化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3063-3073.DOI: 10.11949/0438-1157.20201732
收稿日期:2020-12-01
修回日期:2021-03-16
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
张安运
作者简介:侯林怡(1996—),女,硕士,基金资助:
HOU Linyi( ),WANG Yining,ZHANG Anyun(
),WANG Yining,ZHANG Anyun( ),SU Jiatian
),SU Jiatian
Received:2020-12-01
Revised:2021-03-16
Online:2021-06-05
Published:2021-06-05
Contact:
ZHANG Anyun
摘要:
稀有金属铷和铯是重要战略资源,盐湖卤水中含有一定量铷和铯。因其组成与碱性介质的复杂性,铷铯的分离与回收极富挑战性,尚未有效解决。本文基于固定化与真空活化灌注技术将超分子衍生物杯[4]双冠6(BC6)和杯[4]双冠5(BC5)负载于高分子介孔载体XAD-7孔道中,制备与表征了新型超分子识别材料BC6/XAD-7和BC5/XAD-7。研究了水相pH和温度变化对BC6/XAD-7和BC5/XAD-7吸附典型碱金属和碱土金属离子性能的影响,考察了BC6/XAD-7和BC5/XAD-7随接触时间变化吸附铷和铯动力学行为,获得了最佳吸附条件,明确了提取钾后余液中以超分子识别材料吸附分离铯和铷的技术可行性,提出了有效吸附分离铯和铷的CREC技术流程,为应用新型超分子识别材料于盐湖卤水中吸附分离铯和铷提供理论与实验依据。
中图分类号:
侯林怡, 王一宁, 张安运, 苏佳天. 新型calix[4]biscrown超分子识别材料制备及其吸附铷和铯性能研究[J]. 化工学报, 2021, 72(6): 3063-3073.
HOU Linyi, WANG Yining, ZHANG Anyun, SU Jiatian. Preparation of new calix[4]biscrown supramolecular recognition materials for the adsorption of rubidium and cesium[J]. CIESC Journal, 2021, 72(6): 3063-3073.

图5 BC6/XAD-7、BC5/XAD-7和XAD-7于77 K条件下的N2吸附-脱附曲线和孔径分布
Fig.5 N2 adsorption-desorption curves and pore size distribution of BC6/XAD-7, BC5/XAD-7 and XAD-7 at 77 K
| 样品 | 比表面积/(m2/g) | 最可几孔径/nm | 孔体积/(cm3/g) |
|---|---|---|---|
| XAD-7 | 348.5 | 39.48 | 0.70 |
| BC6/XAD-7 | 204.9 | 31.22 | 0.64 |
| BC5/XAD-7 | 188.78 | 31.18 | 0.67 |
表1 XAD-7、BC6/XAD-7和BC5/XAD-7的比表面积、孔径及孔体积
Table 1 Specific surface area, pore size and pore volume of XAD-7, BC6/XAD-7 and BC5/XAD-7
| 样品 | 比表面积/(m2/g) | 最可几孔径/nm | 孔体积/(cm3/g) |
|---|---|---|---|
| XAD-7 | 348.5 | 39.48 | 0.70 |
| BC6/XAD-7 | 204.9 | 31.22 | 0.64 |
| BC5/XAD-7 | 188.78 | 31.18 | 0.67 |
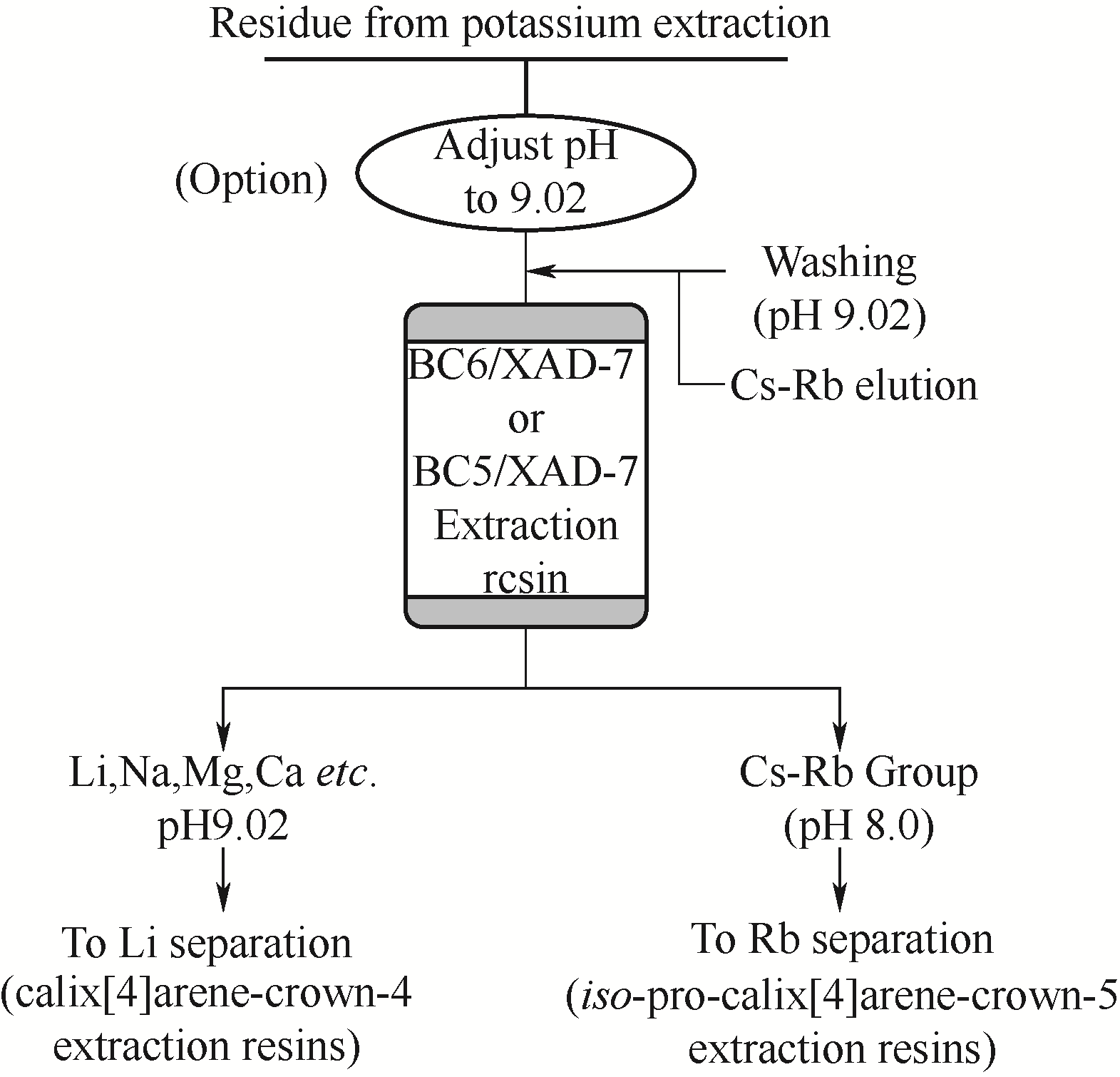
图11 超分子识别材料BC6/XAD-7或BC5/XAD-7吸附分离铯和铷的CREC流程
Fig.11 An advanced CREC process for cesium and rubidium separation by extraction chromatography by BC6/XAD-7 or BC5/XAD-7
| 1 | 胡开宝, 潘存峰, 张荣. 吉兰泰盐湖资源开采与地质环境问题探讨[J]. 盐业与化工, 2011, 40(1): 49-51. |
| Hu K B, Pan C F, Zhang R. Jilantai salt lake resource development and discussion on the geological environment[J]. Journal of Salt and Chemical Industry, 2011, 40(1): 49-51. | |
| 2 | Zhang N, Gao D L, Liu M M, et al. Rubidium and cesium recovery from brine resources[J]. Advanced Materials Research, 2014, 1015: 417-420. |
| 3 | 孙艳, 王瑞江, 亓锋, 等. 世界铷资源现状及我国铷开发利用建议[J]. 中国矿业, 2013, 22(9): 11-13. |
| Sun Y, Wang R J, Qi F, et al. The global status of rubidium resource and suggestions on its development and utilization in China[J]. China Mining Magazine, 2013, 22(9): 11-13. | |
| 4 | 孙海霞, 保英莲, 曹红翠, 等. 青海盐湖卤水铷铯资源及分析方法研究进展[J]. 广东化工, 2012, 39(4): 11-12. |
| Sun H X, Bao Y L, Cao H C, et al. Saline rubidium and cesium resource and research progress of analysis method in Qinghai[J]. Guangdong Chemical Industry, 2012, 39(4): 11-12. | |
| 5 | 马喆, 韩凤清, 易磊, 等. 柴达木盆地昆特依盐湖沉积特征及其盐类资源评价[J]. 盐湖研究, 2020, 28(1): 86-95. |
| Ma Z, Han F Q, Yi L, et al. Sedimentary characteristics and assessment of salt mineral resources in Kunteyi salt lake, Qaidam basin[J]. Journal of Salt Lake Research, 2020, 28(1): 86-95. | |
| 6 | 马培华. 中国盐湖资源的开发利用与科技问题[J]. 地球科学进展, 2000, 15(4): 365-375. |
| Ma P H. Comprehensive utilization of salt lake resources[J]. Advance in Earth Sciences, 2000, 15(4): 365-375. | |
| 7 | Sun Y, Wang Q, Wang Y H, et al. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine[J]. Separation and Purification Technology, 2021, 256: 117807. |
| 8 | 郑绵平, 张永生, 刘喜方, 等. 中国盐湖科学技术研究的若干进展与展望[J]. 地质学报, 2016, 90(9): 2123-2166. |
| Zheng M P, Zhang Y S, Liu X F, et al. Progress and prospects of salt lake research in China[J]. Acta Geologica Sinica, 2016, 90(9): 2123-2166. | |
| 9 | Zheng M P. Resources and eco-environmental protection of salt lakes in China[J]. Environmental Earth Sciences, 2011, 64(6): 1537-1546. |
| 10 | 乜贞, 卜令忠, 刘建华, 等. 我国盐湖钾盐资源现状及提钾工艺技术进展[J]. 地球学报, 2010, 31(6): 869-874. |
| Nie Z, Bu L Z, Liu J H, et al. Status of potash resources in salt lakes and progress in potash technologies in China[J]. Acta Geoscientica Sinica, 2010, 31(6): 869-874. | |
| 11 | 李小松. 察尔汗盐湖光卤石矿制备氯化钾工艺过程与工程化研究[D]. 上海: 华东理工大学, 2012. |
| Li X S. Production of potassium chloride by cold decomposition and recrystallization of carnallite[D]. Shanghai: East China University of Science and Technology, 2012. | |
| 12 | 刘有智, 白梅, 申红艳, 等. 均相沉淀法制备超细氢氧化镁[J]. 无机盐工业, 2012, 44(3): 30-32. |
| Liu Y Z, Bai M, Shen H Y, et al. Preparation of superfine magnesium hydroxide by homogeneous precipitation method[J]. Inorganic Chemicals Industry, 2012, 44(3): 30-32. | |
| 13 | 张建忠, 徐永杰, 王金晶. 水热法制备氢氧化镁阻燃剂研究[J]. 盐科学与化工, 2018, 47(6): 10-13. |
| Zhang J Z, Xu Y J, Wang J J. Development of preparation of flame retardant for magnesium hydroxide by hydrothermal method[J]. Journal of Salt Science and Chemical Industry, 2018, 47(6): 10-13. | |
| 14 | 李颖, 董金美, 肖学英, 等. 利用盐湖常见元素的助烧结作用绿色制备氧化镁及磷酸镁水泥[J]. 盐湖研究, 2020, 28(2): 15-25. |
| Li Y, Dong J M, Xiao X Y, et al. Green preparation of magnesia and magnesium phosphate cement by utilizing common elements in salt lake as sintering aid[J]. Journal of Salt Lake Research, 2020, 28(2): 15-25. | |
| 15 | 李国祥. 氨法生产氢氧化镁和氧化镁的工艺流程分析[J]. 纯碱工业, 2019, (1): 8-12. |
| Li G X. Process analysis of production of magnesium hydroxide and magnesium oxide by ammonia process[J]. Soda Industry, 2019, (1): 8-12. | |
| 16 | 靳军宝, 白光祖, 田晓阳, 等. 盐湖锂镁分离提取技术国际态势分析[J]. 盐湖研究, 2014, 22(1): 61-67. |
| Jin J B, Bai G Z, Tian X Y, et al. International patent analysis for separating technique of lithium and magnesium from salt lake brines[J]. Journal of Salt Lake Research, 2014, 22(1): 61-67. | |
| 17 | 孙犁, 黄建成, 叶秀深, 等. 柴达木盆地盐湖卤水中硼的分离提取研究进展[J]. 无机盐工业, 2017, 49(10): 1-5. |
| Sun L, Huang J C, Ye X S, et al. Research progress in separation and extraction of boron from salt lakes brine of Qaidam basin[J]. Inorganic Chemicals Industry, 2017, 49(10): 1-5. | |
| 18 | Mosin D N, Marks E A, Burmakin E I, et al. Electroconduction of potassium, rubidium, and cesium orthophosphates[J]. Russian Journal of Electrochemistry, 2001, 37(8): 863-864. |
| 19 | Lee J Y, Kim H J, Jung J H, et al. Networking of calixcrowns: from heteronuclear endo/exocyclic coordination polymers to a photoluminescence switch[J]. Journal of the American Chemical Society, 2008, 130(42): 13838-13839. |
| 20 | Soukiassian P, Bakshi M H, Hurych Z. Exceptionally large enhancement of InP (110) oxidation rate by cesium catalyst[J]. Journal of Applied Physics, 1987, 61(7): 2679-2681. |
| 21 | Durojaiye T, Hayes J, Potassium Goudy A., rubidium and cesium hydrides as dehydrogenation catalysts for the lithium amide/magnesium hydride system[J]. International Journal of Hydrogen Energy, 2015, 40(5): 2266-2273. |
| 22 | Lukas R J, Cullen M J. An isotopic rubidium ion efflux assay for the functional characterization of nicotinic acetylcholine receptors on clonal cell lines[J]. Analytical Biochemistry, 1988, 175(1): 212-218. |
| 23 | Gao L, Ma G H, Zheng Y X, et al. Research trends on separation and extraction of rare alkali metal from salt lake brine: rubidium and cesium[J]. Solvent Extraction and Ion Exchange, 2020, 38(7): 753-776. |
| 24 | Tomar J, Awasthy A, Sharma U. Synthetic ionophores for the separation of Li+,Na+,K+,Ca2+,Mg2+ metal ions using liquid membrane technology[J]. Desalination, 2008, 232(1/2/3): 102-109. |
| 25 | Wang J L, Zhuang S T. Cesium separation from radioactive waste by extraction and adsorption based on crown ethers and calixarenes[J]. Nuclear Engineering and Technology, 2020, 52(2): 328-336. |
| 26 | Stoll I, Eberhard J, Brodbeck R, et al. A new fluorescent calix crown ether: synthesis and complex formation with alkali metal ions[J]. Chemistry - A European Journal, 2008, 14(4): 1155-1163. |
| 27 | Asfari Z, Böhmer V, Harrowfield J, et al. Calixarenes 2001[M]. Dordrecht: Kluwer Academic Publishers, 2002. |
| 28 | Asfari Z, Wenger S, Vicens J. Calixcrowns and related molecules[J]. Journal of Inclusion Phenomena and Molecular Recognition in Chemistry, 1994, 19(1/2/3/4): 137-148. |
| 29 | Ludwig R, Dzung N. Calixarene-based molecules for cation recognition[J]. Sensors, 2002, 2(10): 397-416. |
| 30 | Mokhtari B, Pourabdollah K. Binding and extraction of alkali and alkaline earth metals by nano-baskets of calix[4]arene-1,2-crown-3[J]. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2012, 73(1/2/3/4): 269-277. |
| 31 | Zhang A Y, Chai Z F. Adsorption property of cesium onto modified macroporous silica-calix[4]arene-crown based supramolecular recognition materials[J]. Industrial & Engineering Chemistry Research, 2012, 51(17): 6196-6204. |
| 32 | Riddle C L, Baker J D, Law J D, et al. Fission product extraction (FPEX): development of a novel solvent for the simultaneous separation of strontium and cesium from acidic solutions[J]. Solvent Extraction and Ion Exchange, 2005, 23(3): 449-461. |
| 33 | Mincher B J, Mezyk S P, Bauer W F, et al. FPEX γ-radiolysis in the presence of nitric acid[J]. Solvent Extraction and Ion Exchange, 2007, 25(5): 593-601. |
| 34 | Walker D D, Norato M A, Campbell S G, et al. Cesium removal from savannah river site radioactive waste using the caustic-side solvent extraction (CSSX) process[J]. Separation Science and Technology, 2005, 40(1/2/3): 297-309. |
| 35 | Bonnesen P V, Delmau L H, Moyer B A, et al. A robust alkaline-side CSEX solvent suitable for removing cesium from Savannah river high level waste[J]. Solvent Extraction and Ion Exchange, 2000, 18(6): 1079-1107. |
| 36 | Dozol J F, Lamare V, Bressot C, et al. Crown calix[4]arenes, method of preparation and use for selective extraction of caesium: US6156282[P]. 2000-12-05. |
| 37 | Dozol J F, Simon N, Lamare V, et al. A solution, for cesium removal from high-salinity acidic or alkaline liquid waste: the crown calix[4]arenes[J]. Separation Science and Technology, 1999, 34(6/7): 877-909. |
| 38 | Lamare V, Bressot C, Dozol J F, et al. Selective extraction of cesium at tracer level concentration from a sodium nitrate solution with calix-crowns, molecular modeling study of the Cs+/Na+ selectivity[J]. Separation Science and Technology, 1997, 32(1/2/3/4): 175-191. |
| 39 | Hill C, Dozol J F, Lamare V, et al. Nuclear waste treatment by means of supported liquid membranes containing calixcrown compounds[J]. Journal of Inclusion Phenomena and Molecular Recognition in Chemistry, 1994, 19(1/2/3/4): 399-408. |
| 40 | Zhang A Y, Dai Y, Xu L, et al. Extraction behavior of cesium and some typical fission and non-fission products with a new 1, 3-di(1-decyloxy)-2, 4-crown-6-calix[4]arene[J]. Radiochimica Acta, 2014, 102(1/2): 135-142. |
| 41 | Ellis R J, Reinhart B, Williams N J, et al. Capping the calix: how toluene completes cesium (Ⅰ) coordination with calix[4]pyrrole[J]. Chemical Communications, 2017, 53(41): 5610-5613. |
| 42 | Zhang W W, Zhang A Y, Xu L, et al. Extraction of cesium and some typical metals with a supramolecular recognition agent 1, 3-bis(1-nonyloxy)-2,4-crown-6-calix[4]arene[J]. Journal of Chemical & Engineering Data, 2013, 58(1): 167-175. |
| 43 | Mohite B S, Khopkar S M. Solvent extraction separation of rubidium with dicyclohexano-18-crown-6[J]. Talanta, 1985, 32(7): 565-567. |
| 44 | Zhang A Y, Hu Q H, Chai Z F. SPEC: a new process for strontium and cesium partitioning utilizing two macroporous silica-based supramolecular recognition agents impregnated polymeric composites[J]. Separation Science and Technology, 2009, 44(9): 2146-2168. |
| 45 | Zhang A Y, Li J Y, Dai Y, et al. Development of a new simultaneous separation of cesium and strontium by extraction chromatograph utilization of a hybridized macroporous silica-based functional material[J]. Separation and Purification Technology, 2014, 127: 39-45. |
| 46 | Zhang A Y, Zhang W W, Wang Y N, et al. Effective separation of cesium with a new silica-calix[4]biscrown material by extraction chromatography[J]. Separation and Purification Technology, 2016, 171: 17-25. |
| 47 | Zhang A Y, Hu Q H, Chai Z F. Chromatographic partitioning of cesium by a macroporous silica-calix[4]arene-crown supramolecular recognition composite[J]. AIChE Journal, 2010, 56(10): 2632-2640. |
| 48 | Xiao C L, Zhang A Y, Chai Z F. Synthesis and characterization of novel macroporous silica-polymer-calixcrown hybrid supramolecular recognition materials for effective separation of cesium[J]. Journal of Hazardous Materials, 2014, 267: 109-118. |
| 49 | Zhang A Y, Xiao C L, Liu Y H, et al. Preparation of macroporous silica-based crown ether materials for strontium separation[J]. Journal of Porous Materials, 2010, 17(2): 153-161. |
| 50 | Zhang A Y, Hu Q H, Chai Z F. Synthesis of a novel macroporous silica-calix[4]arene-crown polymeric composite and its adsorption for alkali metals and alkaline-earth metals[J]. Industrial & Engineering Chemistry Research, 2010, 49(5): 2047-2054. |
| 51 | 陈春梅. 几种大孔硅基超分子识别材料制备及其吸附铯的基础特性研究[D]. 杭州: 浙江大学, 2011. |
| Chen C M. Synthesis of a few macroporous silica-based supermolecular recognition materials and their properties adsorption for cesium[D]. Hangzhou: Zhejiang University, 2011. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [7] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [8] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [9] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [10] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [11] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [12] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [13] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| [14] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [15] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号