化工学报 ›› 2021, Vol. 72 ›› Issue (12): 6291-6297.DOI: 10.11949/0438-1157.20211079
张瑞1,2( ),钟静1,2,林森1,2(
),钟静1,2,林森1,2( ),于建国1,2(
),于建国1,2( )
)
收稿日期:2021-08-02
修回日期:2021-11-19
出版日期:2021-12-05
发布日期:2021-12-22
通讯作者:
林森,于建国
作者简介:张瑞(1998—),女,博士研究生,基金资助:
Rui ZHANG1,2( ),Jing ZHONG1,2,Sen LIN1,2(
),Jing ZHONG1,2,Sen LIN1,2( ),Jianguo YU1,2(
),Jianguo YU1,2( )
)
Received:2021-08-02
Revised:2021-11-19
Online:2021-12-05
Published:2021-12-22
Contact:
Sen LIN,Jianguo YU
摘要:
铝系锂吸附剂成型颗粒在盐湖卤水提锂工业应用过程中存在吸附容量低、吸附速率慢和吸附剂粉末脱落等问题。基于现有反溶剂法挤压成型工艺,对盐湖铝系提锂吸附剂成型条件的影响进行了系统性研究。实验结果显示吸附剂成型颗粒粒径越小,达到吸附平衡越快,当颗粒直径d<1 mm时,吸附剂颗粒可在24 h左右达到吸附平衡;降低黏结剂浓度可有效加快吸附剂颗粒的吸附速率,但黏结剂浓度过低会导致其对粉末的包裹性下降;吸附剂颗粒的吸附速率与致孔剂添加比例成正比,当致孔剂添加比例为20%时,吸附剂颗粒能在4 h内完成快速吸附阶段,吸附平衡时对察尔汗高镁锂比盐湖卤水中锂的吸附容量可达4.97 mg·g-1。
中图分类号:
张瑞, 钟静, 林森, 于建国. 盐湖铝系提锂吸附剂成型条件的影响研究[J]. 化工学报, 2021, 72(12): 6291-6297.
Rui ZHANG, Jing ZHONG, Sen LIN, Jianguo YU. Study on the influence of granulation conditions on Li/Al-LDHs for lithium recovery from low grade brine[J]. CIESC Journal, 2021, 72(12): 6291-6297.
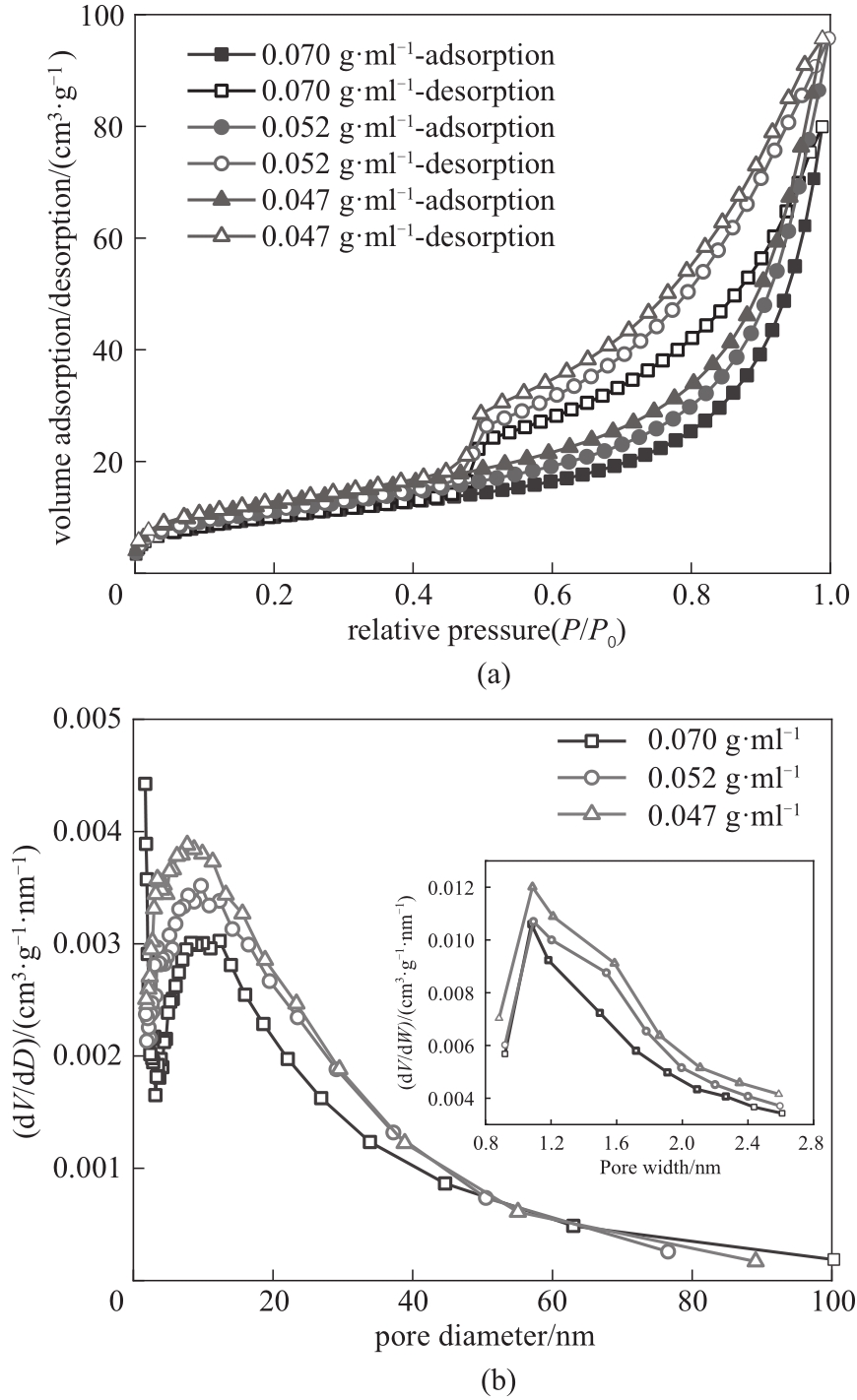
图3 77 K下不同黏结剂浓度下吸附剂小球的N2吸附/解吸等温线(a)和孔径分布(b)
Fig.3 N2 adsorption/desorption isotherm (a) and pore size distributions (b) of granulated Li/Al-LDHs with different adhesive concentrations at 77 K
| 黏结剂DMF浓度/(g·ml-1) | 比表面积/(m2·g-1) | 孔容/(cm3·g-1) | 平均孔径/nm |
|---|---|---|---|
| 0.070 | 36.85 | 0.12 | 8.22 |
| 0.052 | 40.55 | 0.14 | 7.94 |
| 0.047 | 45.31 | 0.14 | 7.84 |
表1 不同黏结剂浓度下铝系吸附剂小球的孔结构参数
Table 1 Pore structure parameters of granulated Li/Al-LDHs with different adhesive concentrations
| 黏结剂DMF浓度/(g·ml-1) | 比表面积/(m2·g-1) | 孔容/(cm3·g-1) | 平均孔径/nm |
|---|---|---|---|
| 0.070 | 36.85 | 0.12 | 8.22 |
| 0.052 | 40.55 | 0.14 | 7.94 |
| 0.047 | 45.31 | 0.14 | 7.84 |

图5 吸附剂小球在摇瓶实验中的粉末损失情况(1为直接黏结成型;2~4为反溶剂法成型,黏结剂浓度分别为0.047、0.052、0.070 g·ml-1)
Fig.5 Powder loss of granulated Li/Al-LDHs in shake flask experiment(1 is direct bonding granulation; 2,3,4 are anti-solvent granulation with the adhesive concentrations of 0.047,0.052 and 0.070 g·ml-1)
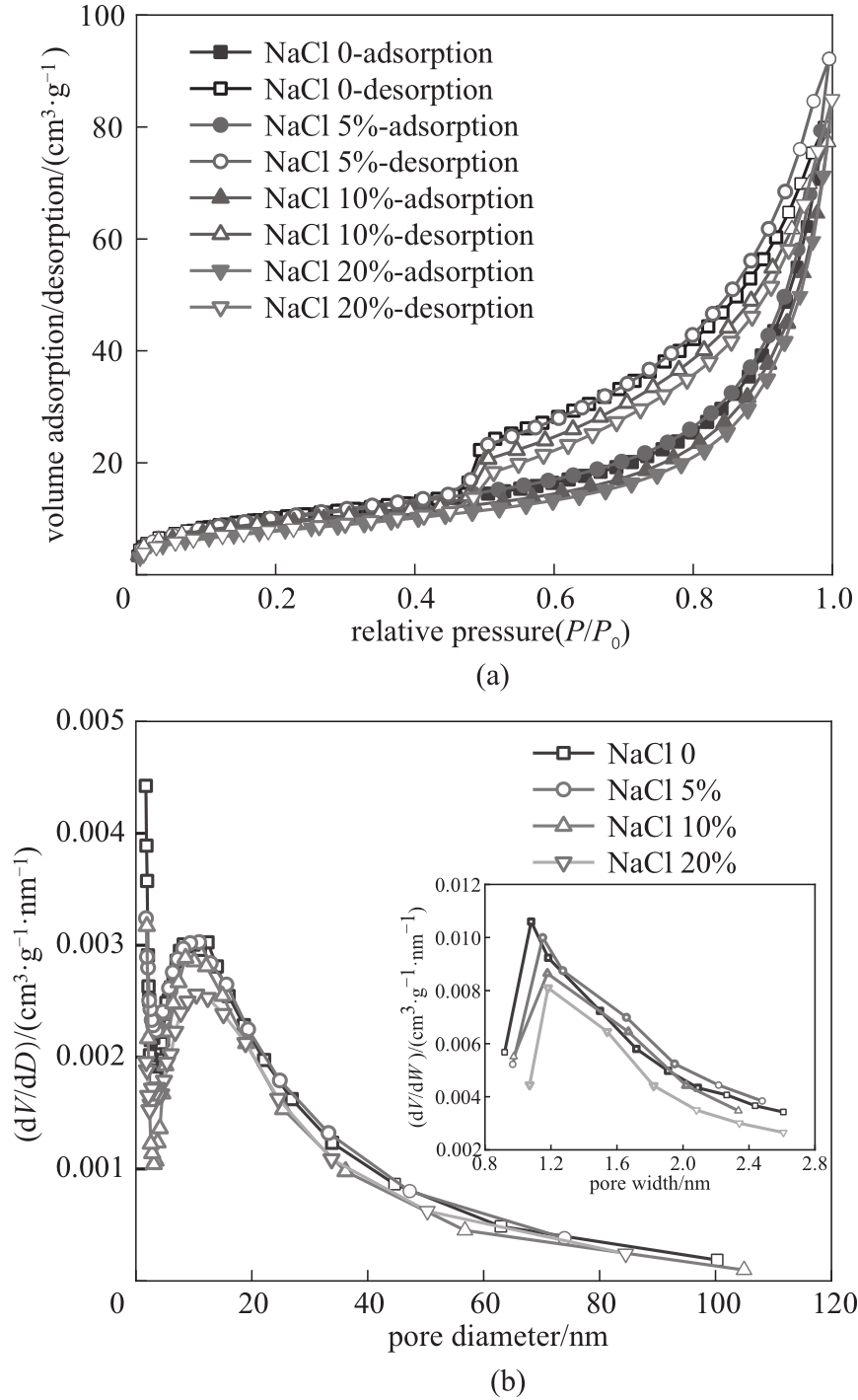
图7 77K下不同致孔剂NaCl含量下吸附剂小球的N2吸附-解吸等温线(a)和孔径分布(b)
Fig.7 N2 adsorption/desorption isotherm (a) and pore size distributions (b) of granulated Li/Al-LDHs with different NaCl contents at 77K
| NaCl含量/% | 比表面积/(m2·g-1) | 孔容/(cm3·g-1) | 平均孔径/nm |
|---|---|---|---|
| 0 | 36.85 | 0.12 | 8.22 |
| 5 | 36.84 | 0.12 | 8.75 |
| 10 | 33.18 | 0.12 | 8.64 |
| 20 | 28.44 | 0.11 | 9.29 |
表2 不同致孔剂NaCl含量下吸附剂小球的孔结构参数
Table 2 Pore structure parameters of granulated Li/Al-LDHs with different NaCl contents
| NaCl含量/% | 比表面积/(m2·g-1) | 孔容/(cm3·g-1) | 平均孔径/nm |
|---|---|---|---|
| 0 | 36.85 | 0.12 | 8.22 |
| 5 | 36.84 | 0.12 | 8.75 |
| 10 | 33.18 | 0.12 | 8.64 |
| 20 | 28.44 | 0.11 | 9.29 |
| 1 | Bernhardt D,Reilly I I.Minerals Commodity Summaries 2019[R].Reston: US Geological Survey, 2019. |
| 2 | 韩佳欢, 乜贞, 伍倩, 等. 中国锂资源供需现状分析[J]. 无机盐工业, 2021, 53 (12): 61-66. |
| Han J H, Nie Z, Wu Q, et al. Analysis of supply and demand on China's lithium resources[J]. Inorganic Chemicals Industry, 2021, 53 (12): 61-66. | |
| 3 | 熊增华, 王兴富, 王石军, 等. 青海盐湖锂资源综合利用规模探讨[J]. 盐湖研究, 2020, 28(4): 125-131. |
| Xiong Z H, Wang X F, Wang S J, et al. Discussion on comprehensive utilization scale of lithium resources in Qinghai salt lakes[J]. Journal of Salt Lake Research, 2020, 28(4): 125-131. | |
| 4 | 王翘楚, 孙鑫, 郝瀚, 等. 锂的城市矿产利用:前景、挑战及政策建议[J]. 科技导报, 2020, 38(15): 6-15. |
| Wang Q C, Sun X, Hao H, et al. Urban mining of lithium: prospects, challenges and policy recommendations[J]. Science & Technology Review, 2020, 38(15): 6-15. | |
| 5 | 苏彤, 郭敏, 刘忠, 等. 全球锂资源综合评述[J]. 盐湖研究, 2019, 27(3): 104-111. |
| Su T, Guo M, Liu Z, et al. Comprehensive review of global lithium resources[J]. Journal of Salt Lake Research, 2019, 27(3): 104-111. | |
| 6 | 高春亮, 余俊清, 闵秀云, 等. 全球盐湖卤水锂矿床的分布特征及其控制因素[J]. 盐湖研究, 2020, 28(4): 48-55. |
| Gao C L, Yu J Q, Min X Y, et al. Distribution characteristics and controlling factors of lithium brine deposits in the world[J]. Journal of Salt Lake Research, 2020, 28(4): 48-55. | |
| 7 | 曹兆江, 高敏, 宁占玉, 等. 青海盐湖锂资源及提锂技术概述[J]. 化工设计通讯, 2019, 45(6): 190, 207. |
| Cao Z J, Gao M, Ning Z Y, et al. Lithium resources and lithium extraction technology in Qinghai salt lake[J]. Chemical Engineering Design Communications, 2019, 45(6): 190, 207. | |
| 8 | Liu X H, Zhong M L, Chen X Y, et al. Separating lithium and magnesium in brine by aluminum-based materials[J]. Hydrometallurgy, 2018, 176: 73-77. |
| 9 | 于潇, 孙淑英, 于建国. 层状吸附剂提锂过程研究[J]. 无机盐工业, 2017, 49(12): 23-26, 49. |
| Yu X, Sun S Y, Yu J G. Extraction of lithium from solution with layered adsorbent[J]. Inorganic Chemicals Industry, 2017, 49(12): 23-26, 49. | |
| 10 | 陈念, 钟辉, 颜辉. 国内外卤水提锂工艺技术现状[J]. 盐业与化工, 2014, 43(3): 1-4. |
| Chen N, Zhong H, Yan H. Present situation of the process and technique of lithium recovery from brine around the world[J]. Journal of Salt and Chemical Industry, 2014, 43(3): 1-4. | |
| 11 | 顾俊杰, 李增荣, 唐发满, 等. 用于盐湖卤水吸附法提锂的连续离子交换工艺研究[J]. 无机盐工业, 2020, 52(7): 46-51. |
| Gu J J, Li Z R, Tang F M, et al. Study on continuous ion exchange process applied in lithium extraction from salt lake brine with adsorption method[J]. Inorganic Chemicals Industry, 2020, 52(7): 46-51. | |
| 12 | 柏春, 郭敏, 张慧芳, 等. 离子筛型锂吸附剂吸附法从盐湖卤水/海水中提锂的研究进展[J]. 化工进展, 2017, 36(3): 802-809. |
| Bai C, Guo M, Zhang H F, et al. The research progress of extracting lithium from brine by lithium ion sieve[J]. Chemical Industry and Engineering Progress, 2017, 36(3): 802-809. | |
| 13 | 吴静, 任秀莲, 魏琦峰. 盐湖卤水中锂的分离提取研究进展[J]. 无机盐工业, 2020, 52(12): 1-6. |
| Wu J, Ren X L, Wei Q F. Research progress on separation and extraction of lithium from salt-lake brine[J]. Inorganic Chemicals Industry, 2020, 52(12): 1-6. | |
| 14 | 许乃才, 史丹丹, 黎四霞, 等. 利用吸附技术提取盐湖卤水中锂的研究进展[J]. 材料导报, 2017, 31(17): 116-121. |
| Xu N C, Shi D D, Li S X, et al. Advances in extracting lithium from salt-lake brines by adsorption technique[J]. Materials Review, 2017, 31(17): 116-121. | |
| 15 | 刘东帆, 孙淑英, 于建国. 盐湖卤水提锂技术研究与发展[J]. 化工学报, 2018, 69(1): 141-155. |
| Liu D F, Sun S Y, Yu J G. Research and development on technique of lithium recovery from salt lake brine[J]. CIESC Journal, 2018, 69(1): 141-155. | |
| 16 | Chitrakar R, Makita Y, Ooi K, et al. Synthesis of iron-doped manganese oxides with an ion-sieve property: lithium adsorption from Bolivian brine[J]. Industrial & Engineering Chemistry Research, 2014, 53(9): 3682-3688. |
| 17 | Ryu T, Shin J, Ghoreishian S M, et al. Recovery of lithium in seawater using a titanium intercalated lithium manganese oxide composite[J]. Hydrometallurgy, 2019, 184: 22-28. |
| 18 | Wang S L, Zhang M, Zhang Y, et al. High adsorption performance of the Mo-doped titanium oxide sieve for lithium ions[J]. Hydrometallurgy, 2019, 187: 30-37. |
| 19 | Isupov V P, Kotsupalo N P, Nemudry A P, et al. Aluminium hydroxide as selective sorbent of lithium salts from brines and technical solutions[M]//Studies in Surface Science and Catalysis. Amsterdam: Elsevier, 1999: 621-652. |
| 20 | 吴志坚, 郭敏, 李权, 等. 氢氧化铝基锂吸附剂从卤水中吸附锂的机理[J]. 盐湖研究, 2018, 26(3): 1-6. |
| Wu Z J, Guo M, Li Q, et al. Adsorption mechanisms for the recovery of lithium from brines using aluminum hydroxide based adsorbent[J]. Journal of Salt Lake Research, 2018, 26(3): 1-6. | |
| 21 | Kotsupalo N P, Ryabtsev A D, Poroshina I A, et al. Effect of structure on the sorption properties of chlorine-containing form of double aluminum lithium hydroxide[J]. Russian Journal of Applied Chemistry, 2013, 86(4): 482-487. |
| 22 | Paranthaman M P, Li L, Luo J Q, et al. Recovery of lithium from geothermal brine with lithium-aluminum layered double hydroxide chloride sorbents[J]. Environmental Science & Technology, 2017, 51(22): 13481-13486. |
| 23 | Jiang H X, Yang Y, Yu J G. Application of concentration-dependent HSDM to the lithium adsorption from brine in fixed bed columns[J]. Separation and Purification Technology, 2020, 241: 116682. |
| 24 | Zhao B, Zhang Y, Dou X M, et al. Granulation of Fe-Al-Ce trimetal hydroxide as a fluoride adsorbent using the extrusion method[J]. Chemical Engineering Journal, 2012, 185/186: 211-218. |
| 25 | Wu X M, Zhang Y, Dou X M, et al. Fluoride removal performance of a novel Fe-Al-Ce trimetal oxide adsorbent[J]. Chemosphere, 2007, 69(11): 1758-1764. |
| 26 | Park M J, Nisola G M, Beltran A B, et al. Recyclable composite nanofiber adsorbent for Li+ recovery from seawater desalination retentate[J]. Chemical Engineering Journal, 2014, 254: 73-81. |
| 27 | 刘文涛, 刘亦凡. 锂离子交换体Li1.5Ti1.625O4 的研究(Ⅲ): Li1.5Ti1.625O4的造粒、改型及油田咸水中锂的回收[J]. 离子交换与吸附, 2011, 27(4): 353-358. |
| Liu W T, Liu Y F. Study on the lithium ion permutoid Li1.5Ti1.625O4(Ⅲ):Pelleting & modification of the precursor Li1.5Ti1.625O4, and recovery of lithium from the salty water in oil fields[J]. Ion Exchange and Adsorption, 2011, 27(4): 353-358. | |
| 28 | Ryabtsev A D, Menzheres L T, Ten A V. Sorption of lithium from brine onto granular LiCl·2Al(OH)3·mH2O sorbent under dynamic conditions[J]. Russian Journal of Applied Chemistry, 2002, 75(7): 1069-1074. |
| 29 | Xiao G P, Tong K F, Zhou L S, et al. Adsorption and desorption behavior of lithium ion in spherical PVC-MnO2 ion sieve[J]. Industrial & Engineering Chemistry Research, 2012, 51(33): 10921-10929. |
| 30 | Hu F P, Lin S, Li P, et al. Quantitative effects of desorption intensity on structural stability and readsorption performance of lithium/aluminum layered double hydroxides in cyclic Li+ extraction from brines with ultrahigh Mg/Li ratio[J]. Industrial & Engineering Chemistry Research, 2020, 59(30): 13539-13548. |
| [1] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [2] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [3] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [4] | 侯林怡, 王一宁, 张安运, 苏佳天. 新型calix[4]biscrown超分子识别材料制备及其吸附铷和铯性能研究[J]. 化工学报, 2021, 72(6): 3063-3073. |
| [5] | 李燕, 王敏, 赵有璟, 王怀有, 杨红军, 祝增虎. 纳滤膜对高镁锂比盐湖卤水镁锂分离性能研究[J]. 化工学报, 2021, 72(6): 3130-3139. |
| [6] | 王琪, 赵有璟, 刘洋, 王云昊, 王敏, 项顼. 高镁锂比盐湖镁锂分离与锂提取技术研究进展[J]. 化工学报, 2021, 72(6): 2905-2921. |
| [7] | 李栋婵, 王嘉宇, 王士强. 四元体系(Li+, Mg2+//Cl-, borate-H2O)308.15 K相平衡与相图研究[J]. 化工学报, 2021, 72(6): 3170-3178. |
| [8] | 孟庆伟, 张峰, 陈璐, 夏永生, 居沈贵, 邢卫红. 离子筛吸附与陶瓷膜耦合用于盐湖卤水提锂[J]. 化工学报, 2017, 68(5): 1899-1905. |
| [9] | 熊妍, 鲍宗必, 邢华斌, 苏宝根, 杨亦文, 任其龙. 1,3-二元脂肪醇萃取硼酸的平衡特性[J]. 化工学报, 2012, 63(11): 3546-3552. |
| [10] | 熊 妍,鲍宗必,邢华斌,苏宝根,杨亦文,任其龙. 盐湖卤水提硼萃取剂的研究进展[J]. 化工进展, 2012, 31(08): 1647-1655. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号