化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3368-3379.DOI: 10.11949/0438-1157.20201483
纪燕男1,2( ),孙培茁2,马强2,张玮琦2,苏华能2,徐谦2(
),孙培茁2,马强2,张玮琦2,苏华能2,徐谦2( )
)
收稿日期:2020-10-26
修回日期:2020-12-23
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
徐谦
作者简介:纪燕男(1994—),女,硕士,助教,基金资助:
JI Yannan1,2( ),SUN Peizhuo2,MA Qiang2,ZHANG Weiqi2,SU Huaneng2,XU Qian2(
),SUN Peizhuo2,MA Qiang2,ZHANG Weiqi2,SU Huaneng2,XU Qian2( )
)
Received:2020-10-26
Revised:2020-12-23
Online:2021-06-05
Published:2021-06-05
Contact:
XU Qian
摘要:
非水系氧化还原液流电池(NARFB)的广泛应用受制于其较低的性能。在电解液中加入一些金属离子添加剂是一种可能的解决方案。实验研究了Sb3+离子对低共熔溶剂(DES)电解液液流电池电化学性能的影响。结果表明,添加Sb3+离子可以强化V(Ⅲ)/V(Ⅱ)氧化还原离子对的电化学反应动力学(最高可达22.6%)过程,钒离子在DES中的扩散系数提高了63.3%,并且电荷转移电阻降低了11.9%。场发射扫描电子显微镜表明,Sb3+离子电沉积在石墨毡的表面,对电化学反应起催化作用,从而改善了电化学性能。考虑增强的动力学和降低的活性比表面积之间的平衡,确定了Sb3+的最佳浓度为15 mmol·L-1。此外,当使用含有Sb3+的负极电解液液流电池时,液流电池的功率密度提高了31.2%,从含原始电解质的3.08 mW·cm-2到含15 mmol·L-1 Sb3+离子的4.04 mW·cm-2。这些结果为改善NARFB的电池性能提供了一个便捷而有前景的方法。
中图分类号:
纪燕男, 孙培茁, 马强, 张玮琦, 苏华能, 徐谦. 锑离子添加剂对低共熔溶剂(DES)电解液液流电池的性能改善研究[J]. 化工学报, 2021, 72(6): 3368-3379.
JI Yannan, SUN Peizhuo, MA Qiang, ZHANG Weiqi, SU Huaneng, XU Qian. Improving the performance of a deep eutectic solvent (DES)-electrolyte non-aqueous redox flow battery by antimony ion additive[J]. CIESC Journal, 2021, 72(6): 3368-3379.

图1 含有不同浓度SbCl3的VCl3电解液A—原始溶液; B—5 mmol·L-1; C—10 mmol·L-1; D—15 mmol·L-1;
Fig.1 Electrolytes with different concentrations of SbCl3E—20 mmol·L-1; F—25 mmol·L-1
| Sb3+浓度/(mmol·L-1) | 黏度/(mPa·s) | 电导率/(mS·cm-1) |
|---|---|---|
| pristine | 62.4 | 6.64 |
| 5 | 61.6 | 6.70 |
| 10 | 61.5 | 6.72 |
| 15 | 61.2 | 6.75 |
| 20 | 61.9 | 6.69 |
表1 含有不同浓度Sb3+离子的电解液的黏度和电导率值
Table 1 Viscosity and conductivity of electrolytes with different concentrations of Sb3+ ions
| Sb3+浓度/(mmol·L-1) | 黏度/(mPa·s) | 电导率/(mS·cm-1) |
|---|---|---|
| pristine | 62.4 | 6.64 |
| 5 | 61.6 | 6.70 |
| 10 | 61.5 | 6.72 |
| 15 | 61.2 | 6.75 |
| 20 | 61.9 | 6.69 |
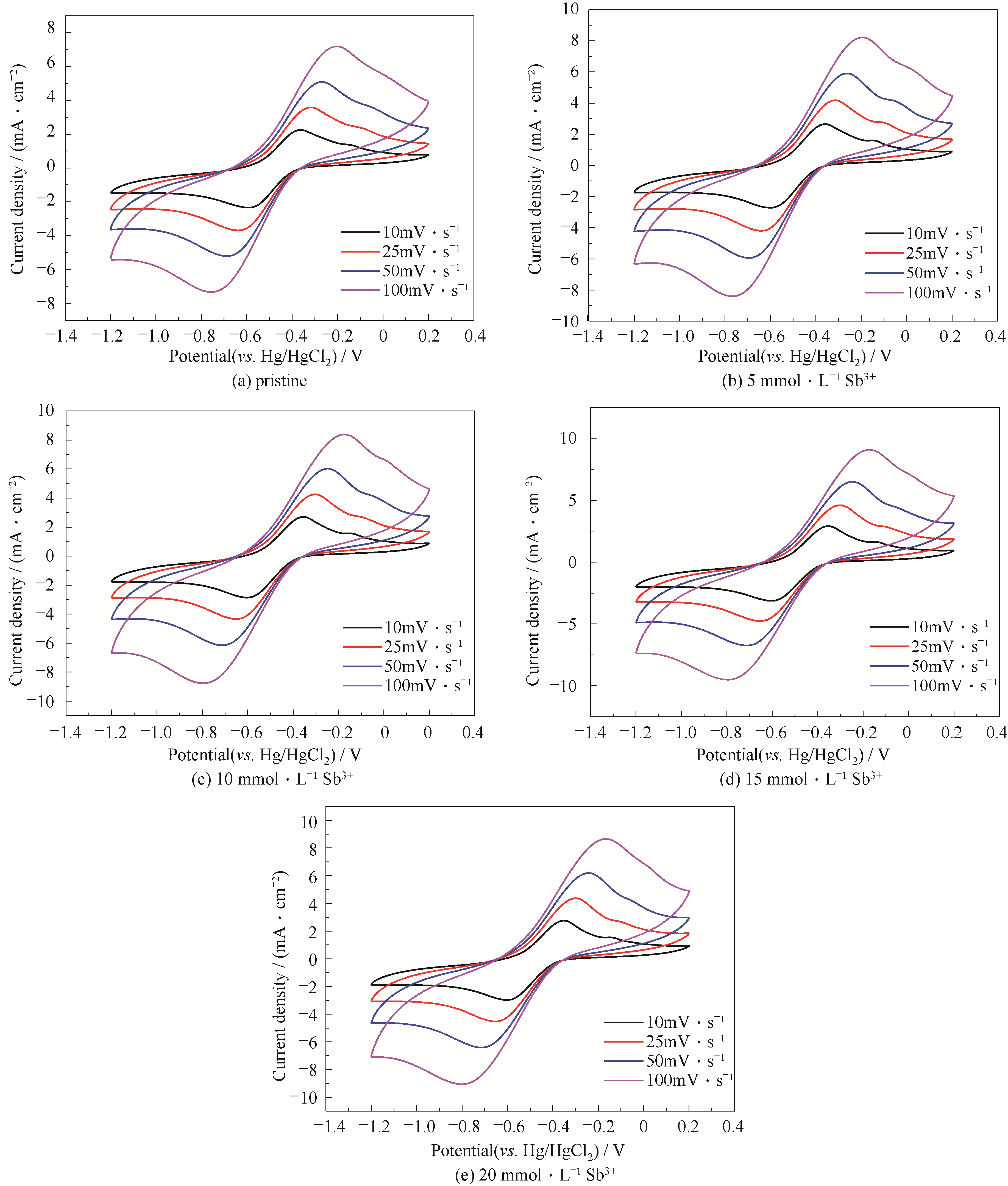
图4 含不同浓度Sb3+离子0.1 mol·L-1 V(Ⅲ)电解质在不同扫描速率下的CV曲线
Fig.4 CV curves of 0.1 mol·L-1 V(Ⅲ) electrolyte with different concentrations of Sb3+ ions at different scanning rates
| Sb3+浓度/(mmol·L-1) | 氧化峰电流密度/(mA·cm-2) | 还原峰电流密度/(mA·cm-2) | ||||||
|---|---|---|---|---|---|---|---|---|
| 10 mV·s-1 | 25 mV·s-1 | 50 mV·s-1 | 100 mV·s-1 | 10 mV·s-1 | 25 mV·s-1 | 50 mV·s-1 | 100 mV·s-1 | |
| pristine | 2.252 | 3.583 | 5.085 | 7.189 | -2.343 | -3.691 | -5.211 | -7.340 |
| 5 | 2.650 | 4.176 | 5.901 | 8.221 | -2.717 | -4.197 | -5.940 | -8.406 |
| 10 | 2.703 | 4.254 | 6.029 | 8.389 | -2.860 | -4.342 | -6.146 | -8.770 |
| 15 | 2.898 | 4.589 | 6.481 | 9.072 | -3.128 | -4.764 | -6.742 | -9.514 |
| 20 | 2.754 | 4.371 | 6.192 | 8.645 | -2.977 | -4.526 | -6.412 | -9.065 |
表2 不同扫描速率下的峰电流密度
Table 2 Peak current density at different scanning rates
| Sb3+浓度/(mmol·L-1) | 氧化峰电流密度/(mA·cm-2) | 还原峰电流密度/(mA·cm-2) | ||||||
|---|---|---|---|---|---|---|---|---|
| 10 mV·s-1 | 25 mV·s-1 | 50 mV·s-1 | 100 mV·s-1 | 10 mV·s-1 | 25 mV·s-1 | 50 mV·s-1 | 100 mV·s-1 | |
| pristine | 2.252 | 3.583 | 5.085 | 7.189 | -2.343 | -3.691 | -5.211 | -7.340 |
| 5 | 2.650 | 4.176 | 5.901 | 8.221 | -2.717 | -4.197 | -5.940 | -8.406 |
| 10 | 2.703 | 4.254 | 6.029 | 8.389 | -2.860 | -4.342 | -6.146 | -8.770 |
| 15 | 2.898 | 4.589 | 6.481 | 9.072 | -3.128 | -4.764 | -6.742 | -9.514 |
| 20 | 2.754 | 4.371 | 6.192 | 8.645 | -2.977 | -4.526 | -6.412 | -9.065 |
Sb3+浓度/ (mmol·L-1) | Dre/(cm2·s-1) | Dirre/(cm2·s-1) |
|---|---|---|
| pristine | 7.009×10-7 | 1.362×10-6 |
| 5 | 9.705×10-7 | 1.951×10-6 |
| 10 | 1.010×10-6 | 2.142×10-6 |
| 15 | 1.161×10-6 | 2.472×10-6 |
| 20 | 1.048×10-6 | 2.241×10-6 |
表3 具有不同浓度Sb3+离子的0.1 mol·L-1 V(Ⅲ)离子的扩散系数
Table 3 Diffusion coefficients of 0.1 mol·L-1 V(Ⅲ) ions with different concentrations of Sb3+ ions
Sb3+浓度/ (mmol·L-1) | Dre/(cm2·s-1) | Dirre/(cm2·s-1) |
|---|---|---|
| pristine | 7.009×10-7 | 1.362×10-6 |
| 5 | 9.705×10-7 | 1.951×10-6 |
| 10 | 1.010×10-6 | 2.142×10-6 |
| 15 | 1.161×10-6 | 2.472×10-6 |
| 20 | 1.048×10-6 | 2.241×10-6 |
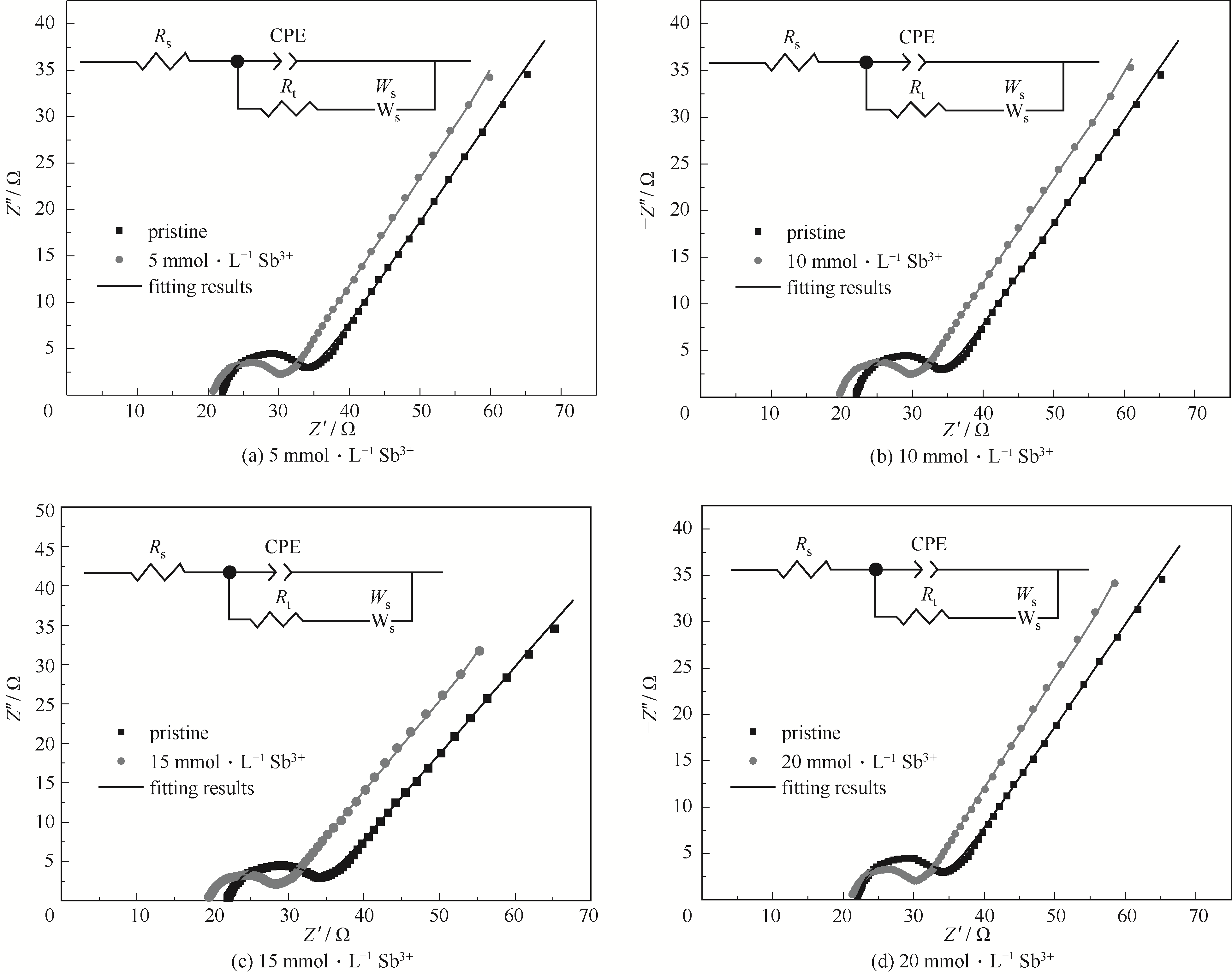
图6 0.1 mol·L-1 V(Ⅲ)电解质在不同浓度Sb3+下的Nyquist图及相应的等效电路
Fig.6 Nyquist plot of 0.1 mol·L-1 V(Ⅲ) electrolyte with different concentrations of Sb3+ ions and corresponding equivalent circuit
| Sb3+浓度/(mmol·L-1) | Rs/Ω | Rt/Ω | CPE/F | Ws |
|---|---|---|---|---|
| pristine | 22.03 | 11.57 | 7.22×10-4 | 0.533 |
| 5 | 20.72 | 9.89 | 9.86×10-4 | 0.538 |
| 10 | 20.67 | 9.50 | 1.01×10-3 | 0.548 |
| 15 | 19.41 | 8.95 | 1.15×10-3 | 0.558 |
| 20 | 21.15 | 9.11 | 1.06×10-3 | 0.549 |
表4 用等效电路拟合EIS图得到的参数
Table 4 The parameters obtained from fitting the EIS plots with the equivalent circuit
| Sb3+浓度/(mmol·L-1) | Rs/Ω | Rt/Ω | CPE/F | Ws |
|---|---|---|---|---|
| pristine | 22.03 | 11.57 | 7.22×10-4 | 0.533 |
| 5 | 20.72 | 9.89 | 9.86×10-4 | 0.538 |
| 10 | 20.67 | 9.50 | 1.01×10-3 | 0.548 |
| 15 | 19.41 | 8.95 | 1.15×10-3 | 0.558 |
| 20 | 21.15 | 9.11 | 1.06×10-3 | 0.549 |
| Sb3+浓度/(mmol·L-1) | 内阻/Ω | 能量效率/% |
|---|---|---|
| pristine | 14.38 | 78.39 |
| 5 | 11.71 | 80.57 |
| 10 | 11.39 | 82.86 |
| 15 | 10.34 | 87.01 |
| 20 | 11.28 | 84.01 |
表5 在Nafion 115膜组装下含有不同浓度Sb3+离子电解质的电池的内阻及能量效率
Table 5 Internal resistance and energy efficiency of batteries with different concentrations of Sb3+ ions electrolyte under Nafion 115 membrane assembly
| Sb3+浓度/(mmol·L-1) | 内阻/Ω | 能量效率/% |
|---|---|---|
| pristine | 14.38 | 78.39 |
| 5 | 11.71 | 80.57 |
| 10 | 11.39 | 82.86 |
| 15 | 10.34 | 87.01 |
| 20 | 11.28 | 84.01 |
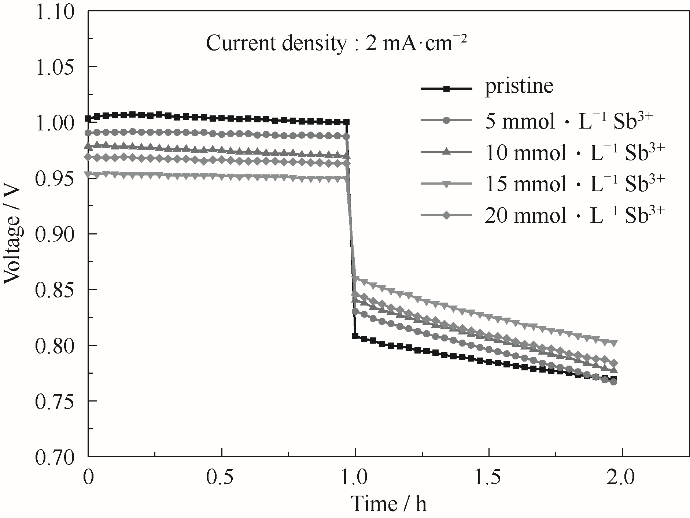
图8 在Nafion 115膜组装下含有不同浓度Sb3+离子电解质的电池充放电曲线
Fig.8 The charge-discharge curves of batteries using electrolyte with different concentrations of Sb3+ ions under Nafion 115 membrane assembly

图9 在Nafion 115膜组装下含有不同浓度Sb3+离子电解质的电池的极化曲线(a)和最大功率密度(b)
Fig.9 Polarization curves(a) and maximum power densities (b) of batteries with different concentrations of Sb3+ ions under Nafion 115 membrane assembly
| Sb3+浓度/(mmol·L-1) | 能量效率/% | ||
|---|---|---|---|
| 第一次循环 | 第二次循环 | 第三次循环 | |
| pristine | 78.39 | 75.42 | 73.50 |
| 5 | 80.57 | 78.26 | 76.06 |
| 10 | 82.86 | 80.90 | 78.99 |
| 15 | 87.01 | 86.52 | 84.93 |
| 20 | 84.01 | 83.48 | 82.11 |
表6 含有不同浓度Sb3+离子电解质的电池在三次充放电循环中的能量效率
Table 6 Energy efficiency of batteries with different concentrations of Sb3+ ions electrolyte in three charge-discharge cycles
| Sb3+浓度/(mmol·L-1) | 能量效率/% | ||
|---|---|---|---|
| 第一次循环 | 第二次循环 | 第三次循环 | |
| pristine | 78.39 | 75.42 | 73.50 |
| 5 | 80.57 | 78.26 | 76.06 |
| 10 | 82.86 | 80.90 | 78.99 |
| 15 | 87.01 | 86.52 | 84.93 |
| 20 | 84.01 | 83.48 | 82.11 |
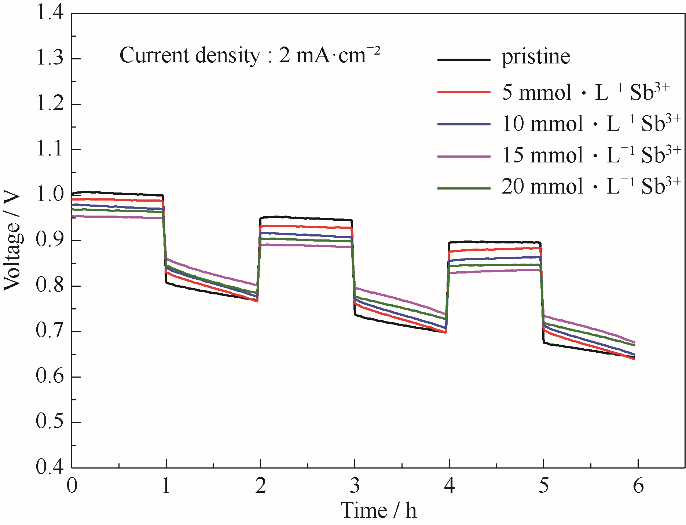
图10 使用含有不同浓度Sb3+离子电解质的电池在2 mA·cm-2电流密度下的充放电曲线
Fig.10 The charge-discharge curves of batteries using electrolyte with different concentrations of Sb3+ ions at a current density of 2 mA·cm-2
| 1 | Leung P, Li X, Poncedeleon C, et al. Progress in redox flow batteries, remaining challenges and their applications in energy storage[J]. RSC Advances, 2012, 2(27): 10125-10156. |
| 2 | Xu Q, Zhao T S. Fundamental models for flow batteries[J]. Progress in Energy & Combustion Science, 2015, 49: 40-58. |
| 3 | Xu Q, Zhao T S, Zhang C. Effects of SOC-dependent electrolyte viscosity on performance of vanadium redox flow batteries[J]. Applied Energy, 2014, 130: 139-147. |
| 4 | Soloveichik G L. Flow batteries: current status and trends[J]. Chemical Reviews, 2015, 115: 11533-11558. |
| 5 | Thaller L H. Electrically rechargeable redox flow cells[C]//Intersociety Energy Conversion Engineering Conference, 1974: 924-928. |
| 6 | Chalamala B R, Soundappan T, Fisher G R, et al. Redox flow batteries: an engineering perspective[J]. Proceedings of the IEEE, 2014, 102: 976-999. |
| 7 | Skyllas-Kazacos M, Chakrabarti M H, Hajimolana S A, et al. Progress in flow battery research and development[J]. Journal of the Electrochemical Society, 2011, 158: R55-R79. |
| 8 | Galus Z. Electrochemical reactions in nonaqueous and mixed solvents[M]//Advances in Electrochemical Science and Engineering: Volume 4. Wiley‐VCH Verlag GmbH, 2008. |
| 9 | Wei X, Duan W, Huang J, et al. A high-current, stable nonaqueous organic redox flow battery[J]. ACS Energy Letters, 2016, 1(4): 705-711. |
| 10 | Escalante-García I L, Wainright J S, Thompson L T, et al. Performance of a non-aqueous vanadium acetylacetonate prototype redox flow battery: examination of separators and capacity decay[J]. Journal of the Electrochemical Society, 2014, 162: A363-A372. |
| 11 | Abbott A P, Mckenzie K J. Application of ionic liquids to the electrodeposition of metals[J]. Physical Chemistry Chemical Physics, 2006, 8: 4265-4279. |
| 12 | Lin I J B, Vasam C S. Metal-containing ionic liquids and ionic liquid crystals based on imidazolium moiety[J]. Journal of Organometallic Chemistry, 2005, 690: 3498-3512. |
| 13 | Abbott A P, Barron J C, Ryder K S, et al. Eutectic-based ionic liquids with metal-containing anions and cations[J]. Chemistry - A European Journal, 2007, 13: 6495-6501. |
| 14 | Zhang Q, Vigier K D O, Royer S, et al. Deep eutectic solvents: syntheses, properties and applications[J]. Chemical Society Reviews, 2012, 41: 7108-7146. |
| 15 | Ding Y, Zhang C, Zhang L, et al. Molecular engineering of organic electroactive materials for redox flow batteries[J]. Chemical Society Reviews, 2018, 47: 69-103. |
| 16 | Zhang C K, Zhang L Y, Ding Y, et al. Progress and prospects of next-generation redox flow batteries[J]. Energy Storage Materials, 2018, 15: 324-350. |
| 17 | Lloyd D, Vainikka T, Murtomäki L, et al. The kinetics of the Cu2+/Cu+ redox couple in deep eutectic solvents[J]. Electrochimica Acta, 2011, 56: 4942-4948. |
| 18 | Xu Q, Zhao T S, Wei L, et al. Electrochemical characteristics and transport properties of Fe(Ⅱ)/Fe(Ⅲ) redox couple in a non-aqueous reline deep eutectic solvent[J]. Electrochimica Acta, 2015, 154: 462-467. |
| 19 | Xu Q, Qin L, Su H, et al. Electrochemical and transport characteristics of V(Ⅱ)/V(Ⅲ) redox couple in a nonaqueous deep eutectic solvent: temperature effect[J]. Journal of Energy Engineering, 2017, 143: 04017051. |
| 20 | Jiang H R, Zeng Y K, Wu M C, et al. A uniformly distributed bismuth nanoparticle-modified carbon cloth electrode for vanadium redox flow batteries[J]. Applied Energy, 2019, 240: 226-235. |
| 21 | Hwang J, Ha H Y. Method for preparing electrolyte for redox flow battery including organic molecules as additive and redox flow battery using the same: US20180013163[P]. 2018-01-11. |
| 22 | Leung P, Shah A A, Sanz L, et al. Recent developments in organic redox flow batteries: a critical review[J]. Journal of Power Sources, 2017, 360: 243-283. |
| 23 | Jiang J, Materna K, Hedström S, et al. Molecular antimony complexes for electrocatalysis: activity of a main group element in proton reduction[J]. Angewandte Chemie International Edition, 2017, 56: 9111-9115. |
| 24 | Bradwell D J, Kim H, Sirk A H C, et al. Magnesium-antimony liquid metal battery for stationary energy storage[J]. Journal of the American Chemical Society, 2012, 134: 1895-1897. |
| 25 | Shen J, Liu S, He Z, et al. Influence of antimony ions in negative electrolyte on the electrochemical performance of vanadium redox flow batteries[J]. Electrochimica Acta, 2015, 151: 297-305. |
| 26 | Liang X, Peng S, Lei Y, et al. Effect of L-glutamic acid on the positive electrolyte for all-vanadium redox flow battery[J]. Electrochimica Acta, 2013, 95: 80-86. |
| 27 | Xu Q, Ji Y N, Qin L Y, et al. Effect of carbon dioxide additive on the characteristics of a deep eutectic solvent (DES) electrolyte for non-aqueous redox flow batteries[J]. Chemical Physics Letters, 2018, 708: 48-53. |
| [1] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [2] | 张童, 杨扬, 叶丁丁, 陈蓉, 朱恂, 廖强. 催化剂分布对可渗透阳极微流体燃料电池性能特性影响的研究[J]. 化工学报, 2022, 73(9): 4156-4162. |
| [3] | 叶丁丁,李 俊,朱 恂,廖 强,黄桂兰,付 乾. 自呼吸式直接甲醇燃料电池性能及其传质特性 [J]. CIESC Journal, 2009, 28(6): 948-. |
| [4] | 涂海涛, 孙文策, 解茂昭, 阿布里提. 质子交换膜燃料电池双层扩散层特性三维分析 [J]. 化工学报, 2008, 59(1): 182-188. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号