均电子亲和能 |
| Nd1 、Nd2 | M1和M2的d电子数 | (Nd1+Nd2)/2 | 平均d电子数 |
| WF1 、WF2 | M1和M2的功函数[ | (WF1+WF2)/2 | 平均功函数 |
| r1 、r2 | M1和M2的原子半径[ | (r1+r2)/2 | 平均原子半径 |
| R1 、R2 | M1和M2的范德华半径[ | (R1+R2)/2 | 平均范德华半径 |
| IE1/Nd1、IE2/Nd2 | 第一电离能除以d电子数 | [(Z1+Z2)/2]2 | 平均原子序数的平方值 |
| EA1/Nd1、EA2/Nd2 | 电子亲和能除以d电子数 | [(N1+N2)/2]2 | 平均价电子数的平方值 |
| PE1/Nd1、PE2/Nd2 | 电负性除以d电子数 | [(PE1+PE2)/2]2 | 平均电负性的平方值 |
| PE1×Nd1、PE2×Nd2 | 电负性与d电子数之积 | [(IE1+IE2)/2]2 | 平均第一电离能的平方值 |
| PE1+PE2、PE1-PE2 | 电负性之和、电负性之差 | [(EA1+EA2)/2]2 | 平均电子亲和能的平方值 |
| r1+r2 | 原子半径之和 | [(Nd1+Nd2)/2]2 | 平均d电子数的平方值 |
| R1+R2 | 范德华半径之和 | [(WF1+WF2)/2]2 | 平均功函数的平方值 |
| WF1/Nd1、WF2/Nd2 | 功函数除以d电子数 | [(r1+r2)/2]2 | 平均原子半径的平方值 |
| WF1+WF2、WF1-WF2 | 功函数之和、功函数之差 | [(R1+R2)/2]2 | 平均范德华半径的平方值 |
表2 机器学习的完整52个特征
Table 2 Complete feature space of 52 features for ML
| 特征 | 定义 | 特征 | 定义 |
|---|---|---|---|
| Z1 、Z2 | M1和M2的原子序数 | (Z1+Z2)/2 | 平均原子序数 |
| N1 、N2 | M1和M2的价电子数 | (N1+N2)/2 | 平均价电子数 |
| PE1 、PE2 | M1和M2的电负性[ | (PE1+PE2)/2 | 平均电负性 |
| IE1 、IE2 | M1和M2的第一电离能[ | (IE1+IE2)/2 | 平均第一电离能 |
| EA1 、EA2 | M1和M2的电子亲和能[ | (EA1+EA2)/2 | 平均电子亲和能 |
| Nd1 、Nd2 | M1和M2的d电子数 | (Nd1+Nd2)/2 | 平均d电子数 |
| WF1 、WF2 | M1和M2的功函数[ | (WF1+WF2)/2 | 平均功函数 |
| r1 、r2 | M1和M2的原子半径[ | (r1+r2)/2 | 平均原子半径 |
| R1 、R2 | M1和M2的范德华半径[ | (R1+R2)/2 | 平均范德华半径 |
| IE1/Nd1、IE2/Nd2 | 第一电离能除以d电子数 | [(Z1+Z2)/2]2 | 平均原子序数的平方值 |
| EA1/Nd1、EA2/Nd2 | 电子亲和能除以d电子数 | [(N1+N2)/2]2 | 平均价电子数的平方值 |
| PE1/Nd1、PE2/Nd2 | 电负性除以d电子数 | [(PE1+PE2)/2]2 | 平均电负性的平方值 |
| PE1×Nd1、PE2×Nd2 | 电负性与d电子数之积 | [(IE1+IE2)/2]2 | 平均第一电离能的平方值 |
| PE1+PE2、PE1-PE2 | 电负性之和、电负性之差 | [(EA1+EA2)/2]2 | 平均电子亲和能的平方值 |
| r1+r2 | 原子半径之和 | [(Nd1+Nd2)/2]2 | 平均d电子数的平方值 |
| R1+R2 | 范德华半径之和 | [(WF1+WF2)/2]2 | 平均功函数的平方值 |
| WF1/Nd1、WF2/Nd2 | 功函数除以d电子数 | [(r1+r2)/2]2 | 平均原子半径的平方值 |
| WF1+WF2、WF1-WF2 | 功函数之和、功函数之差 | [(R1+R2)/2]2 | 平均范德华半径的平方值 |
| 1 | Herron J A, Kim J, Upadhye A A, et al. A general framework for the assessment of solar fuel technologies[J]. Energy & Environmental Science, 2015, 8(1): 126-157. |
| 2 | Davis S J, Caldeira K, Matthews H D. Future CO2 emissions and climate change from existing energy infrastructure[J]. Science, 2010, 329(5997): 1330-1333. |
| 3 | Wu J H, Huang Y, Ye W, et al. CO2 reduction: from the electrochemical to photochemical approach[J]. Advanced Science, 2017, 4(11): 1700194. |
| 4 | Bonetto R, Crisanti F, Sartorel A. Carbon dioxide reduction mediated by iron catalysts: mechanism and intermediates that guide selectivity[J]. ACS Omega, 2020, 5(34): 21309-21319. |
| 5 | Li F W, Chen L, Xue M Q, et al. Towards a better Sn: efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets[J]. Nano Energy, 2017, 31: 270-277. |
| 6 | Chen C, Khosrowabadi Kotyk J F, Sheehan S W. Progress toward commercial application of electrochemical carbon dioxide reduction[J]. Chem, 2018, 4(11): 2571-2586. |
| 7 | Lu Q, Jiao F. Electrochemical CO2 reduction: electrocatalyst, reaction mechanism, and process engineering[J]. Nano Energy, 2016, 29: 439-456. |
| 8 | Hui S R, Shaigan N M, Neburchilov V, et al. Three-dimensional cathodes for electrochemical reduction of CO2: from macro-to nano-engineering[J]. Nanomaterials, 2020, 10(9): 1884. |
| 9 | Gao W L, Liang S Y, Wang R J, et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| 10 | Yang M R, Li H J, Luo N D, et al. Electro-chemical reduction of carbon dioxide into ethylene: catalyst, conditions and mechanism[J]. Progress in Chemistry, 2019, 31(2/3): 245-257. |
| 11 | Chen S H, Su Y Q, Deng P L, et al. Highly selective carbon dioxide electroreduction on structure-evolved copper perovskite oxide toward methane production[J]. ACS Catalysis, 2020, 10(8): 4640-4646. |
| 12 | Yu J L, Liu H Y, Song S Q, et al. Electrochemical reduction of carbon dioxide at nanostructured SnO2/carbon aerogels: the effect of tin oxide content on the catalytic activity and formate selectivity[J]. Applied Catalysis A: General, 2017, 545: 159-166. |
| 13 | Agarwal A S, Zhai Y M, Hill D, et al. The electrochemical reduction of carbon dioxide to formate/formic acid: engineering and economic feasibility[J]. ChemSusChem, 2011, 4(9): 1301-1310. |
| 14 | Loges B, Boddien A, Gärtner F, et al. Catalytic generation of hydrogen from formic acid and its derivatives: useful hydrogen storage materials[J]. Topics in Catalysis, 2010, 53(13/14): 902-914. |
| 15 | Li J, Jiao J Q, Zhang H C, et al. Two-dimensional SnO2 nanosheets for efficient carbon dioxide electroreduction to formate[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(12): 4975-4982. |
| 16 | Mou K W, Chen Z P, Yao S Y, et al. Enhanced electrochemical reduction of carbon dioxide to formate with in situ grown indium-based catalysts in an aqueous electrolyte[J]. Electrochimica Acta, 2018, 289: 65-71. |
| 17 | Fu Y S, Li Y N, Zhang X, et al. Novel hierarchical SnO2 microsphere catalyst coated on gas diffusion electrode for enhancing energy efficiency of CO2 reduction to formate fuel[J]. Applied Energy, 2016, 175: 536-544. |
| 18 | Chen Y H, Kanan M W. Tin oxide dependence of the CO2 reduction efficiency on tin electrodes and enhanced activity for tin/tin oxide thin-film catalysts[J]. Journal of the American Chemical Society, 2012, 134(4): 1986-1989. |
| 19 | Pander J E III, Baruch M F, Bocarsly A B. Probing the mechanism of aqueous CO2 reduction on post-transition-metal electrodes using ATR-IR spectroelectrochemistry[J]. ACS Catalysis, 2016, 6(11): 7824-7833. |
| 20 | Zhu X R, Yan J X, Gu M, et al. Activity origin and design principles for oxygen reduction on dual-metal-site catalysts: a combined density functional theory and machine learning study[J]. The Journal of Physical Chemistry Letters, 2019, 10(24): 7760-7766. |
| 21 | He Q, Yu B, Li Z H, et al. Density functional theory for battery materials[J]. Energy & Environmental Materials, 2019, 2(4): 264-279. |
| 22 | Li M R, Garg S, Chang X X, et al. Toward excellence of transition metal-based catalysts for CO2 electrochemical reduction: an overview of strategies and rationales[J]. Small Methods, 2020, 4(7): 2000033. |
| 23 | Yang Z, Gao W, Jiang Q. A machine learning scheme for the catalytic activity of alloys with intrinsic descriptors[J]. Journal of Materials Chemistry A, 2020, 8(34): 17507-17515. |
| 24 | Chen Y, Huang Y, Cheng T, et al. Identifying active sites for CO2 reduction on dealloyed gold surfaces by combining machine learning with multiscale simulations[J]. Journal of the American Chemical Society, 2019, 141(29): 11651-11657. |
| 25 | Zhong M, Tran K, Min Y, et al. Accelerated discovery of CO2 electrocatalysts using active machine learning[J]. Nature, 2020, 581(7807): 178-183. |
| 26 | Ma X, Li Z, Achenie L E, et al. Machine-learning-augmented chemisorption model for CO2 electroreduction catalyst screening[J]. The Journal of Physical Chemistry Letters, 2015, 6(18): 3528-3533. |
| 27 | Mayer F D, Hosseini-Benhangi P, Sánchez-Sánchez C M, et al. Scanning electrochemical microscopy screening of CO2 electroreduction activities and product selectivities of catalyst arrays[J]. Communications Chemistry, 2020, 3: 155. |
| 28 | Wu D H, Zhang J Y, Cheng M J, et al. Machine learning investigation of supplementary adsorbate influence on copper for enhanced electrochemical CO2 reduction performance[J]. The Journal of Physical Chemistry C, 2021, 125(28): 15363-15372. |
| 29 | Zhang H C, Goddard W A, Lu Q, et al. The importance of grand-canonical quantum mechanical methods to describe the effect of electrode potential on the stability of intermediates involved in both electrochemical CO2 reduction and hydrogen evolution[J]. Physical Chemistry Chemical Physics, 2018, 20(4): 2549-2557. |
| 30 | Ma W C, Xie S J, Zhang X G, et al. Promoting electrocatalytic CO2 reduction to formate via sulfur-boosting water activation on indium surfaces[J]. Nature Communications, 2019, 10: 892. |
| 31 | Hastie T, Tibshirani R, Friedman J H, et al. The elements of statistical learning: data mining, inference, and prediction[J]. The Mathematical Intelligencer, 2004, 27(2): 83-85. |
| 32 | Friedman J H. Greedy function approximation: a gradient boosting machine[J]. The Annals of Statistics, 2001, 29(5): 1189-1232. |
| 33 | Kim J, Jung H, Jung S M, et al. Tailoring binding abilities by incorporating oxophilic transition metals on 3D nanostructured Ni arrays for accelerated alkaline hydrogen evolution reaction[J]. Journal of the American Chemical Society, 2021, 143(3): 1399-1408. |
| 34 | Speight J G. Lange's Handbook of Chemistry[M]. New York: McGraw-Hill, 2005: 1.132-1.156. |
| 35 | 喻典, 梁国明. 元素电子亲和势的密度泛函理论计算[J]. 重庆师范大学学报(自然科学版), 2005, 22(1): 39-42. |
| Yu D, Liang G M. A study of electron affinities of the elements by density functional theory[J]. Journal of Chongqing Teachers College (Natural Science Edition), 2005, 22(1): 39-42. | |
| 36 | Alvarez S. A cartography of the van der Waals territories[J]. Dalton Transactions, 2013, 42(24): 8617. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [6] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [7] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [10] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [11] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [12] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [15] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号