化工学报 ›› 2023, Vol. 74 ›› Issue (2): 599-616.DOI: 10.11949/0438-1157.20221088
李敏1,2,3( ), 阎雪茹1,2,3, 刘新磊1,2,3(
), 阎雪茹1,2,3, 刘新磊1,2,3( )
)
收稿日期:2022-10-09
修回日期:2022-12-01
出版日期:2023-02-05
发布日期:2023-03-21
通讯作者:
刘新磊
作者简介:李敏(1998—),女,硕士研究生,limin1013@tju.edu.cn
基金资助:
Min LI1,2,3( ), Xueru YAN1,2,3, Xinlei LIU1,2,3(
), Xueru YAN1,2,3, Xinlei LIU1,2,3( )
)
Received:2022-10-09
Revised:2022-12-01
Online:2023-02-05
Published:2023-03-21
Contact:
Xinlei LIU
摘要:
强的链内、链间作用力和丰富的官能团赋予苯并咪唑连接聚合物优异的化学稳定性、热稳定性、高孔隙率、高比表面积、介电性以及良好的力学性能,因此被用作高效分离材料。本文讨论了苯并咪唑连接聚合物的种类及性能,综述了其在吸附剂和膜分离领域的应用,总结了研究现状,并对未来发展进行了展望。
中图分类号:
李敏, 阎雪茹, 刘新磊. 苯并咪唑连接聚合物吸附剂和膜研究进展[J]. 化工学报, 2023, 74(2): 599-616.
Min LI, Xueru YAN, Xinlei LIU. Advances in benzimidazole-linked polymer adsorbents and membranes[J]. CIESC Journal, 2023, 74(2): 599-616.
| 聚合物 | SABET①/(m2/g) | 吸附量②/(mg/g) | 选择性③ | 文献 | |||
|---|---|---|---|---|---|---|---|
| CO2 at 1 bar | N2 at 1 bar | CH4 at 1 bar | CO2/N2 | CO2/CH4 | |||
| BILP-1 | 1172 | 188(131) | 5.0(7.3) | 23(16) | 70(36) | 10(7) | [ |
| BILP-2 | 708 | 149(104) | 3.4(2.5) | 14(9) | 113(71) | 17(12) | [ |
| BILP-3 | 1306 | 225(145) | 3.3(2.4) | 24(17) | 59(31) | 8(5) | [ |
| BILP-4 | 1135 | 235(158) | 1.1(3.8) | 26(18) | 79(32) | 10(7) | [ |
| BILP-5 | 599 | 128(87) | 0.2(2.7) | 15(10) | 95(36) | 10(6) | [ |
| BILP-6 | 1261 | 211(121) | 6.8(6.7) | 27(19) | 63(39) | 8(5) | [ |
| BILP-6-NH2 | 1185 | 244.8(164.7) | — | — | — | —(8.4) | [ |
| BILP-7 | 1122 | 193(122) | 5.63(5.40) | 19(12) | 62(34) | 9(7) | [ |
| BILP-10 | 787 | 177(111) | — | 16(11) | 111(59) | 14(7) | [ |
| BILP-11 | 658 | 136(88) | — | 16(10) | 103(55) | 11(7) | [ |
| BILP-12 | 1497 | 223(140) | — | 24(15) | 56(31) | 8(6) | [ |
| BILP-13 | 677 | 113(79) | — | 12(9) | 103(38) | 9(6) | [ |
| BILP-14 | 1005 | 170(—) | — | — | 56(49) | 10(9) | [ |
| BILP-15 | 448 | 118(80) | 5.7(3.8) | 13.5(7.2) | 83(63) | 9(8) | [ |
| BILP-16 | 435 | 118.7(80.7) | 6.5(4.3) | 13.5(8.0) | 53(49) | 10(8) | [ |
| BILP-15(AC) | 862 | 151(101) | 7.2(5.8) | 16.4(10.3) | 61(50) | 9(7) | [ |
| BILP-16(AC) | 643 | 152(102.2) | 8.0(4.8) | 17.4(10.3) | 49(61) | 9(7) | [ |
| BILP-17 | 952 | — | — | — | — | — | [ |
| BILP-18 | 947 | — | — | — | — | — | [ |
| BILP-19 | 144 | 2630.95bar(229) | —(3) | 14.1(8.2) | —(59.1) | 11.9(12.3) | [ |
| BILP-101 | —(1070.15bar) | — | — | —(80) | — | [ | |
| PPN-101 | 226.2(—) | — | — | 199(—) | — | [ | |
| IBFNP-1 | 232(148) | — | — | 65(44) | 11(8) | [ | |
| IBLP | 48.06 | 46.5(30.7) | — | — | — | — | [ |
| IBLP-600 | 161.06 | 85.1(59.9) | — | — | — | — | [ |
| IBLP-700 | 270.64 | 121.8(90.7) | — | — | — | — | [ |
| IBLP-800 | 339 | 130.4(98.4) | — | — | — | — | [ |
| BINP-1 | 64 | 7.8(—) | — | — | — | — | [ |
| BINP-2 | 32 | 7(—) | — | — | — | — | [ |
| TBILP-1 | 330 | 117(78) | — | — | —(63) | —(9) | [ |
| TBILP-2 | 1080 | 228(146) | — | — | —(40) | —(7) | [ |
| CTF-BI-3 | 677 | 77.51.1bar(—) | 1.4(—) | — | 88.5(—) | — | [ |
| CTF-BI-4 | 1025 | 108.7(—) | 7.1(—) | — | 44.0(—) | — | [ |
| CTF-BI-5 | 836 | 100.6(—) | 6.9(—) | — | 35.6(—) | — | [ |
| CTF-BI-6 | 759 | 75.2(—) | 6.3(—) | — | 34.5(—) | — | [ |
| CTF-BI-7 | 642 | 56.3(—) | 5.3(—) | — | 39.7(—) | — | [ |
| CTF-BI-9 | 885 | 96.1(—) | 5.4(—) | — | 67.4(—) | — | [ |
| CTF-BI-10 | 1099 | 99.9(—) | 9.0(—) | — | 31.3(—) | — | [ |
| CTF-BI-11 | 1549 | 110.5(—) | 9.6(—) | — | 34.3(—) | — | [ |
| CTF-BIB-1 | 97.6(56.8) | 10.3(5.1) | 30.3(16.2) | 29.3(—) | 6.8(—) | [ | |
| CTF-BIB-2 | 1714 | 92.3(55.6) | 10.0(5.3) | 31.9(17.5) | 33.1(—) | 7.3(—) | [ |
| CTF-BIB-3 | 2088 | 86.4(48.5) | 9.8(3.9) | 27.3(16.2) | 21.2(—) | 4.7(—) | [ |
| CTF-DI-2 | 420 | 33.5(19.3) | — | — | 35(—) | 14(—) | [ |
| CTF-DI-3 | 1877 | 80.4(44.7) | — | — | 26(—) | 6(—) | [ |
| CTF-DI-4 | 1769 | 67.6(39.3) | — | — | 42(—) | 8(—) | [ |
| CTF-DI-5 | 1207 | 50.9(29.4) | — | — | 27(—) | 6(—) | [ |
| CTF-DI-6 | 697 | 62.8(40.0) | — | — | 53(—) | 15(—) | [ |
| CTF-DI-7 | 1300 | 89.2(54.2) | — | — | 41(—) | 11(—) | [ |
| CTF-DI-8 | 1749 | 77.8(44.5) | — | — | 35(—) | 8(—) | [ |
| CTF-DI-9 | 1228 | 59.7(35.4) | — | — | 14(—) | 6(—) | [ |
| PBILP | 121.4(79.2) | — | — | 72(—) | 7.2(—) | [ | |
表1 苯并咪唑连接聚合物的BET比表面积、气体吸附量及选择性(CO2/N2和CO2/CH4)
Table 1 BET surface area, gas uptake and selectivity (CO2/N2 and CO2/CH4) of benzimidazole-linked polymers
| 聚合物 | SABET①/(m2/g) | 吸附量②/(mg/g) | 选择性③ | 文献 | |||
|---|---|---|---|---|---|---|---|
| CO2 at 1 bar | N2 at 1 bar | CH4 at 1 bar | CO2/N2 | CO2/CH4 | |||
| BILP-1 | 1172 | 188(131) | 5.0(7.3) | 23(16) | 70(36) | 10(7) | [ |
| BILP-2 | 708 | 149(104) | 3.4(2.5) | 14(9) | 113(71) | 17(12) | [ |
| BILP-3 | 1306 | 225(145) | 3.3(2.4) | 24(17) | 59(31) | 8(5) | [ |
| BILP-4 | 1135 | 235(158) | 1.1(3.8) | 26(18) | 79(32) | 10(7) | [ |
| BILP-5 | 599 | 128(87) | 0.2(2.7) | 15(10) | 95(36) | 10(6) | [ |
| BILP-6 | 1261 | 211(121) | 6.8(6.7) | 27(19) | 63(39) | 8(5) | [ |
| BILP-6-NH2 | 1185 | 244.8(164.7) | — | — | — | —(8.4) | [ |
| BILP-7 | 1122 | 193(122) | 5.63(5.40) | 19(12) | 62(34) | 9(7) | [ |
| BILP-10 | 787 | 177(111) | — | 16(11) | 111(59) | 14(7) | [ |
| BILP-11 | 658 | 136(88) | — | 16(10) | 103(55) | 11(7) | [ |
| BILP-12 | 1497 | 223(140) | — | 24(15) | 56(31) | 8(6) | [ |
| BILP-13 | 677 | 113(79) | — | 12(9) | 103(38) | 9(6) | [ |
| BILP-14 | 1005 | 170(—) | — | — | 56(49) | 10(9) | [ |
| BILP-15 | 448 | 118(80) | 5.7(3.8) | 13.5(7.2) | 83(63) | 9(8) | [ |
| BILP-16 | 435 | 118.7(80.7) | 6.5(4.3) | 13.5(8.0) | 53(49) | 10(8) | [ |
| BILP-15(AC) | 862 | 151(101) | 7.2(5.8) | 16.4(10.3) | 61(50) | 9(7) | [ |
| BILP-16(AC) | 643 | 152(102.2) | 8.0(4.8) | 17.4(10.3) | 49(61) | 9(7) | [ |
| BILP-17 | 952 | — | — | — | — | — | [ |
| BILP-18 | 947 | — | — | — | — | — | [ |
| BILP-19 | 144 | 2630.95bar(229) | —(3) | 14.1(8.2) | —(59.1) | 11.9(12.3) | [ |
| BILP-101 | —(1070.15bar) | — | — | —(80) | — | [ | |
| PPN-101 | 226.2(—) | — | — | 199(—) | — | [ | |
| IBFNP-1 | 232(148) | — | — | 65(44) | 11(8) | [ | |
| IBLP | 48.06 | 46.5(30.7) | — | — | — | — | [ |
| IBLP-600 | 161.06 | 85.1(59.9) | — | — | — | — | [ |
| IBLP-700 | 270.64 | 121.8(90.7) | — | — | — | — | [ |
| IBLP-800 | 339 | 130.4(98.4) | — | — | — | — | [ |
| BINP-1 | 64 | 7.8(—) | — | — | — | — | [ |
| BINP-2 | 32 | 7(—) | — | — | — | — | [ |
| TBILP-1 | 330 | 117(78) | — | — | —(63) | —(9) | [ |
| TBILP-2 | 1080 | 228(146) | — | — | —(40) | —(7) | [ |
| CTF-BI-3 | 677 | 77.51.1bar(—) | 1.4(—) | — | 88.5(—) | — | [ |
| CTF-BI-4 | 1025 | 108.7(—) | 7.1(—) | — | 44.0(—) | — | [ |
| CTF-BI-5 | 836 | 100.6(—) | 6.9(—) | — | 35.6(—) | — | [ |
| CTF-BI-6 | 759 | 75.2(—) | 6.3(—) | — | 34.5(—) | — | [ |
| CTF-BI-7 | 642 | 56.3(—) | 5.3(—) | — | 39.7(—) | — | [ |
| CTF-BI-9 | 885 | 96.1(—) | 5.4(—) | — | 67.4(—) | — | [ |
| CTF-BI-10 | 1099 | 99.9(—) | 9.0(—) | — | 31.3(—) | — | [ |
| CTF-BI-11 | 1549 | 110.5(—) | 9.6(—) | — | 34.3(—) | — | [ |
| CTF-BIB-1 | 97.6(56.8) | 10.3(5.1) | 30.3(16.2) | 29.3(—) | 6.8(—) | [ | |
| CTF-BIB-2 | 1714 | 92.3(55.6) | 10.0(5.3) | 31.9(17.5) | 33.1(—) | 7.3(—) | [ |
| CTF-BIB-3 | 2088 | 86.4(48.5) | 9.8(3.9) | 27.3(16.2) | 21.2(—) | 4.7(—) | [ |
| CTF-DI-2 | 420 | 33.5(19.3) | — | — | 35(—) | 14(—) | [ |
| CTF-DI-3 | 1877 | 80.4(44.7) | — | — | 26(—) | 6(—) | [ |
| CTF-DI-4 | 1769 | 67.6(39.3) | — | — | 42(—) | 8(—) | [ |
| CTF-DI-5 | 1207 | 50.9(29.4) | — | — | 27(—) | 6(—) | [ |
| CTF-DI-6 | 697 | 62.8(40.0) | — | — | 53(—) | 15(—) | [ |
| CTF-DI-7 | 1300 | 89.2(54.2) | — | — | 41(—) | 11(—) | [ |
| CTF-DI-8 | 1749 | 77.8(44.5) | — | — | 35(—) | 8(—) | [ |
| CTF-DI-9 | 1228 | 59.7(35.4) | — | — | 14(—) | 6(—) | [ |
| PBILP | 121.4(79.2) | — | — | 72(—) | 7.2(—) | [ | |
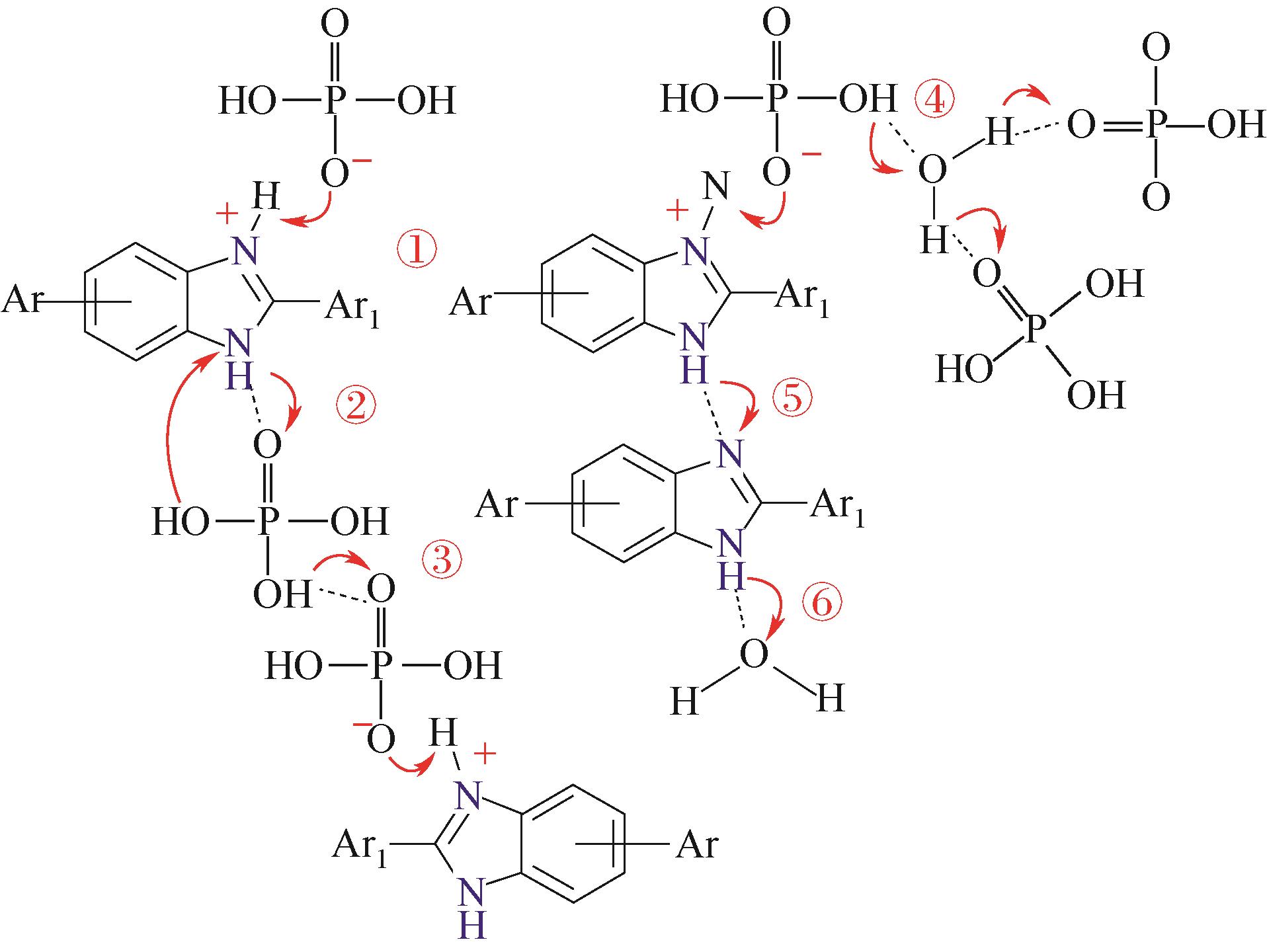
图10 苯并咪唑连接聚合物质子传递膜的分离机理[63]① 酸质子化咪唑;② 咪唑与酸之间质子跳跃;③ 酸与酸之间质子跳跃;④ 酸与水之间质子跳跃;⑤ 咪唑之间质子跳跃;⑥咪唑与水之间质子跳跃
Fig.10 Separation mechanism of benzimidazole-linked polymer proton transfer membranes[63]
| 聚合物 | 电导率 σ/( | 温度 T/K | 湿度 RH/% | 活化能 Ea/eV | 文献 |
|---|---|---|---|---|---|
| DPBIB | 9.3 | 393 | 100 | — | [ |
| PON-4 | 9.7 | 393 | 0 | 0.6 | [ |
| PON-5 | 2.0 | 393 | 0 | 0.36 | [ |
| PON-6 | 4.9 | 393 | 0 | 0.46 | [ |
| BIP | 3.2 | 368 | 95 | 0.34 | [ |
| BILP-10 | 1.2 | 368 | 95 | — | [ |
| 2.4 | 393 | 0 | 0.16 | [ | |
| H3PO4@TPB-DABI-COF[66%(质量分数)] | 1.52 | 433 | 0 | 0.17 | [ |
| Nafion 112 | 9.4 | 393 | 100 | — | [ |
| Nafion 115 | 1.3 | 363 | 100 | — | [ |
| Nafion 117 | 4.5 | 383 | 0 | — | [ |
表2 苯并咪唑连接聚合物与商业Nafion膜的质子传导率
Table 2 Proton conductivity of benzimidazole-linked polymers and commercial Nafion membranes
| 聚合物 | 电导率 σ/( | 温度 T/K | 湿度 RH/% | 活化能 Ea/eV | 文献 |
|---|---|---|---|---|---|
| DPBIB | 9.3 | 393 | 100 | — | [ |
| PON-4 | 9.7 | 393 | 0 | 0.6 | [ |
| PON-5 | 2.0 | 393 | 0 | 0.36 | [ |
| PON-6 | 4.9 | 393 | 0 | 0.46 | [ |
| BIP | 3.2 | 368 | 95 | 0.34 | [ |
| BILP-10 | 1.2 | 368 | 95 | — | [ |
| 2.4 | 393 | 0 | 0.16 | [ | |
| H3PO4@TPB-DABI-COF[66%(质量分数)] | 1.52 | 433 | 0 | 0.17 | [ |
| Nafion 112 | 9.4 | 393 | 100 | — | [ |
| Nafion 115 | 1.3 | 363 | 100 | — | [ |
| Nafion 117 | 4.5 | 383 | 0 | — | [ |
| 1 | Trewin A, Cooper A I. Porous organic polymers: distinction from disorder?[J]. Angewandte Chemie International Edition, 2010, 49(9): 1533-1535. |
| 2 | Das S, Heasman P, Ben T, et al. Porous organic materials: strategic design and structure-function correlation[J]. Chemical Reviews, 2017, 117(3): 1515-1563. |
| 3 | Dawson R, Cooper A I, Adams D J. Nanoporous organic polymer networks[J]. Progress in Polymer Science, 2012, 37(4): 530-563. |
| 4 | Slater A G, Cooper A I. Function-led design of new porous materials[J]. Science, 2015, 348(6238): aaa8075. |
| 5 | Liang J, Huang Y-B, Cao R. Metal-organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates[J]. Coordination Chemistry Reviews, 2019, 378: 32-65. |
| 6 | Karmakar A, Illathvalappil R, Anothumakkool B, et al. Hydrogen-bonded organic frameworks (HOFs): a new class of porous crystalline proton-conducting materials[J]. Angewandte Chemie International Edition, 2016, 55(36): 10667-10671. |
| 7 | Geng K, He T, Liu R, et al. Covalent organic frameworks: design, synthesis, and functions[J]. Chemical Reviews, 2020, 120(16): 8814-8933. |
| 8 | Chen Y Z, Zhang R, Jiao L, et al. Metal-organic framework-derived porous materials for catalysis[J]. Coordination Chemistry Reviews, 2018, 362: 1-23. |
| 9 | Diercks C S, Yaghi O M. The atom, the molecule, and the covalent organic framework[J]. Science, 2017, 355(6328): eaal1585. |
| 10 | Liu M, Jiang K, Ding X, et al. Controlling monomer feeding rate to achieve highly crystalline covalent triazine frameworks[J]. Advanced Materials, 2019, 31(19): 1807865. |
| 11 | Veldhuizen H, Elzen L, Mahon T, et al. Charge-transfer-complexed conjugated microporous polymers (CT-CMPs)[J]. Macromolecular Chemistry and Physics, 2020, 221(9): 1900415. |
| 12 | Cai C, Hou Z, Huang T, et al. Preparation of monodisperse hyper-crosslinking polymer nanoparticles for highly efficient CO2 adsorption[J]. Macromolecular Chemistry and Physics, 2017, 218(7): 1700001. |
| 13 | Budd P M, Ghanem B S, Makhseed S, et al. Polymers of intrinsic microporosity (PIMs): robust, solution-processable, organic nanoporous materials[J]. Chemical Communications, 2004(2): 230-231. |
| 14 | Ben T, Ren H, Ma S, et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area[J]. Angewandte Chemie International Edition, 2009, 48(50): 9457-9460. |
| 15 | Mukherjee S, Das M, Manna A, et al. Newly designed 1,2,3-triazole functionalized covalent triazine frameworks with exceptionally high uptake capacity for both CO2 and H2 [J]. Journal of Materials Chemistry A, 2019, 7(3): 1055-1068. |
| 16 | Liu M, Guo L, Jin S, et al. Covalent triazine frameworks: synthesis and applications[J]. Journal of Materials Chemistry A, 2019, 7(10): 5153-5172. |
| 17 | Bhadra S, Kim N H, Choi J S, et al. Hyperbranched poly(benzimidazole-co-benzene) with honeycomb structure as a membrane for high-temperature proton-exchange membrane fuel cells[J]. Journal of Power Sources, 2010, 195(9): 2470-2477. |
| 18 | Rabbani M G, El-Kaderi H M. Template-free synthesis of a highly porous benzimidazole-linked polymer for CO2 capture and H2 storage[J]. Chemistry of Materials, 2011, 23(7): 1650-1653. |
| 19 | Rabbani M G, El-Kaderi H M. Synthesis and characterization of porous benzimidazole-linked polymers and their performance in small gas storage and selective uptake[J]. Chemistry of Materials, 2012, 24(8): 1511-1517. |
| 20 | Rabbani M G, Reich T E, Kassab R M, et al. High CO2 uptake and selectivity by triptycene-derived benzimidazole-linked polymers[J]. Chemical Communications, 2012, 48(8): 1141-1143. |
| 21 | Rabbani M G, Sekizkardes A K, El-Kadri O M, et al. Pyrene-directed growth of nanoporous benzimidazole-linked nanofibers and their application to selective CO2 capture and separation[J]. Journal of Materials Chemistry, 2012, 22(48): 25409-25417. |
| 22 | Sekizkardes A K, İslamoğlu T, Kahveci Z, et al. Application of pyrene-derived benzimidazole-linked polymers to CO2 separation under pressure and vacuum swing adsorption settings[J]. Journal of Materials Chemistry A, 2014, 2(31): 12492-12500. |
| 23 | Altarawneh S, Behera S, Jena P, et al. New insights into carbon dioxide interactions with benzimidazole-linked polymers[J]. Chemical Communication, 2014, 50(27): 3571-3574. |
| 24 | Altarawneh S, İslamoğlu T, Sekizkardes A K, et al. Effect of acid-catalyzed formation rates of benzimidazole-linked polymers on porosity and selective CO2 capture from gas mixtures[J]. Environmental Science & Technology, 2015, 49(7): 4715-4723. |
| 25 | Klumpen C, Radakovitsch F, Jess A, et al. BILP-19—an ultramicroporous organic network with exceptional carbon dioxide uptake[J]. Molecules, 2017, 22(8): 1343. |
| 26 | Sekizkardes A K, Culp J T, Islamoglu T, et al. An ultra-microporous organic polymer for high performance carbon dioxide capture and separation[J]. Chemical Communications, 2015, 51(69): 13393-13396. |
| 27 | Islamoglu T, Behera S, Kahveci Z, et al. Enhanced carbon dioxide capture from landfill gas using bifunctionalized benzimidazole-linked polymers[J]. ACS Applied Materials & Interfaces, 2016, 8(23): 14648-14655. |
| 28 | Sekizkardes A K, Altarawneh S, Kahveci Z, et al. Highly selective CO2 capture by triazine-based benzimidazole-linked polymers[J]. Macromolecules, 2014, 47(23): 8328-8334. |
| 29 | Tao L, Niu F, Wang C, et al. Benzimidazole functionalized covalent triazine frameworks for CO2 capture[J]. Journal of Materials Chemistry A, 2016, 4(30): 11812-11820. |
| 30 | Du J, Liu Y, Krishna R, et al. Enhancing gas sorption and separation performance via bisbenzimidazole functionalization of highly porous covalent triazine frameworks[J]. ACS Applied Materials & Interfaces, 2018, 10(31): 26678-26686. |
| 31 | Du J, Cui Y, Liu Y, et al. Preparation of benzodiimidazole-containing covalent triazine frameworks for enhanced selective CO2 capture and separation[J]. Microporous and Mesoporous Materials, 2019, 276: 213-222. |
| 32 | Neti V S P K, Wang J, Deng S, et al. Selective CO2 adsorption in a porphyrin polymer with benzimidazole linkages[J]. RSC Advances, 2015, 5(15): 10960-10963. |
| 33 | Muhammad R, Rekha P, Mohanty P. Facile synthesis of a thermally stable imine and benzimidazole functionalized nanoporous polymer (IBFNP) for CO2 capture application[J]. Greenhouse Gases: Science and Technology, 2016, 6(1): 150-157. |
| 34 | Rehman A, Park S J. Facile synthesis of nitrogen-enriched microporous carbons derived from imine and benzimidazole-linked polymeric framework for efficient CO2 adsorption[J]. Journal of CO2 Utilization, 2017, 21: 503-512. |
| 35 | Ranjeesh K C, Illathvalappil R, Veer S D, et al. Imidazole-linked crystalline two-dimensional polymer with ultrahigh proton-conductivity[J]. Journal of the American Chemical Society, 2019, 141(38): 14950-14954. |
| 36 | Li J, Wang J, Wu Z, et al. Ultrafast and stable proton conduction in polybenzimidazole covalent organic frameworks via confinement and activation[J]. Angewandte Chemie International Edition, 2021, 60(23): 12918-12923. |
| 37 | Sekizkardes A K, Kusuma V A, Dahe G, et al. Separation of carbon dioxide from flue gas by mixed matrix membranes using dual phase microporous polymeric constituents[J]. Chemical Communications, 2016, 52(79): 11768-11771. |
| 38 | Song C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing[J]. Catalysis Today, 2006, 115(1/2/3/4): 2-32. |
| 39 | Challa P, Paleti G, Madduluri V R, et al. Trends in emission and utilization of CO2: sustainable feedstock in the synthesis of value-added fine chemicals[J]. Catalysis Surveys from Asia, 2022, 26(2): 80-91. |
| 40 | 何利梅, 姜伟丽, 李继聪, 等. CO2吸附材料的研究进展[J].石油化工, 2022,51(1):83-91. |
| He L M, Jiang W L, Li J C, et al. Research progress in the adsorption materials of CO2 [J]. Petrochemical Technology, 2022, 51(1): 83-91. | |
| 41 | Gao W, Liang S, Wang R, et al. Industrial carbon dioxide capture and utilization: state of the art and future challenges[J]. Chemical Society Reviews, 2020, 49(23): 8584-8686. |
| 42 | Rochelle G T. Amine scrubbing for CO2 capture[J]. Science, 2009, 325(5948): 1652-1654. |
| 43 | Dutcher B, Fan M, Russell A G. Amine-based CO2 capture technology development from the beginning of 2013—a review[J]. ACS Applied Materials & Interfaces, 2015, 7(4): 2137-2148. |
| 44 | 江涛, 魏小娟, 王胜平, 等. 固体吸附剂捕集CO2的研究进展[J]. 洁净煤技术, 2022, 28(1): 42-57. |
| Jiang T, Wei X J, Wang S P, et al. Research progress on solid sorbents for CO2 capture[J]. Clean Coal Technology, 2022, 28(1): 42-57. | |
| 45 | Mukhtar A, Saqib S, Mellon N B, et al. A review on CO2 capture via nitrogen-doped porous polymers and catalytic conversion as a feedstock for fuels[J]. Journal of Cleaner Production, 2020, 277: 123999. |
| 46 | D'Alessandro D M, Smit B, Long J R. Carbon dioxide capture: prospects for new materials[J]. Angewandte Chemie International Edition, 2010, 49(35): 6058-6082. |
| 47 | Altarawneh S, Nahar L, Arachchige I U, et al. Highly porous and photoluminescent pyrene-quinoxaline-derived benzimidazole-linked polymers[J]. Journal of Materials Chemistry A, 2015, 3(6): 3006-3010. |
| 48 | Zhang M, Perry Z, Park J, et al. Stable benzimidazole-incorporated porous polymer network for carbon capture with high efficiency and low cost[J]. Polymer, 2014, 55(1): 335-339. |
| 49 | Maruthapandi M, Eswaran L, Cohen R, et al. Silica-supported nitrogen-enriched porous benzimidazole-linked and triazine-based polymers for the adsorption of CO2 [J]. Langmuir, 2020, 36(16): 4280-4288. |
| 50 | Nguyen T S, Yavuz C T. Quantifying the nitrogen effect on CO2 capture using isoporous network polymers[J]. Chemical Communications, 2020, 56(31): 4273-4275. |
| 51 | Chowdhury S, Mazumder M A J, Al-Attas O, et al. Heavy metals in drinking water: occurrences, implications, and future needs in developing countries[J]. Science of the Total Environment, 2016, 569/570: 476-488. |
| 52 | Fu Z, Xi S. The effects of heavy metals on human metabolism[J]. Toxicology Mechanisms and Methods, 2020, 30(3): 167-176. |
| 53 | Castro-Muñoz R, González-Melgoza L L, García-Depraect O. Ongoing progress on novel nanocomposite membranes for the separation of heavy metals from contaminated water[J]. Chemosphere, 2021, 270: 129421. |
| 54 | Joseph L, Jun B-M, Flora J R V, et al. Removal of heavy metals from water sources in the developing world using low-cost materials: a review[J]. Chemosphere, 2019, 229: 142-159. |
| 55 | Chakraborty R, Asthana A, Singh A K, et al. Adsorption of heavy metal ions by various low-cost adsorbents: a review[J]. International Journal of Environmental Analytical Chemistry, 2022, 102(2): 342-379. |
| 56 | Maruthapandi M, Luong J H T, Gedanken A. Nitrogen-enriched porous benzimidazole-linked polymeric network for the adsorption of La (Ⅲ), Ce (Ⅲ), and Nd (Ⅲ)[J]. The Journal of Physical Chemistry C, 2020, 124(11): 6206-6214. |
| 57 | Li J, Yang X, Bai C, et al. A novel benzimidazole-functionalized 2-D COF material: synthesis and application as a selective solid-phase extractant for separation of uranium[J]. Journal of Colloid and Interface Science, 2015, 437: 211-218. |
| 58 | Cong S, Wang J, Wang Z, et al. Polybenzimidazole (PBI) and benzimidazole-linked polymer (BILP) membranes[J]. Green Chemical Engineering, 2021, 2(1): 44-56. |
| 59 | Tessema T D M, Venna S R, Dahe G, et al. Incorporation of benzimidazole linked polymers into matrimid to yield mixed matrix membranes with enhanced CO2/N2 selectivity[J]. Journal of Membrane Science, 2018, 554: 90-96. |
| 60 | Shan M, Seoane B, Pustovarenko A, et al. Benzimidazole linked polymers (BILPs) in mixed-matrix membranes: influence of filler porosity on the CO2/N2 separation performance[J]. Journal of Membrane Science, 2018, 566: 213-222. |
| 61 | Shan M, Liu X, Wang X, et al. Facile manufacture of porous organic framework membranes for precombustion CO2 capture[J]. Science Advances, 2018, 4(9): eaau1698. |
| 62 | Liu D, Tian C, Shan M, et al. Interface synthesis of flexible benzimidazole-linked polymer molecular-sieving membranes with superior antimicrobial activity[J]. Journal of Membrane Science, 2022, 648: 120344. |
| 63 | Ma Y L, Wainright J S, Litt M H, et al. Conductivity of PBI membranes for high-temperature polymer electrolyte fuel cells[J]. Journal of The Electrochemical Society, 2004, 151(1): A8-A16. |
| 64 | Kim S, Lee Y M. Rigid and microporous polymers for gas separation membranes[J]. Progress in Polymer Science, 2015, 43: 1-32. |
| 65 | Sanders D F, Smith Z P, Guo R, et al. Energy-efficient polymeric gas separation membranes for a sustainable future: a review[J]. Polymer, 2013, 54(18): 4729-4761. |
| 66 | Lee A, Elam J W, Darling S B. Membrane materials for water purification: design, development, and application[J]. Environmental Science: Water Research & Technology, 2016, 2(1): 17-42. |
| 67 | Paul D. Reformulation of the solution-diffusion theory of reverse osmosis[J]. Journal of Membrane Science, 2004, 241(2): 371-386. |
| 68 | Xu Y C, Wang Z X, Cheng X Q, et al. Positively charged nanofiltration membranes via economically mussel-substance-simulated co-deposition for textile wastewater treatment[J]. Chemical Engineering Journal, 2016, 303: 555-564. |
| 69 | Zhu J, Hou J, Yuan S, et al. MOF-positioned polyamide membranes with a fishnet-like structure for elevated nanofiltration performance[J]. Journal of Materials Chemistry A, 2019, 7(27): 16313-16322. |
| 70 | Munirasu S, Haija M A, Banat F. Use of membrane technology for oil field and refinery produced water treatment—a review[J]. Process Safety and Environmental Protection, 2016, 100: 183-202. |
| 71 | Rahimpour A, Jahanshahi M, Mortazavian N, et al. Preparation and characterization of asymmetric polyethersulfone and thin-film composite polyamide nanofiltration membranes for water softening[J]. Applied Surface Science, 2010, 256(6): 1657-1663. |
| 72 | Tang C Y, Yang Z, Guo H, et al. Potable water reuse through advanced membrane technology[J]. Environmental Science & Technology, 2018, 52(18): 10215-10223. |
| 73 | Lin J. Fractionation of direct dyes and salts in aqueous solution using loose nanofiltration membranes[J]. Journal of Membrane Science, 2015, 477: 183-193. |
| 74 | Kim K, Kim H, Lim J H, et al. Development of a desalination membrane bioinspired by mangrove roots for spontaneous filtration of sodium ions[J]. ACS Nano, 2016, 10(12): 11428-11433. |
| 75 | Ang W L, Mohammad A W, Hilal N, et al. A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants[J]. Desalination, 2015, 363: 2-18. |
| 76 | Mohammad A W, Teow Y H, Ang W L, et al. Nanofiltration membranes review: recent advances and future prospects[J]. Desalination, 2015, 356: 226-254. |
| 77 | Luo J, Wan Y. Effects of pH and salt on nanofiltration—a critical review[J]. Journal of Membrane Science, 2013, 438: 18-28. |
| 78 | Zhang R, Liu Y, He M, et al. Antifouling membranes for sustainable water purification: strategies and mechanisms[J]. Chemical Society Reviews, 2016, 45(21): 5888-5924. |
| 79 | Nithya D, Melbiah J S B, Mohan D. Benzimidazole-based dendritic nanofiltration membranes[J]. Iranian Polymer Journal, 2018, 27(4): 225-237. |
| 80 | Marchetti P, Solomon M F J, Szekely G, et al. Molecular separation with organic solvent nanofiltration: a critical review[J]. Chemical Reviews, 2014, 114(21): 10735-10806. |
| 81 | Szekely G, Jimenez-Solomon M F, Marchetti P, et al. Sustainability assessment of organic solvent nanofiltration: from fabrication to application[J]. Green Chemistry, 2014, 16(10): 4440-4473. |
| 82 | Oatley-Radcliffe D L, Walters M, Ainscough T J, et al. Nanofiltration membranes and processes: a review of research trends over the past decade[J]. Journal of Water Process Engineering, 2017, 19: 164-171. |
| 83 | Huang L, Chen J, Gao T, et al. Reduced graphene oxide membranes for ultrafast organic solvent nanofiltration[J]. Advanced Materials, 2016, 28(39): 8669-8674. |
| 84 | Li C, Li S, Tian L, et al. Covalent organic frameworks (COFs)-incorporated thin film nanocomposite (TFN) membranes for high-flux organic solvent nanofiltration (OSN)[J]. Journal of Membrane Science, 2019, 572: 520-531. |
| 85 | Liu J, Jiang J. Microporous benzimidazole-linked polymer and its derivatives for organic solvent nanofiltration[J]. Polymer, 2019, 185: 121932. |
| 86 | Pei P, Chen H. Main factors affecting the lifetime of proton exchange membrane fuel cells in vehicle applications: a review[J]. Applied Energy, 2014, 125: 60-75. |
| 87 | Sharaf O Z, Orhan M F. An overview of fuel cell technology: fundamentals and applications[J]. Renewable and Sustainable Energy Reviews, 2014, 32: 810-853. |
| 88 | Mekhilef S, Saidur R, Safari A. Comparative study of different fuel cell technologies[J]. Renewable and Sustainable Energy Reviews, 2012, 16(1): 981-989. |
| 89 | Rosli R E, Sulong A B, Daud W R W, et al. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system[J]. International Journal of Hydrogen Energy, 2017, 42(14): 9293-9314. |
| 90 | Wang Y, Diaz D F R, Chen K S, et al. Materials, technological status, and fundamentals of PEM fuel cells—a review[J]. Materials Today, 2020, 32: 178-203. |
| 91 | Wu H W. A review of recent development: transport and performance modeling of PEM fuel cells[J]. Applied Energy, 2016, 165: 81-106. |
| 92 | Xiao F, Wang Y, Wu Z, et al. Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells[J]. Advanced Materials, 2021, 33(50): 2006292. |
| 93 | Spendelow J S, Papageorgopoulos D C. Progress in PEMFC MEA component R&D at the DOE fuel cell technologies program[J]. Fuel Cells, 2011, 11(6): 775-786. |
| 94 | Bose S, Kuila T, Nguyen T X H, et al. Polymer membranes for high temperature proton exchange membrane fuel cell: recent advances and challenges[J]. Progress in Polymer Science, 2011, 36(6): 813-843. |
| 95 | Li J, Wu Z, Li H, et al. Layered-structure microporous poly(benzimidazole)-loaded imidazole for non-aqueous proton conduction[J]. New Journal of Chemistry, 2018, 42(3): 1604-1607. |
| 96 | Klumpen C, Winterstein S, Papastavrou G, et al. Anhydrous proton conduction in porous organic networks[J]. Journal of Materials Chemistry A, 2018, 6(43): 21542-21549. |
| 97 | 李英, 张香平. 用于高温质子交换膜燃料电池的聚合物电解质膜研究进展[J]. 化工进展, 2018, 37(9): 3446-3453. |
| Li Y, Zhang X P. Research progress of polymer electrolyte membrane for high temperature proton exchange membrane fuel cell[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3446-3453. | |
| 98 | 侯宏英, 孙公权, 吴智谋, 等. 直接甲醇燃料电池用磷酸氢锆/Nafion115的研究[J].电源技术, 2009, 33(1): 17-20. |
| Hou H Y, Sun G Q, Wu Z M, et al. Study on zirconium hydrogen phosphate/Nafion115 composite membrane for DMFC[J]. Chinese Journal of Power Sources, 2009, 33(1): 17-20. | |
| 99 | Maiti J, Kakati N, Woo S P, et al. Nafion® based hybrid composite membrane containing GO and dihydrogen phosphate functionalized ionic liquid for high temperature polymer electrolyte membrane fuel cell[J]. Composites Science and Technology, 2018, 155: 189-196. |
| [1] | 邵苛苛, 宋孟杰, 江正勇, 张旋, 张龙, 高润淼, 甄泽康. 水平方向上冰中受陷气泡形成和分布实验研究[J]. 化工学报, 2023, 74(S1): 161-164. |
| [2] | 吴延鹏, 李晓宇, 钟乔洋. 静电纺丝纳米纤维双疏膜油性细颗粒物过滤性能实验分析[J]. 化工学报, 2023, 74(S1): 259-264. |
| [3] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [6] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [7] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [8] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [9] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [10] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [11] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [12] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [13] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [14] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [15] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号