化工学报 ›› 2025, Vol. 76 ›› Issue (9): 4862-4871.DOI: 10.11949/0438-1157.20250122
收稿日期:2025-02-10
修回日期:2025-03-24
出版日期:2025-09-25
发布日期:2025-10-23
通讯作者:
赵静
作者简介:张建民(1999—),硕士研究生,zhangjianmin@njtech.edu.cn
基金资助:
Jianmin ZHANG( ), Meigui HE, Wanxin JIA, Jing ZHAO(
), Meigui HE, Wanxin JIA, Jing ZHAO( ), Wanqin JIN
), Wanqin JIN
Received:2025-02-10
Revised:2025-03-24
Online:2025-09-25
Published:2025-10-23
Contact:
Jing ZHAO
摘要:
交联聚氧化乙烯(PEO)是目前用于CO2捕集的热点膜材料之一。然而,基于环氧开环聚合反应形成交联PEO过程中会产生大量羟基,导致分子链间形成丰富的氢键作用,从而降低链的运动性,增大气体分子的渗透阻力,使膜材料渗透性不理想。为了提升交联PEO膜的CO2分离性能,将18-冠-6(C6)小分子引入PEO交联网络中,形成均相混合物,利用C6分子的空腔结构及其对PEO分子链间氢键作用的干扰,促进CO2的溶解和扩散过程。CO2渗透系数提升至PEO膜的4.2倍[636 Barrer(1 Barrer=3.35×10-16 mol·m·m-2·s·Pa)],同时保持了较高的分离选择性(约50),且膜性能在300 h的连续测试中保持稳定。
中图分类号:
张建民, 何美贵, 贾万鑫, 赵静, 金万勤. 聚氧化乙烯/冠醚共混膜及其二氧化碳分离性能[J]. 化工学报, 2025, 76(9): 4862-4871.
Jianmin ZHANG, Meigui HE, Wanxin JIA, Jing ZHAO, Wanqin JIN. Poly(ethylene oxide)/crown ether blend membrane and performance for CO2 separation[J]. CIESC Journal, 2025, 76(9): 4862-4871.
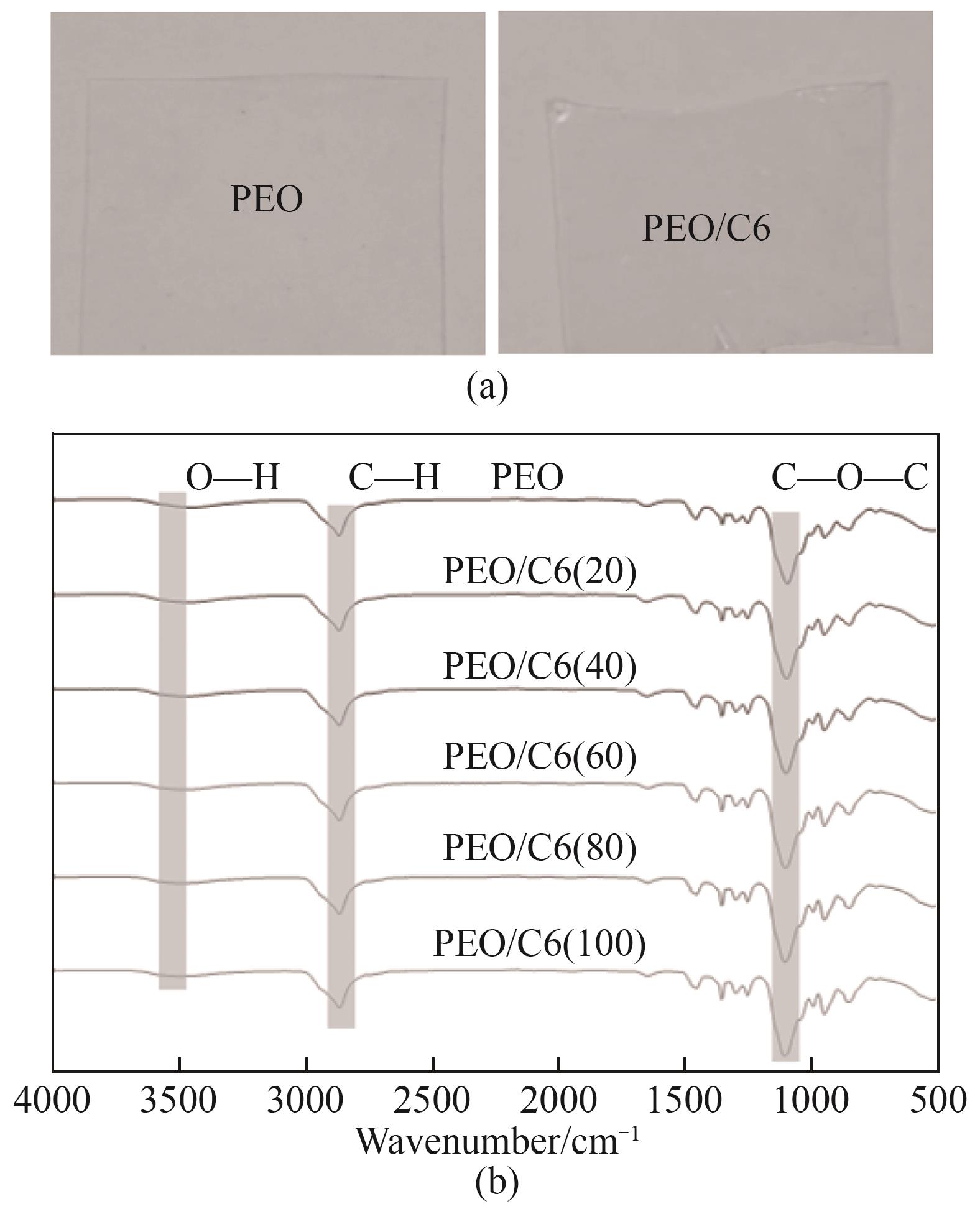
图2 (a)PEO膜和PEO/C6共混膜的照片;(b)PEO膜和PEO/C6共混膜的傅里叶变换红外光谱
Fig.2 (a) Digital photos of PEO and PEO/C6 membranes; (b) FTIR spectra of PEO and PEO/C6 membranes
| 膜 | w | Tg/℃ | Tc/℃ | Tm1/℃ | Tm2/℃ | ΔHm/(J/g) | Xc/% |
|---|---|---|---|---|---|---|---|
| PEO | 0 | -47.57 | — | — | — | — | — |
| PEO/C6(20) | 0.167 | -51.79 | — | — | — | — | — |
| PEO/C6(40) | 0.286 | -54.05 | — | — | — | — | — |
| PEO/C6(60) | 0.375 | -55.62 | -5.7 | 6.58 | 20.64 | 6.89 | 4.22 |
| PEO/C6(80) | 0.444 | -57.03 | -6.02 | 7.35 | 16.73 | 18.03 | 11.03 |
| PEO/C6(100) | 0.500 | -58.67 | -9.17 | — | 15.38 | 34.11 | 20.88 |
表1 PEO膜和PEO/C6膜的Tg、Tc、Tm、ΔHm和Xc
Table 1 Tg, Tc, Tm, ΔHm and Xc of PEO and PEO/C6 membranes
| 膜 | w | Tg/℃ | Tc/℃ | Tm1/℃ | Tm2/℃ | ΔHm/(J/g) | Xc/% |
|---|---|---|---|---|---|---|---|
| PEO | 0 | -47.57 | — | — | — | — | — |
| PEO/C6(20) | 0.167 | -51.79 | — | — | — | — | — |
| PEO/C6(40) | 0.286 | -54.05 | — | — | — | — | — |
| PEO/C6(60) | 0.375 | -55.62 | -5.7 | 6.58 | 20.64 | 6.89 | 4.22 |
| PEO/C6(80) | 0.444 | -57.03 | -6.02 | 7.35 | 16.73 | 18.03 | 11.03 |
| PEO/C6(100) | 0.500 | -58.67 | -9.17 | — | 15.38 | 34.11 | 20.88 |

图5 (a)PEO膜和PEO/C6共混膜的密度及加性模型的拟合曲线;(b)PEO膜和PEO/C6共混膜的自由体积分数
Fig.5 (a) The linear fitting of 1/ρ with w2 according to additive model; (b) Free volume fraction of PEO and PEO/C6 membranes
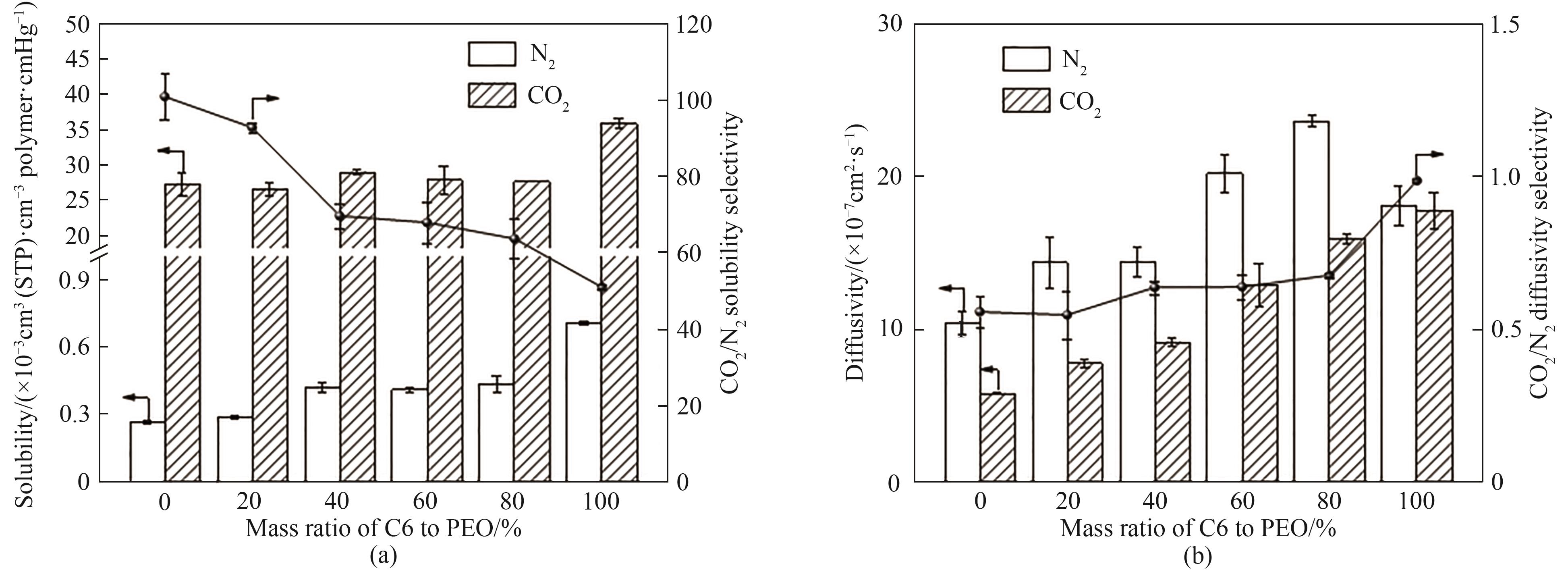
图8 PEO和PEO/C6共混膜的溶解系数和溶解选择性(a)及扩散系数和扩散选择性(b)
Fig.8 Solubility and solubility selectivity (a), diffusivity and diffusivity selectivity (b) of PEO and PEO/C6 membranes
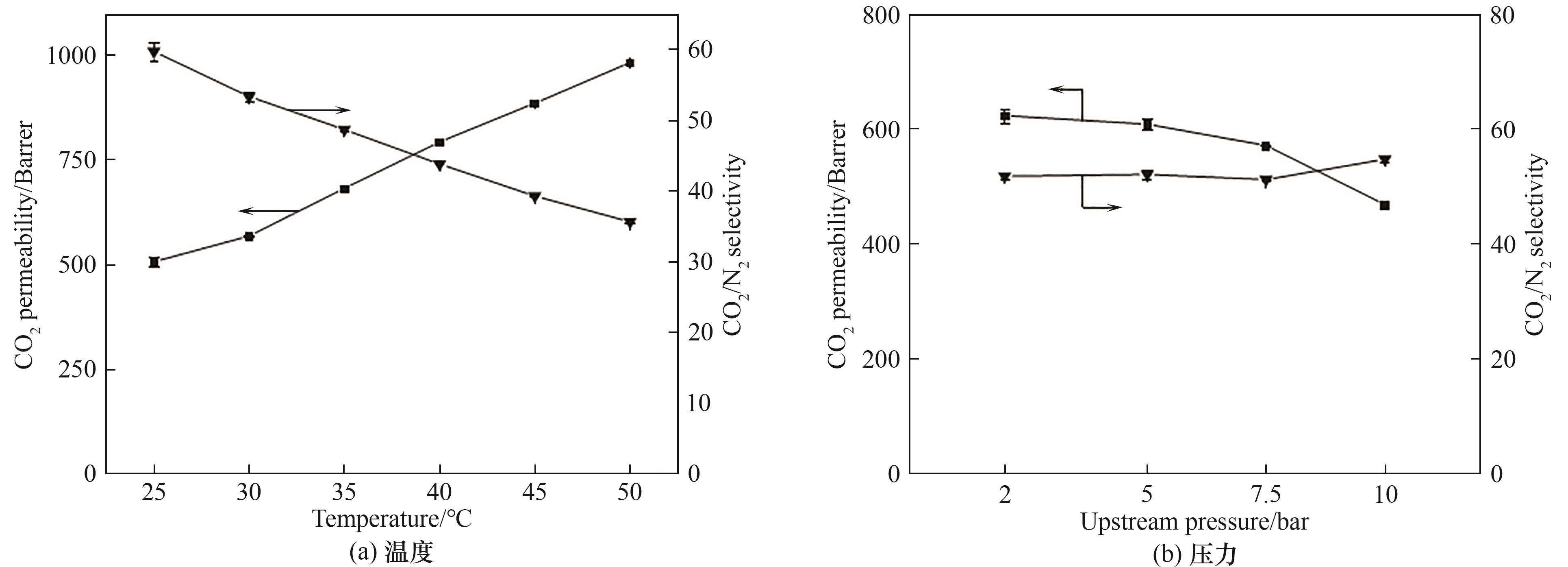
图9 操作条件对 PEO/C6(100)共混膜分离性能的影响(混合气体,CO2∶N2 = 15∶85,体积比)
Fig.9 Effect of operating conditions on the separation performance of PEO/C6(100) membranes (mixed gas, CO2∶N2 = 15∶85, volume ratio)

图10 (a)PEO/C6(100)共混膜的长期稳定性测试(混合气体,CO2∶N2 = 15∶85,体积比);(b)PEO/C6(100)共混膜与目前报道的PEO基聚合物膜的CO2/N2分离性能对比
Fig.10 (a) Long-term stability test of PEO/C6(100) membranes (mixed gas, CO2∶N2 = 15∶85, volume ratio); (b) Comparison of CO2/N2 separation performance of PEO/C6(100) membranes with reported PEO-based polymer membranes
| [1] | Wei Y M, Kang J N, Liu L C, et al. A proposed global layout of carbon capture and storage in line with a 2℃ climate target[J]. Nature Climate Change, 2021, 11: 112-118. |
| [2] | Liang C Z, Chung T S, Lai J Y. A review of polymeric composite membranes for gas separation and energy production[J]. Progress in Polymer Science, 2019, 97: 101141. |
| [3] | Hu C C, Lin C H, Chiao Y H, et al. Mixing effect of ligand on carbon dioxide capture behavior of zeolitic imidazolate framework/poly(amide-b-ethylene oxide) mixed matrix membranes[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15341-15348. |
| [4] | Lilleby Helberg R M, Dai Z D, Ansaloni L, et al. PVA/PVP blend polymer matrix for hosting carriers in facilitated transport membranes: synergistic enhancement of CO2 separation performance[J]. Green Energy & Environment, 2020, 5(1): 59-68. |
| [5] | Roussanaly S, Anantharaman R, Lindqvist K, et al. Membrane properties required for post-combustion CO2 capture at coal-fired power plants[J]. Journal of Membrane Science, 2016, 511: 250-264. |
| [6] | Han H, Scofield J M P, Gurr P A, et al. Ultrathin membrane with robust and superior CO2 permeance by precision control of multilayer structures[J]. Chemical Engineering Journal, 2023, 462: 142087. |
| [7] | Liu S L, Shao L, Chua M L, et al. Recent progress in the design of advanced PEO-containing membranes for CO2 removal[J]. Progress in Polymer Science, 2013, 38(7): 1089-1120. |
| [8] | Zhu B, Jiang X, He S S, et al. Rational design of poly(ethylene oxide) based membranes for sustainable CO2 capture[J]. Journal of Materials Chemistry A, 2020, 8(46): 24233-24252. |
| [9] | Kazarian S G, Vincent M F, Bright F V, et al. Specific intermolecular interaction of carbon dioxide with polymers[J]. Journal of the American Chemical Society, 1996, 118(7): 1729-1736. |
| [10] | Lin H, Freeman B D. Gas solubility, diffusivity and permeability in poly(ethylene oxide)[J]. Journal of Membrane Science, 2004, 239(1): 105-117. |
| [11] | Shao L, Quan S, Cheng X Q, et al. Developing cross-linked poly(ethylene oxide) membrane by the novel reaction system for H2 purification[J]. International Journal of Hydrogen Energy, 2013, 38(12): 5122-5132. |
| [12] | Chen Y, He M G, Zhang J M, et al. Design of ultrathin cross-linked poly(ethylene oxide) selective layer for high-performance CO2 capture[J]. Chemical Engineering Journal, 2023, 478: 147530. |
| [13] | Quan S, Tang Y P, Wang Z X, et al. PEG-imbedded PEO membrane developed by a novel highly efficient strategy toward superior gas transport performance[J]. Macromolecular Rapid Communications, 2015, 36(5): 490-495. |
| [14] | Li S W, Jiang X, Yang Q, et al. Effects of amino functionalized polyhedral oligomeric silsesquioxanes on cross-linked poly(ethylene oxide) membranes for highly-efficient CO2 separation[J]. Chemical Engineering Research and Design, 2017, 122: 280-288. |
| [15] | Kargari A, Rezaeinia S. State-of-the-art modification of polymeric membranes by PEO and PEG for carbon dioxide separation: a review of the current status and future perspectives[J]. Journal of Industrial and Engineering Chemistry, 2020, 84: 1-22. |
| [16] | Amooghin A E, Sanaeepur H, Moghadassi A, et al. Modification of ABS membrane by PEG for capturing carbon dioxide from CO2/N2 streams[J]. Separation Science and Technology, 2010, 45(10): 1385-1394. |
| [17] | Hamrahi Z, Kargari A. Modification of polycarbonate membrane by polyethylene glycol for CO2/CH4 separation[J]. Separation Science and Technology, 2017, 52(3): 544-556. |
| [18] | Castro-Muñoz R, Fíla V, Martin-Gil V, et al. Enhanced CO2 permeability in Matrimid®5218 mixed matrix membranes for separating binary CO2/CH4 mixtures[J]. Separation and Purification Technology, 2019, 210: 553-562. |
| [19] | Car A, Stropnik C, Yave W, et al. PEG modified poly(amide-b-ethylene oxide) membranes for CO2 separation[J]. Journal of Membrane Science, 2008, 307(1): 88-95. |
| [20] | Wu X M, Zhang Q G, Lin P J, et al. Towards enhanced CO2 selectivity of the PIM-1 membrane by blending with polyethylene glycol[J]. Journal of Membrane Science, 2015, 493: 147-155. |
| [21] | Sadeghi M, Chenar M P, Rahimian M, et al. Gas permeation properties of polyvinylchloride/polyethyleneglycol blend membranes[J]. Journal of Applied Polymer Science, 2008, 110(2): 1093-1098. |
| [22] | Ben Hamouda S, Roudesli S. Transport properties of PVA/PEI/PEG composite membranes: sorption and permeation characterizations[J]. Central European Journal of Chemistry, 2008, 6(4): 634-640. |
| [23] | Huang L, Liu J Y, Lin H Q. Thermally stable, homogeneous blends of cross-linked poly(ethylene oxide) and crown ethers with enhanced CO2 permeability[J]. Journal of Membrane Science, 2020, 610: 118253. |
| [24] | Luan B, Elmegreen B, Kuroda M A, et al. Crown nanopores in graphene for CO2 capture and filtration[J]. ACS Nano, 2022, 16(4): 6274-6281. |
| [25] | Wu D Y, Yi C H, Wang Y X, et al. Preparation and gas permeation of crown ether-containing co-polyimide with enhanced CO2 selectivity[J]. Journal of Membrane Science, 2018, 551: 191-203. |
| [26] | Houben M, Borneman Z, Nijmeijer K. Plasticization behavior of crown-ether containing polyimide membranes for the separation of CO2 [J]. Separation and Purification Technology, 2021, 255: 117307. |
| [27] | Zhang Z N, Zhu H, Jin H, et al. Restricting linker rotation in nanocages of ZIF-8 membranes using crown ether “molecular locks” for enhanced propylene/propane separation[J]. Angewandte Chemie International Edition, 2025, 64(3): e202415023. |
| [28] | Zheng S, Bi S, Fu Y B, et al. 3D crown ether covalent organic framework as interphase layer toward high-performance lithium metal batteries[J]. Advanced Materials, 2024, 36(21): 2313076. |
| [29] | Liu Z C, Nalluri S K M, Stoddart J F. Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes[J]. Chemical Society Reviews, 2017, 46(9): 2459-2478. |
| [30] | Li H W, Wang Y, Li T Y, et al. Nanofiltration membrane with crown ether as exclusive Li+ transport channels achieving efficient extraction of lithium from salt lake brine[J]. Chemical Engineering Journal, 2022, 438: 135658. |
| [31] | Jiang X, He S S, Li S W, et al. Penetrating chains mimicking plant root branching to build mechanically robust, ultra-stable CO2-philic membranes for superior carbon capture[J]. Journal of Materials Chemistry A, 2019, 7(28): 16704-16711. |
| [32] | Zhu B, He S S, Wu Y D, et al. One-step synthesis of structurally stable CO2-philic membranes with ultra-high PEO loading for enhanced carbon capture[J]. Engineering, 2023, 26: 220-228. |
| [33] | Sun W S, Yin M J, Zhang W H, et al. Tailor-made microstructures lead to high-performance robust PEO membrane for CO2 capture via green fabrication technique[J]. Green Energy & Environment, 2023, 8(5): 1389-1397. |
| [34] | Dai Z D, Deng L Y. Membranes for CO2 capture and separation: progress in research and development for industrial applications[J]. Separation and Purification Technology, 2024, 335: 126022. |
| [35] | Li S, Chang S M, Yin M J, et al. Build up ‘highway’ in membrane via solvothermal annealing for high-efficient CO2 capture[J]. Journal of Membrane Science, 2022, 652: 120444. |
| [36] | Zhu B, Yang Y, Wang K F, et al. Chemical topology molecular engineering of CO2-philic membranes toward highly efficient carbon capture[J]. Journal of Membrane Science, 2023, 685: 121917. |
| [37] | Hu L Q, Liu J Y, Zhu L X, et al. Highly permeable mixed matrix materials comprising ZIF-8 nanoparticles in rubbery amorphous poly(ethylene oxide) for CO2 capture[J]. Separation and Purification Technology, 2018, 205: 58-65. |
| [38] | Lin H Q, Freeman B D. Materials selection guidelines for membranes that remove CO2 from gas mixtures[J]. Journal of Molecular Structure, 2005, 739(1/2/3): 57-74. |
| [39] | Krevelen D W V. Properties of Polymers[M]. Nuenhuis K T. 4th. Oxford: Elsevier’s Science & Technology Rights Department, 2009: 72-76. |
| [40] | Kim N U, Park B J, Park M S, et al. Semi-interpenetrating polymer network membranes based on a self-crosslinkable comb copolymer for CO2 capture[J]. Chemical Engineering Journal, 2019, 360: 1468-1476. |
| [41] | Liu J Y, Zhang G Y, Clark K, et al. Maximizing ether oxygen content in polymers for membrane CO2 removal from natural gas[J]. ACS Applied Materials & Interfaces, 2019, 11(11): 10933-10940. |
| [42] | Quan S, Li S W, Wang Z X, et al. A bio-inspired CO2-philic network membrane for enhanced sustainable gas separation[J]. Journal of Materials Chemistry A, 2015, 3(26): 13758-13766. |
| [43] | Li S W, Jiang X, Yang X B, et al. Nanoporous framework “reservoir” maximizing low-molecular-weight enhancer impregnation into CO2-philic membranes for highly-efficient CO2 capture[J]. Journal of Membrane Science, 2019, 570: 278-285. |
| [44] | Liu J Y, Zhang S Z, Jiang D E, et al. Highly polar but amorphous polymers with robust membrane CO2/N2 separation performance[J]. Joule, 2019, 3(8): 1881-1894. |
| [1] | 臧子晴, 李修真, 谈莹莹, 刘晓庆. 分凝器对两级分离自复叠制冷循环特性影响研究[J]. 化工学报, 2025, 76(S1): 17-25. |
| [2] | 黄灏, 王文, 贺隆坤. LNG船薄膜型液货舱预冷过程模拟与分析[J]. 化工学报, 2025, 76(S1): 187-194. |
| [3] | 黄博, 黄灏, 王文, 贺隆坤. 薄膜型LNG船液货舱温度场计算分析[J]. 化工学报, 2025, 76(S1): 195-204. |
| [4] | 孙云龙, 徐肖肖, 黄永方, 郭纪超, 陈卫卫. 水平光滑管内CO2流动沸腾的非绝热可视化研究[J]. 化工学报, 2025, 76(S1): 230-236. |
| [5] | 郭纪超, 徐肖肖, 孙云龙. 基于植物工厂中的CO2浓度气流模拟及优化研究[J]. 化工学报, 2025, 76(S1): 237-245. |
| [6] | 裴星亮, 叶翠平, 裴赢丽, 李文英. 碱改性MIL-53(Cr)选择性吸附分离二甲苯异构体[J]. 化工学报, 2025, 76(S1): 258-267. |
| [7] | 李银龙, 刘国强, 晏刚. 分馏与闪蒸分离耦合自复叠制冷循环性能分析[J]. 化工学报, 2025, 76(S1): 26-35. |
| [8] | 孔繁臣, 张硕, 唐明生, 邹慧明, 胡舟航, 田长青. 二氧化碳直线压缩机气体轴承模拟[J]. 化工学报, 2025, 76(S1): 281-288. |
| [9] | 何婷, 张开, 林文胜, 陈利琼, 陈家富. 沼气超临界压力低温脱碳-液化耦合流程研究[J]. 化工学报, 2025, 76(S1): 418-425. |
| [10] | 李文龙, 常程, 吴小林, 姬忠礼. 油水聚结过滤材料中的液体分布特性及过程压降演化研究[J]. 化工学报, 2025, 76(9): 4850-4861. |
| [11] | 王一飞, 李玉星, 欧阳欣, 赵雪峰, 孟岚, 胡其会, 殷布泽, 郭雅琦. 基于裂尖减压特性的CO2管道断裂扩展数值计算[J]. 化工学报, 2025, 76(9): 4683-4693. |
| [12] | 郭旭, 贾继宁, 姚克俭. 基于优化CNN-BiLSTM神经网络的间歇精馏过程建模[J]. 化工学报, 2025, 76(9): 4613-4629. |
| [13] | 王杰, 林渠成, 张先明. 基于分解算法的混合气体多级膜分离系统全局优化[J]. 化工学报, 2025, 76(9): 4670-4682. |
| [14] | 王钰, 冯英楠, 王涛, 赵之平. 原位生长构筑纳米复合纳滤膜:膜制备与应用[J]. 化工学报, 2025, 76(9): 4723-4736. |
| [15] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号