化工学报 ›› 2023, Vol. 74 ›› Issue (8): 3375-3385.DOI: 10.11949/0438-1157.20230375
收稿日期:2023-04-17
修回日期:2023-08-11
出版日期:2023-08-25
发布日期:2023-10-18
通讯作者:
林森
作者简介:盛冰纯(1997—),女,硕士研究生,shengbingchun@163.com
基金资助:
Bingchun SHENG1,2( ), Jianguo YU1,2, Sen LIN1,2(
), Jianguo YU1,2, Sen LIN1,2( )
)
Received:2023-04-17
Revised:2023-08-11
Online:2023-08-25
Published:2023-10-18
Contact:
Sen LIN
摘要:
铝基锂吸附剂由于其解吸条件温和,不发生溶损,是目前唯一一种成功实现工业化生产的盐湖卤水提锂吸附剂,然而其在高钠型地下卤水中的应用可行性还有待考察。使用实验室自制的H-LDHs颗粒吸附剂,系统研究了吸附液进料流速、解吸温度及解吸液中离子浓度对固定床吸附和解吸过程的影响,实验结果表明,在高钠卤水中,当进料流速从1 BV/h(1 BV/h = 0.170 L/h)增加到4 BV/h时,穿透时间缩短了79%,而穿透吸附容量仅降低了17.8%。升高解吸温度可显著提高固定床的Li+解吸量,而增大解吸液中的Na+浓度会抑制Li+的解吸。此外,开发了分段循环解吸工艺,并将其用于四川某地实际地下卤水提锂过程,该工艺能够有效实现解吸工段固定床出料液中Li+的富集。
中图分类号:
盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385.
Bingchun SHENG, Jianguo YU, Sen LIN. Study on lithium resource separation from underground brine with high concentration of sodium by aluminum-based lithium adsorbent[J]. CIESC Journal, 2023, 74(8): 3375-3385.
| 离子 | 浓度/(g/L) |
|---|---|
| Li+ | 0.135 |
| Na+ | 91.905 |
| K+ | 5.145 |
| Ca2+ | 18.100 |
| Mg2+ | 1.970 |
| B3+ | 0.500 |
| Sr2+ | 0.955 |
| Rb+ | 0.008 |
| 195.300 | |
| 0.068 | |
| 0.265 | |
| 0.140 |
表1 四川某地实际地下卤水中主要离子浓度
Table 1 Concentrations of the main existing ions in the real underground brine somewhere in Sichuan
| 离子 | 浓度/(g/L) |
|---|---|
| Li+ | 0.135 |
| Na+ | 91.905 |
| K+ | 5.145 |
| Ca2+ | 18.100 |
| Mg2+ | 1.970 |
| B3+ | 0.500 |
| Sr2+ | 0.955 |
| Rb+ | 0.008 |
| 195.300 | |
| 0.068 | |
| 0.265 | |
| 0.140 |
| 1 | 9.64 | 3.396 | 9.64 |
| 2 | 4.74 | 3.249 | 9.48 |
| 4 | 2.06 | 2.791 | 8.24 |
表2 进料流速对固定床吸附穿透性能的影响
Table 2 Effect of flow rate on the fixed bed adsorption breakthrough performance
| 1 | 9.64 | 3.396 | 9.64 |
| 2 | 4.74 | 3.249 | 9.48 |
| 4 | 2.06 | 2.791 | 8.24 |
| 模型 | A | r/h-1 | 相关系数 | 残差平方和 | ||||
|---|---|---|---|---|---|---|---|---|
| Clark | 0.00004 | 0.45881 | — | — | — | — | 0.9695 | 0.06927 |
| Thomas | — | — | 3.728 | 0.00483 | — | — | 0.9416 | 0.14037 |
| M-D-R | — | — | — | — | 3.136 | 1.73474 | 0.9976 | 0.00356 |
表3 在地下卤水中吸附Li+的穿透曲线经验模型拟合参数
Table 3 Fitting parameters for breakthrough curve empirical models of Li+ adsorption from the underground brine
| 模型 | A | r/h-1 | 相关系数 | 残差平方和 | ||||
|---|---|---|---|---|---|---|---|---|
| Clark | 0.00004 | 0.45881 | — | — | — | — | 0.9695 | 0.06927 |
| Thomas | — | — | 3.728 | 0.00483 | — | — | 0.9416 | 0.14037 |
| M-D-R | — | — | — | — | 3.136 | 1.73474 | 0.9976 | 0.00356 |
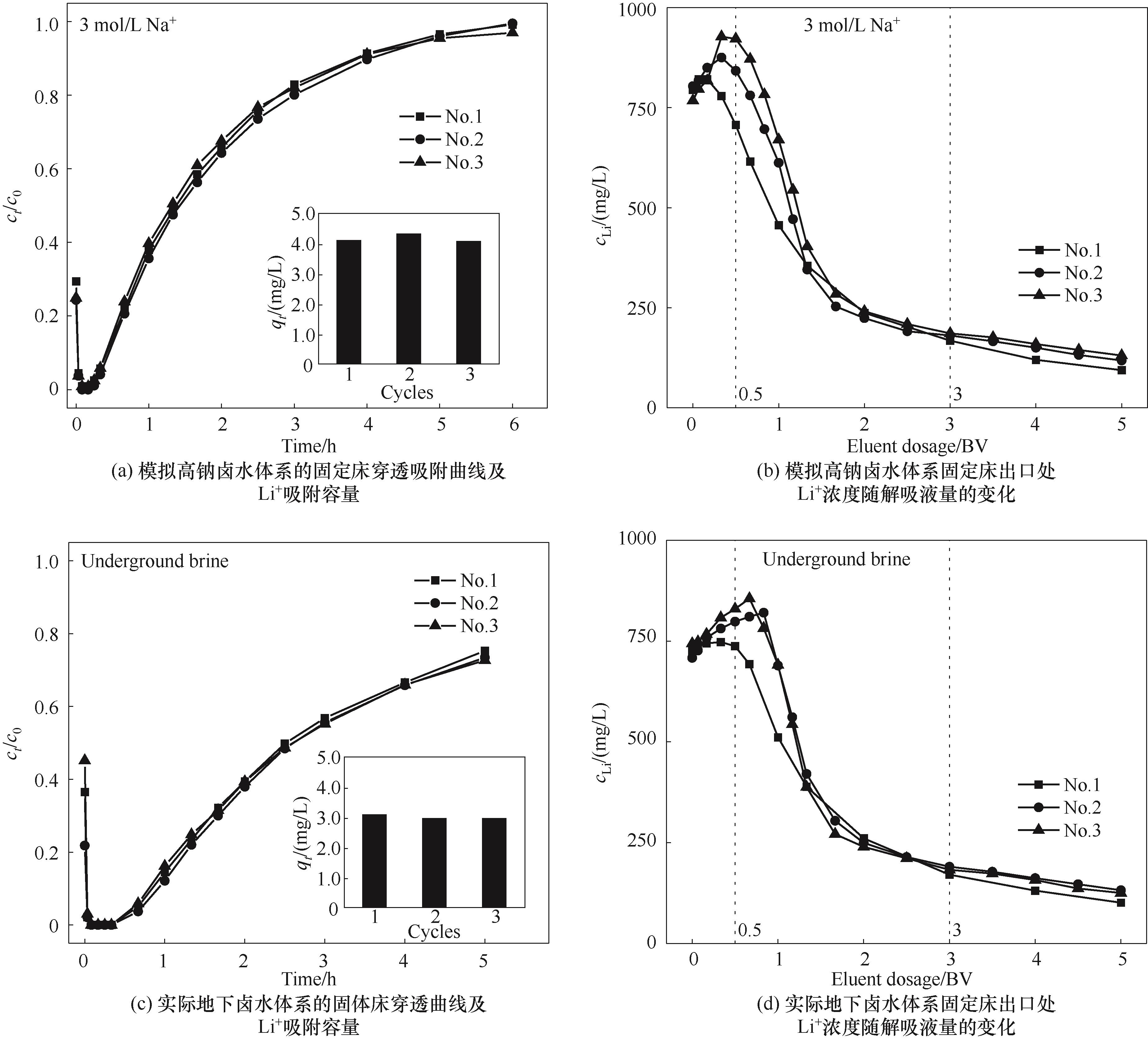
图10 固定床吸附穿透曲线、Li+吸附容量及固定床出口处Li+浓度随解吸液量的变化
Fig.10 Adsorption breakthrough curves over time and adsorption capacities of Li+, as well as outlet concentration curves of Li+ over eluent dosage in the fixed bed
| 项目 | Li+浓度/(mg/L) | ||||
|---|---|---|---|---|---|
| 第一段 | 第二段 | 第三段 | 总收集液 | ||
| 模拟高钠卤水 | 0.1 mol/L Na+ | 786.5 | 371.4 | 119.1 | 284.4 |
| No.1 | 785.0 | 331.6 | 108.1 | — | |
| No.2 | 840.8 | 367.2 | 134.4 | — | |
| No.3 | 865.6 | 408.4 | 137.7 | 336.7 | |
| 实际地下卤水 | 0.1 mol/L Na+ | 729.3 | 343.1 | 113.7 | 254.6 |
| No.1 | 734.9 | 362.3 | 120.2 | — | |
| No.2 | 755.0 | 391.1 | 149.0 | — | |
| No.3 | 756.2 | 381.4 | 146.5 | 309.8 | |
表4 四次解吸过程中各段收集液中的Li+浓度
Table 4 The concentration of Li+ in each stage of eluent during four desorption processes
| 项目 | Li+浓度/(mg/L) | ||||
|---|---|---|---|---|---|
| 第一段 | 第二段 | 第三段 | 总收集液 | ||
| 模拟高钠卤水 | 0.1 mol/L Na+ | 786.5 | 371.4 | 119.1 | 284.4 |
| No.1 | 785.0 | 331.6 | 108.1 | — | |
| No.2 | 840.8 | 367.2 | 134.4 | — | |
| No.3 | 865.6 | 408.4 | 137.7 | 336.7 | |
| 实际地下卤水 | 0.1 mol/L Na+ | 729.3 | 343.1 | 113.7 | 254.6 |
| No.1 | 734.9 | 362.3 | 120.2 | — | |
| No.2 | 755.0 | 391.1 | 149.0 | — | |
| No.3 | 756.2 | 381.4 | 146.5 | 309.8 | |
| 1 | Swain B. Recovery and recycling of lithium: a review[J]. Separation and Purification Technology, 2017, 172: 388-403. |
| 2 | Liu G, Zhao Z W, Ghahreman A. Novel approaches for lithium extraction from salt-lake brines: a review[J]. Hydrometallurgy, 2019, 187: 81-100. |
| 3 | 苏彤, 郭敏, 刘忠, 等. 全球锂资源综合评述[J]. 盐湖研究, 2019, 27(3): 104-111. |
| Su T, Guo M, Liu Z, et al. Comprehensive review of global lithium resources[J]. Journal of Salt Lake Research, 2019, 27(3):104-111. | |
| 4 | 马哲, 李建武. 中国锂资源供应体系研究: 现状、问题与建议[J]. 中国矿业, 2018, 27(10): 1-7. |
| Ma Z, Li J W. Analysis of China's lithium resources supply system: status, issues and suggestions[J]. China Mining Magazine, 2018, 27(10): 1-7. | |
| 5 | Meshram P, Pandey B D, Mankhand T R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: a comprehensive review[J]. Hydrometallurgy, 2014, 150: 192-208. |
| 6 | 王核, 黄亮, 白洪阳, 等. 中国锂资源的主要类型、分布和开发利用现状: 评述和展望[J]. 大地构造与成矿学, 2022, 46(5): 848-866. |
| Wang H, Huang L, Bai H Y, et al. Types, distribution, development and utilization of lithium mineral resources in China: review and perspective[J]. Geotectonica et Metallogenia, 2022, 46(5): 848-866. | |
| 7 | Sanjuan B, Gourcerol B, Millot R, et al. Lithium-rich geothermal brines in Europe: an up-date about geochemical characteristics and implications for potential Li resources[J]. Geothermics, 2022, 101: 102385. |
| 8 | Kumar A, Fukuda H, Hatton T A, et al. Lithium recovery from oil and gas produced water: a need for a growing energy industry[J]. ACS Energy Letters, 2019, 4(6): 1471-1474. |
| 9 | Xu X, Chen Y M, Wan P Y, et al. Extraction of lithium with functionalized lithium ion-sieves[J]. Progress in Materials Science, 2016, 84: 276-313. |
| 10 | Kim J, Oh S, Kwak S Y. Magnetically separable magnetite-lithium manganese oxide nanocomposites as reusable lithium adsorbents in aqueous lithium resources[J]. Chemical Engineering Journal, 2015, 281: 541-548. |
| 11 | Chen S Q, Chen Z S, Wei Z W, et al. Titanium-based ion sieve with enhanced post-separation ability for high performance lithium recovery from geothermal water[J]. Chemical Engineering Journal, 2021, 410: 128320. |
| 12 | Jiang H X, Yang Y, Yu J G. Application of concentration-dependent HSDM to the lithium adsorption from brine in fixed bed columns[J]. Separation and Purification Technology, 2020, 241: 116682. |
| 13 | Xiao J L, Nie X Y, Sun S Y, et al. Lithium ion adsorption-desorption properties on spinel Li4Mn5O12 and pH-dependent ion-exchange model[J]. Advanced Powder Technology, 2015, 26(2): 589-594. |
| 14 | Liu J C, Zhang Y B, Miao Y, et al. Alkaline resins enhancing Li+/H+ ion exchange for lithium recovery from brines using granular titanium-type lithium ion-sieves[J]. Industrial & Engineering Chemistry Research, 2021, 60(45): 16457-16468. |
| 15 | 张瑞, 陆旗玮, 林森, 等. 铝系成型锂吸附剂性能测试评价与对比[J]. 化工学报, 2021, 72(6): 3053-3062. |
| Zhang R, Lu Q W, Lin S, et al. Performance evaluation and comparison of aluminum-based granulated lithium adsorbent[J]. CIESC Journal, 2021, 72(6): 3053-3062. | |
| 16 | Hu F P, Lin S, Li P, et al. Quantitative effects of desorption intensity on structural stability and readsorption performance of lithium/aluminum layered double hydroxides in cyclic Li+ extraction from brines with ultrahigh Mg/Li ratio[J]. Industrial & Engineering Chemistry Research, 2020, 59(30): 13539-13548. |
| 17 | Chen J, Lin S, Yu J G. High-selective cyclic adsorption and magnetic recovery performance of magnetic lithium-aluminum layered double hydroxides (MLDHs) in extracting Li+ from ultrahigh Mg/Li ratio brines[J]. Separation and Purification Technology, 2021, 255: 117710. |
| 18 | Isupov V P, Kotsupalo N P, Nemudry A P, et al. Aluminium hydroxide as selective sorbent of lithium salts from brines and technical solutions[M]//Studies in Surface Science and Catalysis. Amsterdam: Elsevier, 1999: 621-652. |
| 19 | Paranthaman M P, Li L, Luo J Q, et al. Recovery of lithium from geothermal brine with lithium-aluminum layered double hydroxide chloride sorbents[J]. Environmental Science & Technology, 2017, 51(22): 13481-13486. |
| 20 | Yang Y, Jiang H X, Yu J G. Investigation on desorption process in fixed bed for lithium recovery[J]. Separation and Purification Technology, 2022, 281: 119596. |
| 21 | 钟静, 陆旗玮, 林森, 等. 锂铝层状吸附剂超低品位卤水提锂冲洗和解吸过程[J]. 化工进展, 2021, 40(8): 4638-4646. |
| Zhong J, Lu Q W, Lin S, et al. Washing and desorption procedures research on granulated lithium aluminum layered double hydroxides for lithium recovery from low-grade brine[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4638-4646. | |
| 22 | Lee S Y, Choi J W, Song K G, et al. Adsorption and mechanistic study for phosphate removal by rice husk-derived biochar functionalized with Mg/Al-calcined layered double hydroxides via co-pyrolysis[J]. Composites Part B: Engineering, 2019, 176: 107209. |
| 23 | Zhu S D, Khan M A, Kameda T, et al. New insights into the capture performance and mechanism of hazardous metals Cr3+ and Cd2+ onto an effective layered double hydroxide based material[J]. Journal of Hazardous Materials, 2022, 426: 128062. |
| 24 | Stephen H, Krishanamohan C S V, Brian E V, et al. Lithium extraction composition and method of preparation thereof: US8637428[P]. 2014-01-28. |
| 25 | Chen J, Yuan H, Yu J, et al. Regulating lithium extraction based on intercalated S O 4 2 - in Li/Al-LDHs[J]. Journal of Colloid and Interface Science, 2023, 649: 694-702. |
| 26 | Zhong J, Lin S, Yu J G. Lithium recovery from ultrahigh Mg2+/Li+ ratio brine using a novel granulated Li/Al-LDHs adsorbent[J]. Separation and Purification Technology, 2021, 256: 117780. |
| 27 | Ahmed M J, Hameed B H. Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: a review[J]. Ecotoxicology and Environmental Safety, 2018, 149: 257-266. |
| 28 | Clark R M. Evaluating the cost and performance of field-scale granular activated carbon systems[J]. Environmental Science & Technology, 1987, 21(6): 573-580. |
| 29 | Thomas H C. Heterogeneous ion exchange in a flowing system[J]. Journal of the American Chemical Society, 1944, 66(10): 1664-1666. |
| 30 | Yan G Y, Viraraghavan T, Chen M. A new model for heavy metal removal in a biosorption column[J]. Adsorption Science & Technology, 2001, 19(1): 25-43. |
| 31 | Zhong J, Lin S, Yu J G. Effects of excessive lithium deintercalation on Li+ adsorption performance and structural stability of lithium/aluminum layered double hydroxides[J]. Journal of Colloid and Interface Science, 2020, 572: 107-113. |
| [1] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [2] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [3] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [4] | 吴选军, 王超, 曹子健, 蔡卫权. 数据与物理信息混合驱动的固定床吸附穿透深度学习模型[J]. 化工学报, 2023, 74(3): 1145-1160. |
| [5] | 许世佩, 王超, 李庆远, 张炳康, 许世伟, 张雪琴, 王诗颖, 丛梦晓. 氧化钙对油基钻屑热脱附产物影响的研究[J]. 化工学报, 2022, 73(4): 1724-1731. |
| [6] | 杨英杰, 杨赫, 朱家龙, 郭双淇, 尚妍, 李扬, 靳立军, 胡浩权. 淖毛湖煤慢速热解过程官能团相互作用[J]. 化工学报, 2022, 73(2): 865-875. |
| [7] | 许雄飞, 刘鹏龙, 张玮, 许鑫, 张侃, 王俊文. 两段法固定床甲醇制芳烃产物预测多元非线性回归模型[J]. 化工学报, 2022, 73(2): 838-846. |
| [8] | 李艳, 曹进辉, 刘原一, 谭厚章. 生物质固定床热解碳烟结构特征及反应活性[J]. 化工学报, 2022, 73(12): 5564-5571. |
| [9] | 王润涛, 罗泽军, 王储, 朱锡锋. 生物油蒸馏残渣与废弃塑料催化共热解协同作用的研究[J]. 化工学报, 2022, 73(11): 5088-5097. |
| [10] | 彭昌炜, 桑世华, 崔瑞芝, 任红保. 五元体系NaBr-KBr-MgBr2-CaBr2-H2O在298.15 K下的空间立体相图研究[J]. 化工学报, 2022, 73(11): 4850-4858. |
| [11] | 迟子怡, 刘成伟, 张欲凌, 李学刚, 肖文德. CO氧化偶联反应器模拟与优化[J]. 化工学报, 2022, 73(11): 4974-4986. |
| [12] | 毛文发, 郑赛男, 骆念军, 周静红, 曹约强, 周兴贵. 列管固定床反应器内CO氧化偶联制草酸二甲酯反应模拟及优化[J]. 化工学报, 2022, 73(1): 284-293. |
| [13] | 翁俊旗, 刘鑫磊, 余佳豪, 施尧, 叶光华, 屈进, 段学志, 李金兵, 周兴贵. 蜂窝状催化剂中空结构对固定床反应器压降的影响[J]. 化工学报, 2022, 73(1): 266-274. |
| [14] | 张瑞, 陆旗玮, 林森, 于建国. 铝系成型锂吸附剂性能测试评价与对比[J]. 化工学报, 2021, 72(6): 3053-3062. |
| [15] | 余作伟, 刘倩, 钟文琪, 周骏. 烘焙生物质燃烧过程中钾的赋存形态及析出迁移特性[J]. 化工学报, 2021, 72(4): 2258-2266. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号