化工学报 ›› 2024, Vol. 75 ›› Issue (12): 4780-4792.DOI: 10.11949/0438-1157.20240617
肖忠良1( ), 夏宇博1, 宋刘斌1(
), 夏宇博1, 宋刘斌1( ), 向优涛1, 赵亭亭1, 罗静1, 刘远佳1, 邓鹏辉1, 颜群轩2(
), 向优涛1, 赵亭亭1, 罗静1, 刘远佳1, 邓鹏辉1, 颜群轩2( )
)
收稿日期:2024-06-04
修回日期:2024-08-16
出版日期:2024-12-25
发布日期:2025-01-03
通讯作者:
宋刘斌,颜群轩
作者简介:肖忠良(1964—),男,博士,教授,xiaozhongliang@163.com
基金资助:
Zhongliang XIAO1( ), Yubo XIA1, Liubin SONG1(
), Yubo XIA1, Liubin SONG1( ), Youtao XIANG1, Tingting ZHAO1, Jing LUO1, Yuanjia LIU1, Penghui DENG1, Qunxuan YAN2(
), Youtao XIANG1, Tingting ZHAO1, Jing LUO1, Yuanjia LIU1, Penghui DENG1, Qunxuan YAN2( )
)
Received:2024-06-04
Revised:2024-08-16
Online:2024-12-25
Published:2025-01-03
Contact:
Liubin SONG, Qunxuan YAN
摘要:
从废旧磷酸铁锂(LiFePO4)动力电池中回收有价金属,实现其资源化利用是当前亟待解决的问题。提出磷酸-酒石酸混合浸出体系,对废旧LiFePO4的全组分浸出回收工艺进行研究。通过单因素实验结果得到浸出条件的大致范围,再采用响应曲面法对不同浸出条件进行优化得到最佳浸出工艺。结果表明:在磷酸浓度为3.1 mol/L、酒石酸(TA)浓度为1.3 mol/L、液固比6.8∶1、搅拌速度500 r/min、反应温度65℃下反应5 h,Li和Fe的浸出率分别为97.55%和98.67%。实验结果表明,酒石酸可以与磷酸浸出释放的Fe3+进行络合,混合酸的协同作用几乎将废料中所有的Li、Fe都浸出到溶液中。通过调整浸出液中Li∶Fe∶P的摩尔比,采用喷雾干燥-烧结法合成再生磷酸铁锂(RE-LiFePO4)。该工艺在整个回收过程中几乎没有废酸、废气排放,实现了废旧LiFePO4的全组分绿色回收。
中图分类号:
肖忠良, 夏宇博, 宋刘斌, 向优涛, 赵亭亭, 罗静, 刘远佳, 邓鹏辉, 颜群轩. 磷酸-酒石酸体系协同浸出废旧磷酸铁锂工艺[J]. 化工学报, 2024, 75(12): 4780-4792.
Zhongliang XIAO, Yubo XIA, Liubin SONG, Youtao XIANG, Tingting ZHAO, Jing LUO, Yuanjia LIU, Penghui DENG, Qunxuan YAN. Synergistic leaching process of waste LiFePO4 with phosphoric acid-tartaric acid system[J]. CIESC Journal, 2024, 75(12): 4780-4792.
| 药品名称 | 分子式 | 规格 | 厂家 |
|---|---|---|---|
| 磷酸 | H3PO4 | AR | 国药集团有限公司 |
| 酒石酸 | C4H6O6 | AR | 国药集团有限公司 |
| 碳酸锂 | Li2CO3 | 电池级 | 上海麦克林生化科技股份有限公司 |
| 氧化铁 | Fe2O3 | AR | 上海麦克林生化科技股份有限公司 |
表1 实验试剂
Table 1 Experimental reagents
| 药品名称 | 分子式 | 规格 | 厂家 |
|---|---|---|---|
| 磷酸 | H3PO4 | AR | 国药集团有限公司 |
| 酒石酸 | C4H6O6 | AR | 国药集团有限公司 |
| 碳酸锂 | Li2CO3 | 电池级 | 上海麦克林生化科技股份有限公司 |
| 氧化铁 | Fe2O3 | AR | 上海麦克林生化科技股份有限公司 |
| 仪器名称 | 型号 | 生产厂家 |
|---|---|---|
| 恒速电动搅拌器 | JJ-1B | 西城新瑞仪器厂 |
| 集热式恒温加热磁力 搅拌器 | DF-101S | 上海力辰邦西仪器科技公司 |
| 管式炉 | HLG-X-16 | 洛阳恒立窑炉有限公司 |
| X射线衍射分析仪 | D8 Advance | 德国Bruker公司 |
| 电感耦合等离子体发生光谱仪 | ARCOS | 德国SPECTRO公司 |
表2 实验器材
Table 2 Experimental equipments
| 仪器名称 | 型号 | 生产厂家 |
|---|---|---|
| 恒速电动搅拌器 | JJ-1B | 西城新瑞仪器厂 |
| 集热式恒温加热磁力 搅拌器 | DF-101S | 上海力辰邦西仪器科技公司 |
| 管式炉 | HLG-X-16 | 洛阳恒立窑炉有限公司 |
| X射线衍射分析仪 | D8 Advance | 德国Bruker公司 |
| 电感耦合等离子体发生光谱仪 | ARCOS | 德国SPECTRO公司 |
| Element | Composition/% |
|---|---|
| Li | 3.62 |
| Fe | 28.06 |
| Al | 0.72 |
| P | 16.28 |
表3 铁锂粉化学元素组成
Table 3 Chemical element composition of iron-lithium powder
| Element | Composition/% |
|---|---|
| Li | 3.62 |
| Fe | 28.06 |
| Al | 0.72 |
| P | 16.28 |
实验 序号 | 搅拌速度/(r/min) | 磷酸浓度/(mol/L) | 酒石酸浓度/(mol/L) | 液固比/(ml/g) | 温度/℃ | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 200 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 89.60 | 88.78 |
| 2 | 300 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 92.58 | 95.33 |
| 3 | 400 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 92.59 | 96.34 |
| 4 | 500 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 94.83 | 96.67 |
| 5 | 600 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 93.14 | 97.34 |
| 6 | 700 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 91.27 | 93.00 |
表4 搅拌速度对锂、铁浸出率的影响
Table 4 Effect of stirring speed on leaching rate of Li and Fe
实验 序号 | 搅拌速度/(r/min) | 磷酸浓度/(mol/L) | 酒石酸浓度/(mol/L) | 液固比/(ml/g) | 温度/℃ | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 200 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 89.60 | 88.78 |
| 2 | 300 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 92.58 | 95.33 |
| 3 | 400 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 92.59 | 96.34 |
| 4 | 500 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 94.83 | 96.67 |
| 5 | 600 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 93.14 | 97.34 |
| 6 | 700 | 2.90 | 1.33 | 5∶1 | 70 | 5 | 91.27 | 93.00 |
实验 序号 | 磷酸 浓度/(mol/L) | 搅拌 速度/(r/min) | 酒石酸 浓度/(mol/L) | 液固比/(ml/g) | 温度/℃ | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.74 | 500 | 1.33 | 5∶1 | 70 | 5 | 88.93 | 85.38 |
| 2 | 2.32 | 500 | 1.33 | 5∶1 | 70 | 5 | 94.55 | 94.32 |
| 3 | 2.90 | 500 | 1.33 | 5∶1 | 70 | 5 | 96.34 | 96.48 |
| 4 | 3.48 | 500 | 1.33 | 5∶1 | 70 | 5 | 95.97 | 94.44 |
| 5 | 4.06 | 500 | 1.33 | 5∶1 | 70 | 5 | 95.12 | 94.35 |
| 6 | 4.64 | 500 | 1.33 | 5∶1 | 70 | 5 | 95.61 | 74.15 |
表5 磷酸浓度对锂、铁浸出率的影响
Table 5 Effect of H3PO4 concentration on leaching rate of Li and Fe
实验 序号 | 磷酸 浓度/(mol/L) | 搅拌 速度/(r/min) | 酒石酸 浓度/(mol/L) | 液固比/(ml/g) | 温度/℃ | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.74 | 500 | 1.33 | 5∶1 | 70 | 5 | 88.93 | 85.38 |
| 2 | 2.32 | 500 | 1.33 | 5∶1 | 70 | 5 | 94.55 | 94.32 |
| 3 | 2.90 | 500 | 1.33 | 5∶1 | 70 | 5 | 96.34 | 96.48 |
| 4 | 3.48 | 500 | 1.33 | 5∶1 | 70 | 5 | 95.97 | 94.44 |
| 5 | 4.06 | 500 | 1.33 | 5∶1 | 70 | 5 | 95.12 | 94.35 |
| 6 | 4.64 | 500 | 1.33 | 5∶1 | 70 | 5 | 95.61 | 74.15 |
实验 序号 | 酒石酸 浓度/(mol/L) | 搅拌 速度/(r/min) | 磷酸 浓度/(mol/L) | 液固比/(ml/g) | 温度/℃ | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.53 | 500 | 2.90 | 5∶1 | 70 | 5 | 93.21 | 24.94 |
| 2 | 0.80 | 500 | 2.90 | 5∶1 | 70 | 5 | 93.45 | 52.04 |
| 3 | 1.06 | 500 | 2.90 | 5∶1 | 70 | 5 | 94.03 | 81.83 |
| 4 | 1.33 | 500 | 2.90 | 5∶1 | 70 | 5 | 95.11 | 97.08 |
| 5 | 1.60 | 500 | 2.90 | 5∶1 | 70 | 5 | 95.11 | 94.44 |
| 6 | 1.86 | 500 | 2.90 | 5∶1 | 70 | 5 | 94.53 | 93.79 |
表6 酒石酸浓度对锂、铁浸出率的影响
Table 6 Effect of tartaric acid concentration on leaching rate of Li and Fe
实验 序号 | 酒石酸 浓度/(mol/L) | 搅拌 速度/(r/min) | 磷酸 浓度/(mol/L) | 液固比/(ml/g) | 温度/℃ | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.53 | 500 | 2.90 | 5∶1 | 70 | 5 | 93.21 | 24.94 |
| 2 | 0.80 | 500 | 2.90 | 5∶1 | 70 | 5 | 93.45 | 52.04 |
| 3 | 1.06 | 500 | 2.90 | 5∶1 | 70 | 5 | 94.03 | 81.83 |
| 4 | 1.33 | 500 | 2.90 | 5∶1 | 70 | 5 | 95.11 | 97.08 |
| 5 | 1.60 | 500 | 2.90 | 5∶1 | 70 | 5 | 95.11 | 94.44 |
| 6 | 1.86 | 500 | 2.90 | 5∶1 | 70 | 5 | 94.53 | 93.79 |
实验 序号 | 温度/℃ | 搅拌 速度/(r/min) | 磷酸 浓度/(mol/L) | 酒石酸 浓度/(mol/L) | 液固比/(ml/g) | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 88.91 | 84.26 |
| 2 | 50 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 93.71 | 90.03 |
| 3 | 60 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 94.45 | 97.99 |
| 4 | 70 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 95.76 | 99.20 |
| 5 | 80 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 95.15 | 94.49 |
| 6 | 90 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 94.43 | 92.78 |
表7 反应温度对锂、铁浸出率的影响
Table 7 Effect of reaction temperature on leaching rate of Li and Fe
实验 序号 | 温度/℃ | 搅拌 速度/(r/min) | 磷酸 浓度/(mol/L) | 酒石酸 浓度/(mol/L) | 液固比/(ml/g) | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 88.91 | 84.26 |
| 2 | 50 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 93.71 | 90.03 |
| 3 | 60 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 94.45 | 97.99 |
| 4 | 70 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 95.76 | 99.20 |
| 5 | 80 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 95.15 | 94.49 |
| 6 | 90 | 500 | 2.90 | 1.33 | 5∶1 | 5 | 94.43 | 92.78 |
实验 序号 | 液固比/(ml/g) | 搅拌 速度/(r/min) | 磷酸 浓度/(mol/L) | 酒石酸 浓度/(mol/L) | 温度/℃ | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 3∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 83.92 | 77.84 |
| 2 | 4∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 92.01 | 90.71 |
| 3 | 5∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 94.00 | 97.39 |
| 4 | 6∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 97.52 | 98.87 |
| 5 | 8∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 96.53 | 97.71 |
| 6 | 10∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 92.23 | 86.51 |
表8 液固比对锂、铁浸出率的影响
Table 8 Effect of liquid-solid ratio on leaching rate of Li and Fe
实验 序号 | 液固比/(ml/g) | 搅拌 速度/(r/min) | 磷酸 浓度/(mol/L) | 酒石酸 浓度/(mol/L) | 温度/℃ | 时间/h | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 3∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 83.92 | 77.84 |
| 2 | 4∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 92.01 | 90.71 |
| 3 | 5∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 94.00 | 97.39 |
| 4 | 6∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 97.52 | 98.87 |
| 5 | 8∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 96.53 | 97.71 |
| 6 | 10∶1 | 500 | 2.90 | 1.33 | 70 | 5 | 92.23 | 86.51 |
实验 序号 | 时间/h | 搅拌 速度/(r/min) | 磷酸 浓度/(mol/L) | 酒石酸 浓度/(mol/L) | 温度/℃ | 液固比/(ml/g) | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 86.65 | 86.69 |
| 2 | 3 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 91.03 | 91.39 |
| 3 | 4 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 94.92 | 96.83 |
| 4 | 5 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 97.88 | 98.47 |
| 5 | 6 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 98.08 | 97.43 |
| 6 | 7 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 97.69 | 97.29 |
表9 反应时间对锂、铁浸出率的影响
Table 9 Effect of reaction time on leaching rate of Li and Fe
实验 序号 | 时间/h | 搅拌 速度/(r/min) | 磷酸 浓度/(mol/L) | 酒石酸 浓度/(mol/L) | 温度/℃ | 液固比/(ml/g) | Li 浸出率/% | Fe 浸出率/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 86.65 | 86.69 |
| 2 | 3 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 91.03 | 91.39 |
| 3 | 4 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 94.92 | 96.83 |
| 4 | 5 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 97.88 | 98.47 |
| 5 | 6 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 98.08 | 97.43 |
| 6 | 7 | 500 | 2.90 | 1.33 | 70 | 6∶1 | 97.69 | 97.29 |
| Factors | Code value | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| A: H3PO4/(mol/L) | 2 | 3 | 4 |
| B: Tartaric acid/(mol/L) | 0.8 | 1.3 | 1.8 |
| C: L/S/(ml/g) | 4∶1 | 7∶1 | 10∶1 |
| D: Temperature/℃ | 40 | 65 | 90 |
表10 Box-Behnken 实验因素高低水平
Table 10 Box-Behnken experimental factor level
| Factors | Code value | ||
|---|---|---|---|
| -1 | 0 | 1 | |
| A: H3PO4/(mol/L) | 2 | 3 | 4 |
| B: Tartaric acid/(mol/L) | 0.8 | 1.3 | 1.8 |
| C: L/S/(ml/g) | 4∶1 | 7∶1 | 10∶1 |
| D: Temperature/℃ | 40 | 65 | 90 |
| Run | H3PO4 concentration/(mol/L) | Tartaric acid concentration /(mol/L) | Liquid/Solid ratios/(ml/g) | Leaching temperature /℃ | Leaching rate of Li/% | Leaching rate of Fe/% |
|---|---|---|---|---|---|---|
| 1 | 0 | 1 | 0 | 1 | 83.38 | 85.78 |
| 2 | 0 | 0 | 0 | 0 | 97.08 | 98.03 |
| 3 | -1 | -1 | 0 | 0 | 75.33 | 63.17 |
| 4 | -1 | 0 | 1 | 0 | 63.06 | 62.65 |
| 5 | -1 | 0 | 0 | 1 | 72.45 | 70.76 |
| 6 | 0 | 0 | 0 | 0 | 97.41 | 97.48 |
| 7 | 1 | 0 | 0 | 1 | 80.24 | 80.87 |
| 8 | 1 | -1 | 0 | 0 | 76.08 | 71.25 |
| 9 | 0 | 1 | 0 | -1 | 84.37 | 75.74 |
| 10 | 1 | 0 | 1 | 0 | 64.95 | 70.24 |
| 11 | 0 | -1 | 0 | -1 | 80.78 | 65.75 |
| 12 | 0 | 0 | 0 | 0 | 97.63 | 98.21 |
| 13 | -1 | 0 | -1 | 0 | 68.94 | 74.24 |
| 14 | 0 | 1 | -1 | 0 | 77.45 | 85.15 |
| 15 | 0 | -1 | 0 | 1 | 84.98 | 69.54 |
| 16 | 0 | 0 | 1 | 1 | 75.45 | 68.25 |
| 17 | 1 | 0 | -1 | 0 | 75.3 | 81.53 |
| 18 | -1 | 1 | 0 | 0 | 72.45 | 77.47 |
| 19 | 0 | 0 | 1 | -1 | 67.26 | 69.36 |
| 20 | 1 | 1 | 0 | 0 | 78.9 | 83.18 |
| 21 | 0 | 0 | -1 | 1 | 78.93 | 87.32 |
| 22 | 0 | 1 | 1 | 0 | 72.78 | 71.3 |
| 23 | -1 | 0 | 0 | -1 | 72.75 | 67.56 |
| 24 | 0 | 0 | -1 | -1 | 82.38 | 73.69 |
| 25 | 1 | 0 | 0 | -1 | 72.88 | 71.24 |
| 26 | 0 | -1 | 1 | 0 | 70.28 | 64.27 |
| 27 | 0 | -1 | -1 | 0 | 81.05 | 72.53 |
表11 Li、Fe浸出率BBD实验设计
Table 11 BBD experimental design for Li and Fe leaching rate
| Run | H3PO4 concentration/(mol/L) | Tartaric acid concentration /(mol/L) | Liquid/Solid ratios/(ml/g) | Leaching temperature /℃ | Leaching rate of Li/% | Leaching rate of Fe/% |
|---|---|---|---|---|---|---|
| 1 | 0 | 1 | 0 | 1 | 83.38 | 85.78 |
| 2 | 0 | 0 | 0 | 0 | 97.08 | 98.03 |
| 3 | -1 | -1 | 0 | 0 | 75.33 | 63.17 |
| 4 | -1 | 0 | 1 | 0 | 63.06 | 62.65 |
| 5 | -1 | 0 | 0 | 1 | 72.45 | 70.76 |
| 6 | 0 | 0 | 0 | 0 | 97.41 | 97.48 |
| 7 | 1 | 0 | 0 | 1 | 80.24 | 80.87 |
| 8 | 1 | -1 | 0 | 0 | 76.08 | 71.25 |
| 9 | 0 | 1 | 0 | -1 | 84.37 | 75.74 |
| 10 | 1 | 0 | 1 | 0 | 64.95 | 70.24 |
| 11 | 0 | -1 | 0 | -1 | 80.78 | 65.75 |
| 12 | 0 | 0 | 0 | 0 | 97.63 | 98.21 |
| 13 | -1 | 0 | -1 | 0 | 68.94 | 74.24 |
| 14 | 0 | 1 | -1 | 0 | 77.45 | 85.15 |
| 15 | 0 | -1 | 0 | 1 | 84.98 | 69.54 |
| 16 | 0 | 0 | 1 | 1 | 75.45 | 68.25 |
| 17 | 1 | 0 | -1 | 0 | 75.3 | 81.53 |
| 18 | -1 | 1 | 0 | 0 | 72.45 | 77.47 |
| 19 | 0 | 0 | 1 | -1 | 67.26 | 69.36 |
| 20 | 1 | 1 | 0 | 0 | 78.9 | 83.18 |
| 21 | 0 | 0 | -1 | 1 | 78.93 | 87.32 |
| 22 | 0 | 1 | 1 | 0 | 72.78 | 71.3 |
| 23 | -1 | 0 | 0 | -1 | 72.75 | 67.56 |
| 24 | 0 | 0 | -1 | -1 | 82.38 | 73.69 |
| 25 | 1 | 0 | 0 | -1 | 72.88 | 71.24 |
| 26 | 0 | -1 | 1 | 0 | 70.28 | 64.27 |
| 27 | 0 | -1 | -1 | 0 | 81.05 | 72.53 |
| Source | Sum of Squares | Degree of freedom(df) | Mean Square | F | P-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 2097.05 | 14 | 149.79 | 156.62 | <0.0001 | Significant |
| A-H3PO4 | 45.51 | 1 | 45.51 | 118.05 | <0.0001 | |
| B-Tartaric acid | 0.0574 | 1 | 0.0574 | 340.48 | 0.7773 | |
| C-L/S ratios | 210.59 | 1 | 210.59 | 306.26 | <0.0001 | |
| D-Temperature | 18.78 | 1 | 18.78 | 100.51 | 0.0002 | |
| AB | 8.12 | 1 | 8.12 | 1.10 | 0.0049 | |
| AC | 5.00 | 1 | 5.00 | 0.0177 | 0.0194 | |
| AD | 14.67 | 1 | 14.67 | 8.12 | 0.0006 | |
| BC | 9.30 | 1 | 9.30 | 6.14 | 0.0031 | |
| BD | 6.73 | 1 | 6.73 | 7.67 | 0.0086 | |
| CD | 33.87 | 1 | 33.87 | 42.68 | <0.0001 | |
| A2 | 1206.07 | 1 | 1206.07 | 723.00 | <0.0001 | |
| B2 | 259.24 | 1 | 259.50 | 579.92 | <0.0001 | |
| C2 | 1117.53 | 1 | 1117.53 | 638.00 | <0.0001 | |
| D2 | 278.24 | 1 | 278.24 | 570.96 | <0.0001 | |
| Residual | 8.23 | 12 | 0.6860 | |||
| Lack of Fit | 8.08 | 10 | 0.8079 | 10.54 | 0.0897 | Not significant |
| Pure error | 0.1533 | 2 | 0.0766 | |||
| Cor total | 2105.28 | 26 | ||||
| Adeq precision | 55.9368 | |||||
| R2 | 0.9961 | |||||
| Adjusted R2 | 0.9915 | |||||
| Predicted R2 | 0.9777 | |||||
| C.V./% | 1.06 |
表12 Li浸出率BBD模型的方差分析
Table 12 Variance analysis of BBD model for Li leaching rate
| Source | Sum of Squares | Degree of freedom(df) | Mean Square | F | P-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 2097.05 | 14 | 149.79 | 156.62 | <0.0001 | Significant |
| A-H3PO4 | 45.51 | 1 | 45.51 | 118.05 | <0.0001 | |
| B-Tartaric acid | 0.0574 | 1 | 0.0574 | 340.48 | 0.7773 | |
| C-L/S ratios | 210.59 | 1 | 210.59 | 306.26 | <0.0001 | |
| D-Temperature | 18.78 | 1 | 18.78 | 100.51 | 0.0002 | |
| AB | 8.12 | 1 | 8.12 | 1.10 | 0.0049 | |
| AC | 5.00 | 1 | 5.00 | 0.0177 | 0.0194 | |
| AD | 14.67 | 1 | 14.67 | 8.12 | 0.0006 | |
| BC | 9.30 | 1 | 9.30 | 6.14 | 0.0031 | |
| BD | 6.73 | 1 | 6.73 | 7.67 | 0.0086 | |
| CD | 33.87 | 1 | 33.87 | 42.68 | <0.0001 | |
| A2 | 1206.07 | 1 | 1206.07 | 723.00 | <0.0001 | |
| B2 | 259.24 | 1 | 259.50 | 579.92 | <0.0001 | |
| C2 | 1117.53 | 1 | 1117.53 | 638.00 | <0.0001 | |
| D2 | 278.24 | 1 | 278.24 | 570.96 | <0.0001 | |
| Residual | 8.23 | 12 | 0.6860 | |||
| Lack of Fit | 8.08 | 10 | 0.8079 | 10.54 | 0.0897 | Not significant |
| Pure error | 0.1533 | 2 | 0.0766 | |||
| Cor total | 2105.28 | 26 | ||||
| Adeq precision | 55.9368 | |||||
| R2 | 0.9961 | |||||
| Adjusted R2 | 0.9915 | |||||
| Predicted R2 | 0.9777 | |||||
| C.V./% | 1.06 |
| Source | Sum of Squares | Degee of freedom(df) | Mean Square | F | P-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 2790.57 | 14 | 199.33 | 156.62 | <0.0001 | Significant |
| A-H3PO4 | 150.24 | 1 | 150.24 | 118.05 | <0.0001 | |
| B-Tartaric acid | 433.32 | 1 | 433.32 | 340.48 | <0.0001 | |
| C-L/S ratios | 389.77 | 1 | 389.77 | 306.26 | <0.0001 | |
| D-Temperature | 127.92 | 1 | 127.92 | 100.51 | <0.0001 | |
| AB | 1.40 | 1 | 1.40 | 1.10 | 0.3142 | |
| AC | 0.0225 | 1 | 0.0225 | 0.0177 | 0.8964 | |
| AD | 10.34 | 1 | 10.34 | 8.12 | 0.0146 | |
| BC | 7.81 | 1 | 7.81 | 6.14 | 0.0291 | |
| BD | 9.77 | 1 | 9.77 | 7.67 | 0.0170 | |
| CD | 54.32 | 1 | 54.32 | 42.68 | <0.0001 | |
| A2 | 920.15 | 1 | 920.15 | 723.00 | <0.0001 | |
| B2 | 738.06 | 1 | 738.06 | 579.92 | <0.0001 | |
| C2 | 811.97 | 1 | 811.97 | 638.00 | <0.0001 | |
| D2 | 726.65 | 1 | 726.65 | 570.96 | <0.0001 | |
| Residual | 15.27 | 12 | 1.27 | |||
| Lack of Fit | 14.98 | 10 | 1.50 | 10.36 | 0.0912 | Not significant |
| Pure error | 0.2893 | 2 | 0.1446 | |||
| Cor total | 2805.84 | 26 | ||||
| Adeq precision | 41.6701 | |||||
| R2 | 0.9946 | |||||
| Adjusted R2 | 0.9882 | |||||
| Predicted R2 | 0.9690 | |||||
| C.V./% | 1.48 |
表13 Fe浸出率BBD模型的方差分析
Table 13 Variance analysis of BBD model for Fe leaching rate
| Source | Sum of Squares | Degee of freedom(df) | Mean Square | F | P-value Prob>F | |
|---|---|---|---|---|---|---|
| Model | 2790.57 | 14 | 199.33 | 156.62 | <0.0001 | Significant |
| A-H3PO4 | 150.24 | 1 | 150.24 | 118.05 | <0.0001 | |
| B-Tartaric acid | 433.32 | 1 | 433.32 | 340.48 | <0.0001 | |
| C-L/S ratios | 389.77 | 1 | 389.77 | 306.26 | <0.0001 | |
| D-Temperature | 127.92 | 1 | 127.92 | 100.51 | <0.0001 | |
| AB | 1.40 | 1 | 1.40 | 1.10 | 0.3142 | |
| AC | 0.0225 | 1 | 0.0225 | 0.0177 | 0.8964 | |
| AD | 10.34 | 1 | 10.34 | 8.12 | 0.0146 | |
| BC | 7.81 | 1 | 7.81 | 6.14 | 0.0291 | |
| BD | 9.77 | 1 | 9.77 | 7.67 | 0.0170 | |
| CD | 54.32 | 1 | 54.32 | 42.68 | <0.0001 | |
| A2 | 920.15 | 1 | 920.15 | 723.00 | <0.0001 | |
| B2 | 738.06 | 1 | 738.06 | 579.92 | <0.0001 | |
| C2 | 811.97 | 1 | 811.97 | 638.00 | <0.0001 | |
| D2 | 726.65 | 1 | 726.65 | 570.96 | <0.0001 | |
| Residual | 15.27 | 12 | 1.27 | |||
| Lack of Fit | 14.98 | 10 | 1.50 | 10.36 | 0.0912 | Not significant |
| Pure error | 0.2893 | 2 | 0.1446 | |||
| Cor total | 2805.84 | 26 | ||||
| Adeq precision | 41.6701 | |||||
| R2 | 0.9946 | |||||
| Adjusted R2 | 0.9882 | |||||
| Predicted R2 | 0.9690 | |||||
| C.V./% | 1.48 |
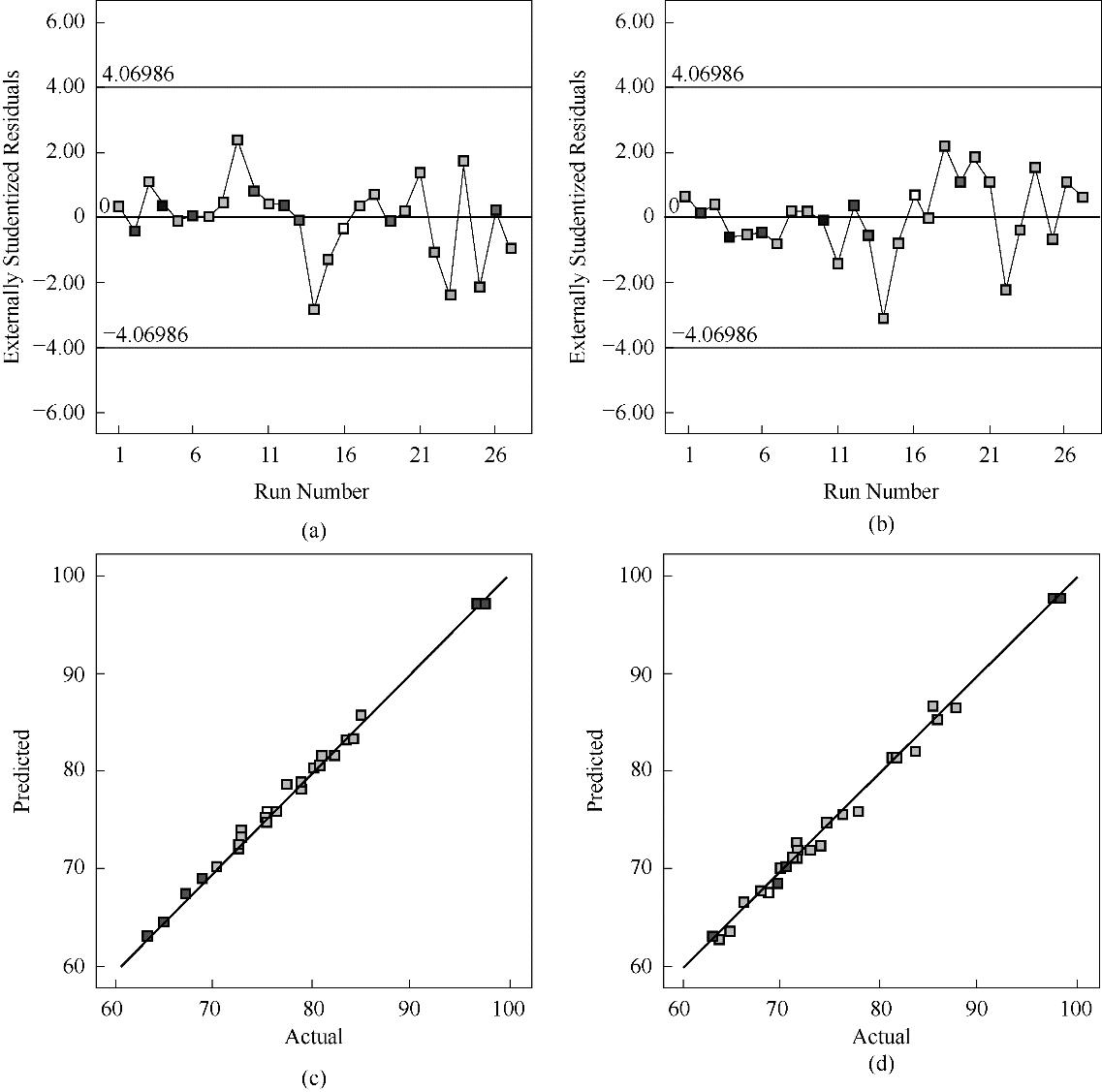
图4 (a)Li响应面模型残差图;(b)Fe响应面模型残差图;(c)Li浸出率实验值与预测值分布图;(d)Fe浸出率实验值与预测值分布图
Fig.4 (a) Residual diagram of Li response surface model; (b) Residual diagram of Fe response surface model; (c) Distribution diagram of experimental value and predicted value of Li leaching rate; (d) Distribution diagram of experimental value and predicted value of Fe leaching

图5 (a)Li浸出率的三维响应曲面图;(b)Fe浸出率的三维响应曲面图;(c)Li浸出率的二维等高线图;(d)Fe浸出率的二维等高线图
Fig.5 (a) Three-dimensional response surface diagram of Li leaching rate; (b) Three-dimensional response surface diagram of Fe leaching rate; (c) Two-dimensional contour map of Li leaching rate; (d) Two-dimensional contour map of Fe leaching rate
| 1 | Liu Z H, Zhou T, Yang H R, et al. A review of the resourceful utilization status for decommissioned power batteries[J]. Energies, 2023, 16(23): 7869. |
| 2 | Xu J J, Cai X Y, Cai S M, et al. High-energy lithium-ion batteries: recent progress and a promising future in applications [J]. Energy & Environmental Materials, 2023, 6(5): 12450. |
| 3 | 李峻, 田阳, 杨斌, 等. 废旧锂离子电池正极材料有价金属回收研究现状[J]. 中国有色金属学报, 2024, 36(6): 1789-1808. |
| Li J, Tian Y, Yang B, et al. Research status of valuable metal recovery of cathode materials of spent lithium-ion batteries[J]. The Chinese Journal of Nonferrous Metals, 2024, 36(6): 1789-1808. | |
| 4 | Zhang L G, Zhang Y, Xu Z M, et al. The foreseeable future of spent lithium-ion batteries: advanced upcycling for toxic electrolyte, cathode, and anode from environmental and technological perspectives[J]. Environmental Science & Technology, 2023, 57(36): 13270-13291. |
| 5 | Fan T, Liang W C, Guo W, et al. Life cycle assessment of electric v e h i c l e s ' lithium-ion batteries reused for energy storage[J]. Journal of Energy Storage, 2023, 71: 108126. |
| 6 | 肖忠良, 尹碧露, 宋刘斌, 等. 废旧锂离子电池回收工艺研究进展及其安全风险分析[J]. 化工学报, 2023, 74(4): 1446-1456. |
| Xiao Z L, Yin B L, Song L B, et al. Research progress of waste lithium-ion battery recycling process and its safety risk analysis[J]. CIESC Journal, 2023, 74(4): 1446-1456. | |
| 7 | 江洋, 彭长宏, 陈伟, 等. 废旧磷酸铁锂粉料综合回收中试研究[J]. 化工学报, 2024, 75(6): 2353-2361. |
| Jiang Y, Peng C H, Chen W, et al. Pilot study on comprehensive recycling of waste lithium iron phosphate powder[J]. CIESC Journal, 2024, 75(6): 2353-2361. | |
| 8 | Naseri T, Mousavi S M. Treatment of spent lithium iron phosphate (LFP) batteries[J]. Current Opinion in Green and Sustainable Chemistry, 2024, 47: 100906. |
| 9 | Saju D, Ebenezer J, Chandran N, et al. Recycling of lithium iron phosphate cathode materials from spent lithium-ion batteries: a mini-review[J]. Industrial & Engineering Chemistry Research, 2023, 62(30): 11768-11783. |
| 10 | Li Y J, Dong L P, Shi P, et al. Selective recovery of lithium from lithium iron phosphate[J]. Journal of Power Sources, 2024, 598: 234158. |
| 11 | Xu Y L, Zhang B C, Ge Z F, et al. Advances and perspectives towards spent LiFePO4 battery recycling[J]. Journal of Cleaner Production, 2024, 434: 140077. |
| 12 | Liang Q, Yue H F, Wang S F, et al. Recycling and crystal regeneration of commercial used LiFePO4 cathode materials[J]. Electrochimica Acta, 2020, 330: 135323. |
| 13 | Qiu X J, Wang C Y, Xie L L, et al. Challenges and perspectives towards direct regeneration of spent LiFePO4 cathode[J]. Journal of Power Sources, 2024, 602: 234365. |
| 14 | Zhang J F, Zou J T, He D, et al. Molten salt infiltration–oxidation synergistic controlled lithium extraction from spent lithium iron phosphate batteries: an efficient, acid free, and closed-loop strategy[J]. Green Chemistry, 2023, 25(15): 6057-6066. |
| 15 | 颜群轩, 罗碧云, 陈嘉鑫, 等. 废旧磷酸铁锂电池可持续回收技术研究进展[J]. 矿冶工程, 2023, 43(4): 174-177, 181. |
| Yan Q X, Luo B Y, Chen J X, et al. Progress in sustainable recycling of spent LiFePO4 batteries[J]. Mining and Metallurgical Engineering, 2023, 43(4): 174-177, 181. | |
| 16 | 张英杰, 许斌, 梁风, 等. 废旧磷酸铁锂电池正极材料的回收研究现状[J]. 人工晶体学报, 2019, 48(5):800-808. |
| Zhang Y J, Xu B, Liang F, et al. Review on recycling cathode materials of spent lithium iron phosphate batteries[J]. Journal of Synthetic Crystals, 2019, 48(5): 800-808. | |
| 17 | Lei S Y, Sun W, Yang Y. Comprehensive technology for recycling and regenerating materials from spent lithium iron phosphate battery[J]. Environmental Science & Technology, 2024, 58(8): 3609-3628. |
| 18 | 肖忠良, 向优涛, 宋刘斌, 等. 机械化学法回收废旧锂离子电池正极材料中有价金属的研究进展[J]. 化工学报, 2023, 74(11): 4419-4432. |
| Xiao Z L, Xiang Y T, Song L B, et al. Progress of mechanochemical recovery of valuable metals from used lithium-ion battery cathode materials[J]. CIESC Journal, 2023, 74(11): 4419-4432. | |
| 19 | Mahandra H, Ghahreman A. A sustainable process for selective recovery of lithium as lithium phosphate from spent LiFePO4 batteries[J]. Resources, Conservation and Recycling, 2021, 175: 105883. |
| 20 | Chen Z, Shen C, Liu F, et al. Selective separation and recovery of Li from spent LiFePO4 cathode materials by oxidation roasting followed by low-acid pressure leaching[J]. Metals, 2023, 13(11): 1884. |
| 21 | Yang Y X, Meng X Q, Cao H B, et al. Selective recovery of lithium from spent lithium iron phosphate batteries: a sustainable process[J]. Green Chemistry, 2018, 20(13): 3121-3133. |
| 22 | Du J W, Qing J L, Fang K Y, et al. The priority leaching of lithium from spent LiFePO4 cathode without the oxidization[J]. Resources, Conservation and Recycling, 2024, 202: 107374. |
| 23 | 鲁俊雀, 黄宁湘, 刘勇奇, 等. 磷酸铁锂正极粉选择性提锂[J]. 有色金属(冶炼部分), 2023, 12: 32-37. |
| Lu J Q, Huang N X, Liu Y Q, et al. Selective extraction of lithium from lithium iron phosphate positive powder[J]. Nonferrous Metals(Extractive Metallurgy), 2023, 12: 32-37. | |
| 24 | Li H, Xing S Z, Liu Y, et al. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 8017-8024. |
| 25 | Wang Y H, Wu J J, Hu G C, et al. Recovery of Li and Fe from spent lithium iron phosphate using organic acid leaching system[J]. Transactions of Nonferrous Metals Society of China, 2024, 34(1): 336-346. |
| 26 | Chai X L, Yu X H, Shen Q F, et al. Study on green closed-loop regeneration of waste lithium iron phosphate based on oxalic acid system[J]. Waste Management, 2024, 181: 168-175. |
| 27 | Zhao T Y, Mahandra H, Choi Y, et al. A clean and sustainable method for recycling of lithium from spent lithium iron phosphate battery powder by using formic acid and oxygen[J]. Science of the Total Environment, 2024, 920: 170930. |
| 28 | Jiang S Q, Li X G, Gao Q, et al. Review on full-component green recycling of spent lithium iron phosphate cathode materials: from the perspective of economy and efficiency[J]. Separation and Purification Technology, 2023, 324: 124630. |
| 29 | Wang T, Wang X L, Lyv W, et al. Regeneration behavior of FePO4·2H2O from spent LiFePO4 under extremely acidic condition (pH0.8): mechanism study and the properties of regenerated LiFePO4 [J]. Separation and Purification Technology, 2024, 330: 125508. |
| 30 | Zeng Y J, Wang Y, Cai S C, et al. All-component recycling and reuse process for spent LiFePO4 cathodes[J]. Industrial & Engineering Chemistry Research, 2024, 63(16): 6847-6856. |
| 31 | Zheng R J, Zhao L, Wang W H, et al. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method[J]. RSC Advances, 2016, 6(49): 43613-43625. |
| 32 | 王猛, 张家靓, 陈永强, 等. 退役磷酸铁锂电池回收技术综述[J]. 有色金属(冶炼部分), 2023, 5: 100-110. |
| Wang M, Zhang J L, Chen Y Q, et al. Review on recycling technology of retired LiFePO4 batteries[J]. Nonferrous Metals(Extractive Metallurgy), 2023, 5: 100-110. | |
| 33 | Kulka A, Braun A, Huang T-W, et al. Evidence for Al doping in lithium sublattice of LiFePO4 [J]. Solid State Ionics, 2015, 270: 33-38. |
| [1] | 赵博超, 聂一凡, 王雪婷, 田向勤, 田祎, 潘涔轩. 不同制液工艺对锰矿锰浸出回收及钙镁铁迁移影响[J]. 化工学报, 2024, 75(S1): 292-299. |
| [2] | 郑晓园, 蔡炎嶙, 应芝, 王波, 豆斌林. 污水污泥磷形态亚临界水热转化研究[J]. 化工学报, 2024, 75(8): 2970-2982. |
| [3] | 秦晓巧, 谭宏博, 温娜. 储能式低温空分系统热力学与经济性分析[J]. 化工学报, 2024, 75(7): 2409-2421. |
| [4] | 吕田田, 原敏, 王江, 高美珍, 杨佳辉, 徐红, 董晋湘, 石琪. ZTIF基疏水微介孔碳的制备及5-羟甲基糠醛吸附分离性能[J]. 化工学报, 2024, 75(4): 1642-1654. |
| [5] | 张天永, 张晶怡, 姜爽, 李彬, 吕东军, 陈都民, 陈雪. 弱酸性蓝AS染料排放的废盐制碳基吸附剂及利用[J]. 化工学报, 2024, 75(3): 890-899. |
| [6] | 王灵洁, 高海龙, 靳继鹏, 王志浩, 李见波. 海水中的污染物对逆电渗析电堆性能的影响[J]. 化工学报, 2024, 75(2): 695-705. |
| [7] | 刘家稳, 夏文成, 武锋, 彭耀丽, 谢广元. 废旧磷酸铁锂电池机械化学固相氧化回收锂机理[J]. 化工学报, 2024, 75(10): 3775-3782. |
| [8] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [9] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [10] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [11] | 肖忠良, 尹碧露, 宋刘斌, 匡尹杰, 赵亭亭, 刘成, 袁荣耀. 废旧锂离子电池回收工艺研究进展及其安全风险分析[J]. 化工学报, 2023, 74(4): 1446-1456. |
| [12] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| [13] | 许万, 陈振斌, 张慧娟, 牛昉昉, 火婷, 刘兴盛. 线性温敏性聚合物嵌段调控的 |
| [14] | 周昉, 刘剑, 张小松. 基于多参数评估原则筛选高温热泵用三元非共沸混合工质[J]. 化工学报, 2023, 74(11): 4487-4500. |
| [15] | 武庭宇, 王超, 秦余涛, 庄钰, 都健. 乙酸乙酯/乙醇/水体系预分离萃取精馏工艺研究[J]. 化工学报, 2023, 74(11): 4578-4586. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号