化工学报 ›› 2023, Vol. 74 ›› Issue (2): 617-629.DOI: 10.11949/0438-1157.20221223
苏伟怡1,2( ), 丁佳慧1,2, 李春利1,2, 王洪海1,2, 姜艳军1,2(
), 丁佳慧1,2, 李春利1,2, 王洪海1,2, 姜艳军1,2( )
)
收稿日期:2022-09-07
修回日期:2022-11-18
出版日期:2023-02-05
发布日期:2023-03-21
通讯作者:
姜艳军
作者简介:苏伟怡(1985—),女,博士,副教授,suweiyi@hebut.edu.cn
基金资助:
Weiyi SU1,2( ), Jiahui DING1,2, Chunli LI1,2, Honghai WANG1,2, Yanjun JIANG1,2(
), Jiahui DING1,2, Chunli LI1,2, Honghai WANG1,2, Yanjun JIANG1,2( )
)
Received:2022-09-07
Revised:2022-11-18
Online:2023-02-05
Published:2023-03-21
Contact:
Yanjun JIANG
摘要:
在“双碳”背景下,绿色可持续的酶促反应正受到工业界的广泛关注,但在实际应用中仍面临着诸多挑战,如反应平衡的限制、不稳定产物的分解、酶的产物抑制等。结晶作为一种高效成熟的分离技术,可通过移除液相产物的方式有效解决上述问题。同时,结晶也是晶体产品的“生成”过程,其与酶促反应耦合可一步实现晶体产品的高效、绿色、可控制备。综述了近年来酶促反应结晶的研究进展,介绍了原位产物结晶(ISPC)技术的发展历程,并讨论了结晶与酶促反应耦合时的相互影响关系;从结晶方式和过程控制角度阐述了酶促反应结晶的实现形式和连续化过程;最后,对酶促反应结晶这一耦合过程的发展和应用进行了总结和展望。
中图分类号:
苏伟怡, 丁佳慧, 李春利, 王洪海, 姜艳军. 酶促反应结晶研究进展[J]. 化工学报, 2023, 74(2): 617-629.
Weiyi SU, Jiahui DING, Chunli LI, Honghai WANG, Yanjun JIANG. Research progress of enzymatic reactive crystallization[J]. CIESC Journal, 2023, 74(2): 617-629.
| 酶 | 目标产物 | 结晶方式 | 调控参数 | 产率 | 文献 |
|---|---|---|---|---|---|
| 转氨酶 | 1-苯基乙胺 | 加成盐剂 | 75% | [ | |
| 加成盐剂 | pH、温度 | 91.5% | [ | ||
| (S)-1-(3-甲氧基苯基)乙胺 | 加成盐剂 | 晶种 | [ | ||
| 加成盐剂 | 晶种 | [ | |||
| 3-(2-萘)- l -丙氨酸 | 自发结晶 | 温度 | 93% | [ | |
| L-邻苯丙氨酸 | 自发结晶 | pH | [ | ||
| 青霉素G酰化酶 | 氨苄西林 | 自发结晶 | 93% | [ | |
| 自发结晶 | 97% | [ | |||
| 自发结晶 | pH | 96% | [ | ||
| 加入晶种 | 晶种 | [ | |||
| 自发结晶 | 晶种、pH | [ | |||
| 自发结晶 | pH | 98% | [ | ||
| 阿莫西林 | 自发结晶 | pH | 98% | [ | |
| 头孢氨苄 | 加络合剂 | 温度、pH | [ | ||
| 头孢克洛 | 加络合剂 | 温度、pH | 80% | [ | |
| 头孢拉定 | 加络合剂 | [ | |||
| 葡萄糖氧化酶 | 葡萄糖酸钙 | 自发结晶 | 温度、pH | [ | |
| 氨基酸消旋酶 | 苏氨酸 | 自发结晶 | [ | ||
| 芳香酸脱羧酶 | 2,6-二羟基苯甲酸 | 加成盐剂 | 97% | [ | |
| 嗜热菌蛋白酶 | Z-阿斯巴甜 | 自发结晶 | pH | 88% | [ |
| 延胡索酸酶 | L-苹果酸钙 | 自发结晶 | 温度 | [ |
表1 酶促反应结晶实现形式和调控手段汇总
Table 1 Summary of the realization and regulation of enzymatic reaction crystallization
| 酶 | 目标产物 | 结晶方式 | 调控参数 | 产率 | 文献 |
|---|---|---|---|---|---|
| 转氨酶 | 1-苯基乙胺 | 加成盐剂 | 75% | [ | |
| 加成盐剂 | pH、温度 | 91.5% | [ | ||
| (S)-1-(3-甲氧基苯基)乙胺 | 加成盐剂 | 晶种 | [ | ||
| 加成盐剂 | 晶种 | [ | |||
| 3-(2-萘)- l -丙氨酸 | 自发结晶 | 温度 | 93% | [ | |
| L-邻苯丙氨酸 | 自发结晶 | pH | [ | ||
| 青霉素G酰化酶 | 氨苄西林 | 自发结晶 | 93% | [ | |
| 自发结晶 | 97% | [ | |||
| 自发结晶 | pH | 96% | [ | ||
| 加入晶种 | 晶种 | [ | |||
| 自发结晶 | 晶种、pH | [ | |||
| 自发结晶 | pH | 98% | [ | ||
| 阿莫西林 | 自发结晶 | pH | 98% | [ | |
| 头孢氨苄 | 加络合剂 | 温度、pH | [ | ||
| 头孢克洛 | 加络合剂 | 温度、pH | 80% | [ | |
| 头孢拉定 | 加络合剂 | [ | |||
| 葡萄糖氧化酶 | 葡萄糖酸钙 | 自发结晶 | 温度、pH | [ | |
| 氨基酸消旋酶 | 苏氨酸 | 自发结晶 | [ | ||
| 芳香酸脱羧酶 | 2,6-二羟基苯甲酸 | 加成盐剂 | 97% | [ | |
| 嗜热菌蛋白酶 | Z-阿斯巴甜 | 自发结晶 | pH | 88% | [ |
| 延胡索酸酶 | L-苹果酸钙 | 自发结晶 | 温度 | [ |
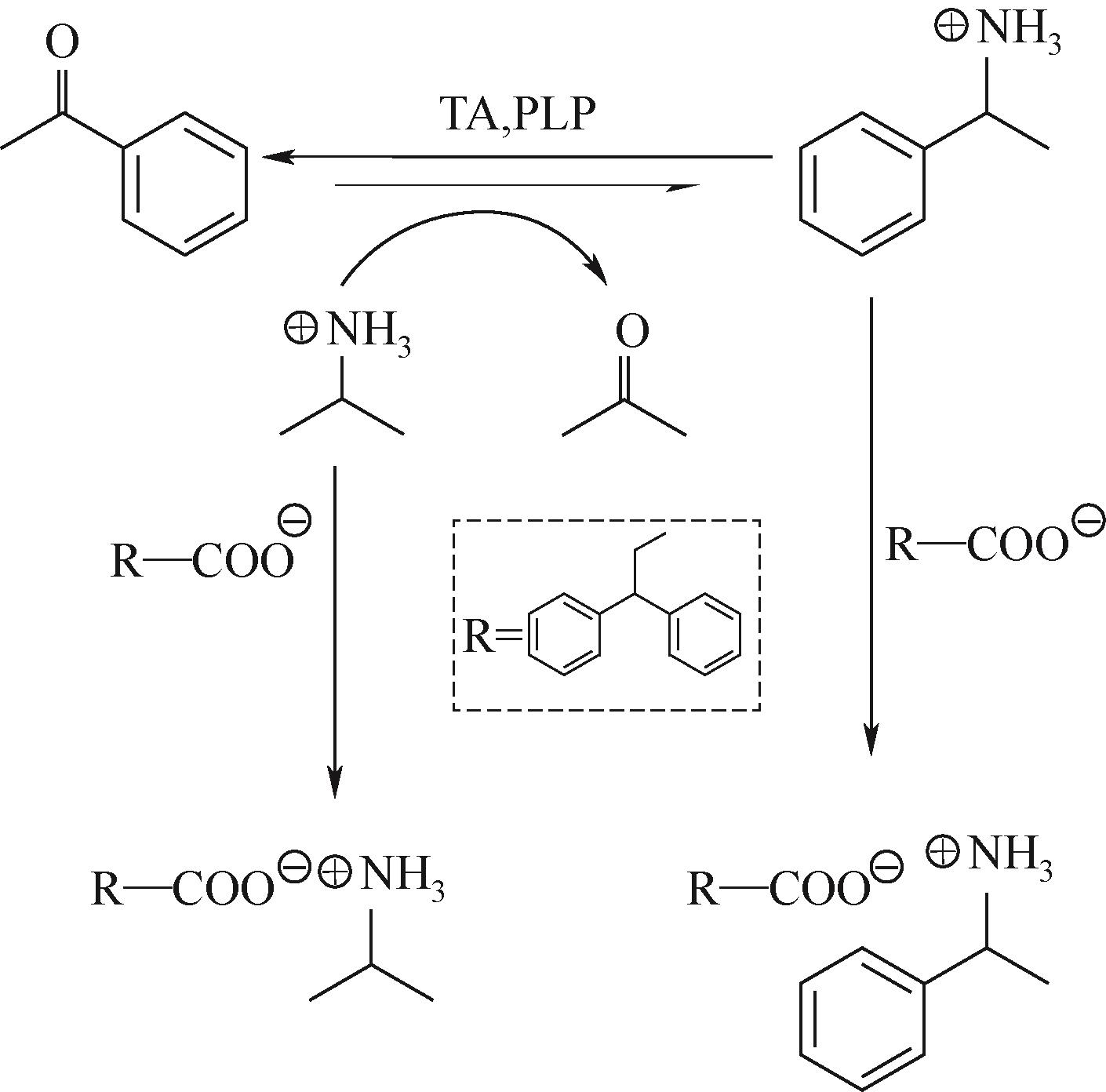
图2 酶促反应结晶用于转氨酶催化的反应,生成一种难溶的1-苯基乙胺-3DPPA盐[18]
Fig.2 In situ product crystallization (ISPC) was combined with the reaction catalyzed by aminotransferase to form insoluble 1-phenylethylamine salt[18]
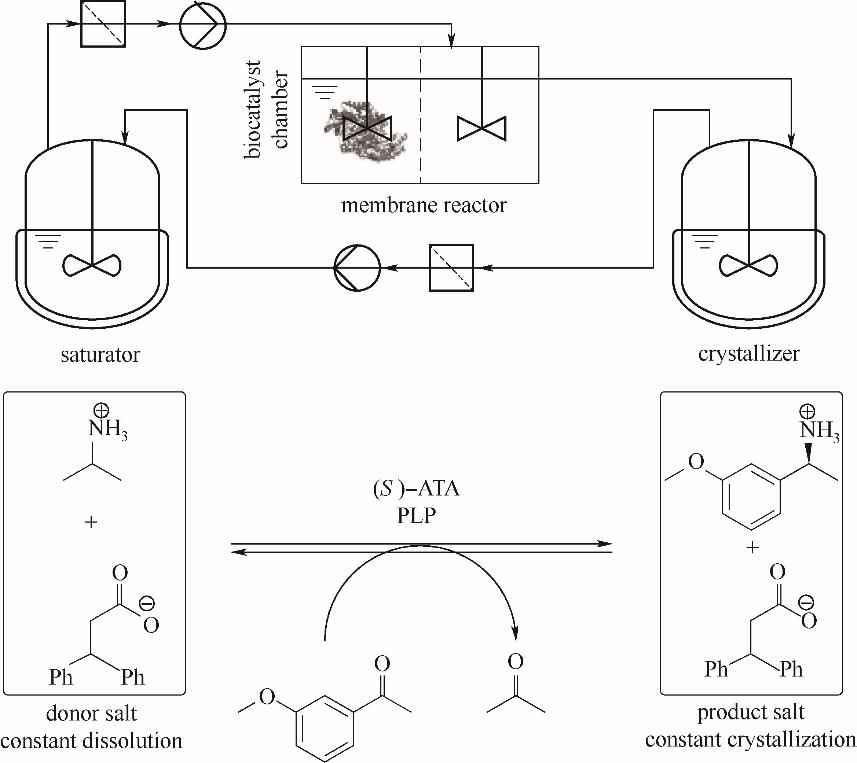
图3 转氨酶催化连续反应过程示意图[包含供体盐在饱和器的溶解(saturator,左)与产物盐在结晶器的结晶(crystallizer,右)][20]
Fig.3 Schematic diagram of a transaminases-catalyzed continuous reaction process [containing the dissolution of donor salts (left) and crystallization of the product salts (right)][20]

图5 过饱和体系构建示意图(实线表示6-APA溶解度,点3对应过饱和溶液中6-APA的浓度)[48]
Fig.5 The schematic pathway for the creation of supersaturated solution(solid line is the thermodynamic solubility of 6-APA, and point 3 corresponds to 6-APA concentration in a supersaturated solution)[48]
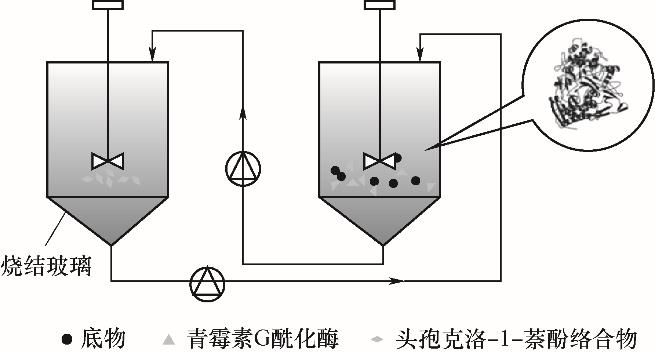
图7 头孢克洛酶促反应结晶(络合结晶)流程图(左为复合反应器,右为酶反应器)[32]
Fig.7 Flow chart of cefaclor enzymatic reactive crystallization (complexation crystallization) (left is the composite reactor, and right is the enzyme reactor)[32]
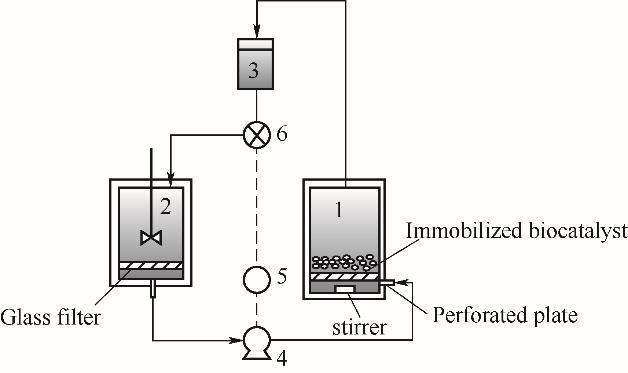
图8 生物反应结晶器流程图[38]1—生物反应器;2—结晶器;3—缓冲罐;4—泵;5—计时器;6—电磁阀
Fig.8 Flow diagram of the bioreactor-crystallizer[38]1—bioreactor; 2—crystallizer; 3—buffer tank; 4—pump; 5—timer; 6—magnetic valve
| 1 | Sheldon R A, van Rantwijk F. Biocatalysis for sustainable organic synthesis[J]. Australian Journal of Chemistry, 2004, 57(4): 281. |
| 2 | Choi J M, Han S S, Kim H S. Industrial applications of enzyme biocatalysis: current status and future aspects[J]. Biotechnology Advances, 2015, 33(7): 1443-1454. |
| 3 | Sheldon R A, Brady D. The limits to biocatalysis: pushing the envelope[J]. Chemical Communications, 2018, 54(48): 6088-6104. |
| 4 | Valeur E, Guéret S M, Adihou H, et al. New modalities for challenging targets in drug discovery[J]. Angewandte Chemie International Edition, 2017, 56(35): 10294-10323. |
| 5 | Garrido-del Solo C, Moruno M A, Havsteen B H, et al. The kinetic effect of product instability in a Michaelis-Menten mechanism with competitive inhibition[J]. Biosystems, 2000, 56(2/3): 75-82. |
| 6 | Satyawali Y, Vanbroekhoven K, Dejonghe W. Process intensification: the future for enzymatic processes? [J]. Biochemical Engineering Journal, 2017, 121: 196-223. |
| 7 | Schügerl K, Hubbuch J. Integrated bioprocesses[J]. Current Opinion in Microbiology, 2005, 8(3): 294-300. |
| 8 | Woodley J M. Bioprocess intensification for the effective production of chemical products[J]. Computers & Chemical Engineering, 2017, 105: 297-307. |
| 9 | McDonald M A, Salami H, Harris P R, et al. Reactive crystallization: a review[J]. Reaction Chemistry & Engineering, 2021, 6(3): 364-400. |
| 10 | Berry D A, Ng K M. Synthesis of reactive crystallization processes[J]. AIChE Journal, 1997, 43(7): 1737-1750. |
| 11 | Fellechner O, Blatkiewicz M, Smirnova I. Reactive separations for in situ product removal of enzymatic reactions: a review[J]. Chemie Ingenieur Technik, 2019, 91(11): 1522-1543. |
| 12 | Hülsewede D, Meyer L E, von Langermann J. Application of in situ product crystallization and related techniques in biocatalytic processes[J]. Chemistry - A European Journal, 2019, 25(19): 4871-4884. |
| 13 | Takamatsu S, Ryu D D Y. Recirculating bioreactor-separator system for simultaneous biotransformation and recovery of product: immobilized L-aspartate β-decarboxylase reactor system[J]. Biotechnology and Bioengineering, 1988, 32(2): 184-191. |
| 14 | Takamatsu S, Ryu D D Y. New recirculating bioreactor-separator combination system for continuous bioconversion and separation of products[J]. Enzyme and Microbial Technology, 1988, 10(10): 593-600. |
| 15 | Renuka Devi K, Srinivasan K. A novel approach to understand the nucleation kinetics of α and γ polymorphs of glycine from aqueous solution in the presence of a selective additive through charge compensation mechanism[J]. CrystEngComm, 2014, 16(4): 707-722. |
| 16 | Black J F B, Cruz-Cabeza A J, Davey R J, et al. The kinetic story of tailor-made additives in polymorphic systems: new data and molecular insights for p-aminobenzoic acid[J]. Crystal Growth & Design, 2018, 18(12): 7518-7525. |
| 17 | Morissette S L, Almarsson Ö, Peterson M L, et al. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids[J]. Advanced Drug Delivery Reviews, 2004, 56(3): 275-300. |
| 18 | Hülsewede D, Tänzler M, Süss P, et al. Development of an in situ-product crystallization (ISPC)-concept to shift the reaction equilibria of selected amine transaminase-catalyzed reactions[J]. European Journal of Organic Chemistry, 2018, 2018(18): 2130-2133. |
| 19 | Hülsewede D, Dohm J N, von Langermann J. Donor amine salt-based continuous in situ-product crystallization in amine transaminase-catalyzed reactions[J]. Advanced Synthesis and Catalysis, 2019, 361(11): 2727-2733. |
| 20 | Hülsewede D, Temmel E, Kumm P, et al. Concept study for an integrated reactor-crystallizer process for the continuous biocatalytic synthesis of (S)-1-(3-methoxyphenyl)ethylamine[J]. Crystals, 2020, 10(5): 345. |
| 21 | Neuburger J, Helmholz F, Tiedemann S, et al. Implementation and scale-up of a semi-continuous transaminase-catalyzed reactive crystallization for the preparation of (S)-(3-methoxyphenyl)ethylamine[J]. Chemical Engineering and Processing - Process Intensification, 2021, 168: 108578. |
| 22 | Hanzawa S, Oe S, Tokuhisa K, et al. Chemo-enzymatic synthesis of 3-(2-naphthyl)- l-alanine by an aminotransferase from the extreme thermophile, thermococcus profundus [J]. Biotechnology Letters, 2001, 23(8): 589-591. |
| 23 | Cho B K, Seo J H, Kang T W, et al. Asymmetric synthesis of L-homophenylalanine by equilibrium-shift using recombinant aromatic L-amino acid transaminase[J]. Biotechnology and Bioengineering, 2003, 83(2): 226-234. |
| 24 | Youshko M I, van Langen L M, de Vroom E, et al. Penicillin acylase-catalyzed synthesis of ampicillin in“aqueous solution-precipitate”systems. High substrate concentration and supersaturation effect[J]. Journal of Molecular Catalysis B: Enzymatic, 2000, 10(5): 509-515. |
| 25 | Youshko M I, van Langen L M, de Vroom E, et al. Highly efficient synthesis of ampicillin in an“aqueous solution-precipitate”system: repetitive addition of substrates in a semicontinuous process[J]. Biotechnology and Bioengineering, 2001, 73(5): 426-430. |
| 26 | Youshko M I, van Langen L M, de Vroom E, et al. Penicillin acylase-catalyzed ampicillin synthesis using a pH gradient: a new approach to optimization[J]. Biotechnology and Bioengineering, 2002, 78(5): 589-593. |
| 27 | Encarnación-Gómez L G, Bommarius A S, Rousseau R W. Reactive crystallization of β-lactam antibiotics: strategies to enhance productivity and purity of ampicillin[J]. Reaction Chemistry & Engineering, 2016, 1(3): 321-329. |
| 28 | McDonald M A, Bommarius A S, Rousseau R W. Enzymatic reactive crystallization for improving ampicillin synthesis[J]. Chemical Engineering Science, 2017, 165: 81-88. |
| 29 | McDonald M A, Bommarius A S, Grover M A, et al. Continuous reactive crystallization of β-lactam antibiotics catalyzed by penicillin G acylase (Part Ⅱ): Case study on ampicillin and product purity[J]. Computers & Chemical Engineering, 2019, 126: 332-341. |
| 30 | McDonald M A, Bommarius A S, Rousseau R W, et al. Continuous reactive crystallization of β-lactam antibiotics catalyzed by penicillin G acylase(Part Ⅰ): Model development[J]. Computers & Chemical Engineering, 2019, 123: 331-343. |
| 31 | Schroën C G P H, Nierstrasz V A, Bosma R, et al. In situ product removal during enzymatic cephalexin synthesis by complexation[J]. Enzyme and Microbial Technology, 2002, 31(3): 264-273. |
| 32 | Yang L, Wei D Z, Zhang Y W. Semi-continuous enzymatic synthesis of cefaclor enhanced by in situ product removal[J]. Journal of Chemical Technology & Biotechnology, 2004, 79(5): 480-485. |
| 33 | Kemperman G J, Dommerholt F J, Zwanenburg B, et al. Complexants for the clathration mediated synthesis of the antibiotic cephradine[J]. Green Chemistry, 2001, 3(4): 189-192. |
| 34 | Bao J, Koumatsu K, Furumoto K, et al. Optimal operation of an integrated bioreaction-crystallization process for continuous production of calcium gluconate using external loop airlift columns[J]. Chemical Engineering Science, 2001, 56(21/22): 6165-6170. |
| 35 | Würges K, Mackfeld U, Pohl M, et al. An efficient route to both enantiomers of allo-threonine by simultaneous amino acid racemase-catalyzed isomerization of threonine and crystallization[J]. Advanced Synthesis & Catalysis, 2011, 353(13): 2431-2438. |
| 36 | Ren J, Yao P Y, Yu S S, et al. An unprecedented effective enzymatic carboxylation of phenols[J]. ACS Catalysis, 2016, 6(2): 564-567. |
| 37 | Halling P J, Eichhorn U, Kuhl P, et al. Thermodynamics of solid-to-solid conversion and application to enzymic peptide synthesis[J]. Enzyme and Microbial Technology, 1995, 17(7): 601-606. |
| 38 | Furui M, Sakata N, Otsuki O, et al. A bioreactor-crystallizer for L-malic acid production[J]. Biocatalysis, 1988, 2(1): 69-77. |
| 39 | Rehn G, Adlercreutz P, Grey C. Supported liquid membrane as a novel tool for driving the equilibrium of ω-transaminase catalyzed asymmetric synthesis[J]. Journal of Biotechnology, 2014, 179: 50-55. |
| 40 | Gundersen M T, Abu R, Schürmann M, et al. Amine donor and acceptor influence on the thermodynamics of ω-transaminase reactions[J]. Tetrahedron: Asymmetry, 2015, 26(10/11): 567-570. |
| 41 | Berge S M, Bighley L D, Monkhouse D C. Pharmaceutical salts[J]. Journal of Pharmaceutical Sciences, 1977, 66(1): 1-19. |
| 42 | Black S N, Collier E A, Davey R J, et al. Structure, solubility, screening, and synthesis of molecular salts[J]. Journal of Pharmaceutical Sciences, 2007, 96(5): 1053-1068. |
| 43 | Buque-Taboada E M, Straathof A J J, Heijnen J J, et al. In situ product removal using a crystallization loop in asymmetric reduction of 4-oxoisophorone by Saccharomyces cerevisiae [J]. Biotechnology and Bioengineering, 2004, 86(7): 795-800. |
| 44 | Zhang J L, Liu A Y, Han Y, et al. Effects of self-assembled monolayers on selective crystallization of tolbutamide[J]. Crystal Growth & Design, 2011, 11(12): 5498-5506. |
| 45 | Yang X C, Sarma B, Myerson A S. Polymorph control of micro/nano-sized mefenamic acid crystals on patterned self-assembled monolayer Islands[J]. Crystal Growth & Design, 2012, 12(11): 5521-5528. |
| 46 | Marešová H, Plačková M, Grulich M, et al. Current state and perspectives of penicillin G acylase-based biocatalyses[J]. Applied Microbiology and Biotechnology, 2014, 98(7): 2867-2879. |
| 47 | Kurochkina V B, Nys P S. Kinetic and thermodynamic approach to design of processes for enzymatic synthesis of betalactams[J]. Biocatalysis and Biotransformation, 2002, 20(1): 35-41. |
| 48 | Youshko M I, Moody H M, Bukhanov A L, et al. Penicillin acylase-catalyzed synthesis of β-lactam antibiotics in highly condensed aqueous systems: beneficial impact of kinetic substrate supersaturation[J]. Biotechnology and Bioengineering, 2004, 85(3): 323-329. |
| 49 | Youshko M I, Chilov G G, Shcherbakova T A, et al. Quantitative characterization of the nucleophile reactivity in penicillin acylase-catalyzed acyl transfer reactions[J]. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 2002, 1599(1/2): 134-140. |
| 50 | Giordano R C, Ribeiro M P A, Giordano R L C. Kinetics of beta-lactam antibiotics synthesis by penicillin G acylase (PGA) from the viewpoint of the industrial enzymatic reactor optimization[J]. Biotechnology Advances, 2006, 24(1): 27-41. |
| 51 | Encarnación-Gómez L G, Bommarius A S, Rousseau R W. Crystallization kinetics of ampicillin using online monitoring tools and robust parameter estimation[J]. Industrial & Engineering Chemistry Research, 2016, 55(7): 2153-2162. |
| 52 | Ottens M, Lebreton B, Zomerdijk M, et al. Crystallization kinetics of ampicillin[J]. Industrial & Engineering Chemistry Research, 2001, 40(22): 4821-4827. |
| 53 | Yuan Y, Zhai R, Li Y, et al. Developing fast enzyme recycling strategy through elucidating enzyme adsorption kinetics on alkali and acid pretreated corn stover[J]. Biotechnology for Biofuels, 2018, 11: 316. |
| 54 | McDonald M, Marshall G D, Bommarius A S, et al. Crystallization kinetics of cephalexin monohydrate in the presence of cephalexin precursors[J]. Crystal Growth & Design, 2019, 19(9): 5065-5074. |
| 55 | McDonald M A, Bommarius A S, Grover M A, et al. Direct observation of growth rate dispersion in the enzymatic reactive crystallization of ampicillin[J]. Processes, 2019, 7(6): 390. |
| 56 | Kubota N, Yokota M, Mullin J W. Supersaturation dependence of crystal growth in solutions in the presence of impurity[J]. Journal of Crystal Growth, 1997, 182(1/2): 86-94. |
| 57 | Ottens M, Lebreton B, Zomerdijk M, et al. Impurity effects on the crystallization kinetics of ampicillin[J]. Industrial & Engineering Chemistry Research, 2004, 43(24): 7932-7938. |
| 58 | Salami H, McDonald M A, Bommarius A S, et al. In situ imaging combined with deep learning for crystallization process monitoring: application to cephalexin production[J]. Organic Process Research & Development, 2021, 25(7): 1670-1679. |
| 59 | Salami H, Lagerman C E, Harris P R, et al. Model development for enzymatic reactive crystallization of β-lactam antibiotics: a reaction-diffusion-crystallization approach[J]. Reaction Chemistry & Engineering, 2020, 5(11): 2064-2080. |
| 60 | Bhagat A A S. Inertial microfluidics for particle separation and filtration[D]. Cincinnati: University of Cincinnati, 2009. |
| 61 | Ferreira A L O, Giordano R L C, Giordano R C. Nonconventional reactor for enzymatic synthesis of semi-synthetic β-lactam antibiotics[J]. Industrial & Engineering Chemistry Research, 2007, 46(23): 7695-7702. |
| 62 | Giordano R L C, Giordano R C, Cooney C L. Performance of a continuous Taylor-Couette-Poiseuille vortex flow enzymic reactor with suspended particles[J]. Process Biochemistry, 2000, 35(10): 1093-1101. |
| 63 | Nguyen A T, Kim J M, Chang S M, et al. Taylor vortex effect on phase transformation of guanosine 5-monophosphate in drowning-out crystallization[J]. Industrial & Engineering Chemistry Research, 2010, 49(10): 4865-4872. |
| 64 | Kim J M, Chang S M, Chang J H, et al. Agglomeration of nickel/cobalt/manganese hydroxide crystals in Couette-Taylor crystallizer[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2011, 384(1/2/3): 31-39. |
| 65 | Giordano R, Giordano R, Ferreira A. Process for protection of insoluble enzymatic biocatalysts, biocatalyst obtained thereof and bioreactor with the immobilized biocatalyst: US10536426[P]. 2003-11-24. |
| 66 | Salami H, Harris P R, Yu D C, et al. Periodic wet milling as a solution to size-based separation of crystal products from biocatalyst for continuous reactive crystallization[J]. Chemical Engineering Research and Design, 2022, 177: 473-483. |
| 67 | 林家伟, 石鹏, 龚俊波, 等. 表面诱导药物多晶型成核的研究进展[J]. 化工学报, 2021, 72(2): 814-827. |
| Lin J W, Shi P, Gong J B, et al. Progress on surface-induced nucleation of drug for controlling polymorphism[J]. CIESC Journal, 2021, 72(2): 814-827. | |
| 68 | Eichhorn U, Bommarius A S, Drauz K, et al. Synthesis of dipeptides by suspension-to-suspension conversion via thermolysin catalysis: from analytical to preparative scale[J]. Journal of Peptide Science, 1997, 3(4): 245-251. |
| 69 | Pesci L, Glueck S M, Gurikov P, et al. Biocatalytic carboxylation of phenol derivatives: kinetics and thermodynamics of the biological Kolbe-Schmitt synthesis[J]. The FEBS Journal, 2015, 282(7): 1334-1345. |
| 70 | Nakao K, Kiefner A, Furumoto K, et al. Production of gluconic acid with immobilized glucose oxidase in airlift reactors[J]. Chemical Engineering Science, 1997, 52(21/22): 4127-4133. |
| 71 | Yoshimoto M, Wang S Q, Arimatsu Y, et al. A kinetic model for glucose oxidation catalyzed by immobilized glucose oxidase-containing liposomes in a mini-scale external loop airlift bubble column[J]. Journal of Chemical Engineering of Japan, 2004, 37(8): 1012-1018. |
| 72 | Yoshimoto M, Furumoto K, Nakao K. Effects of bubble interactions in circulating liquid flow on liquid phase mass transfer coefficient in an external loop airlift bubble column[J]. Journal of Chemical Engineering of Japan, 2012, 45(9): 661-665. |
| 73 | Nakao K, Bao J, Harada T, et al. Measurement and prediction of axial distribution of immobilized glucose oxidase gel beads suspended in bubble column[J]. Journal of Chemical Engineering of Japan, 2000, 33(5): 721-729. |
| 74 | Harris P R, Grover M A, Rousseau R W, et al. Selectivity and kinetic modeling of penicillin G acylase variants for the synthesis of cephalexin under a broad range of substrate concentrations[J]. Biotechnology and Bioengineering, 2022, 119(11): 3117-3126. |
| 75 | Lagerman C E, Grover M A, Rousseau R W, et al. Kinetic model development for α-amino ester hydrolase (AEH)-catalyzed synthesis of β-lactam antibiotics[J]. Chemical Engineering Journal, 2021, 426: 131816. |
| [1] | 敬鹏程, 陈立涛, 闫传梁, 姜传祥, 夏煜翔, 于常宏, 王昊天. 超声波悬浮TBAB溶液液滴表面半笼型水合物生长过程研究[J]. 化工学报, 2022, 73(11): 4893-4902. |
| [2] | 郑海峰, 贾晟哲, 王崧成, 韩瑞, 韩丹丹, 高振国, 龚俊波. 超细晶体的研究进展[J]. 化工学报, 2022, 73(10): 4285-4297. |
| [3] | 景博, 常泽伟, 贾晟哲, 吴送姑, 陈明洋, 高振国, 龚俊波. 熔融结晶的过程强化[J]. 化工学报, 2021, 72(8): 3907-3918. |
| [4] | 王东博, 张蕾蕾, 翟晶焕, 张丽娟, 刘锡建, 朱雪焱, 陆杰. 青霉胺结晶热力学及拆分工艺研究[J]. 化工学报, 2021, 72(4): 1885-1894. |
| [5] | 姜晓滨, 孙国鑫, 贺高红. 高效膜蒸馏结晶过程的研究进展[J]. 化工学报, 2020, 71(9): 3905-3918. |
| [6] | 曹小雪, 吉绍长, 匡雯婕, 廖安平, 蓝平, 张金彦. 甲醇-水溶剂中L-苯丙氨酸结晶热力学[J]. 化工学报, 2019, 70(4): 1255-1262. |
| [7] | 曹小雪, 吉绍长, 匡雯婕, 廖安平, 蓝平, 张金彦. 阿奇霉素二水合物在水-有机溶剂中溶解度及三元相图测定[J]. 化工学报, 2019, 70(3): 817-829. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号