• •
收稿日期:2025-10-10
修回日期:2025-11-03
出版日期:2025-11-04
通讯作者:
马庆明
作者简介:杜福泰(2000—),男,硕士研究生,15910193275@163.com
基金资助:
Futai DU( ), Huan XIN, Huiyuan ZHENG, Weijiang WANG, Qingming MA(
), Huan XIN, Huiyuan ZHENG, Weijiang WANG, Qingming MA( )
)
Received:2025-10-10
Revised:2025-11-03
Online:2025-11-04
Contact:
Qingming MA
摘要:
本研究采用双水相微流控技术成功制备了用于固定化酶的海藻酸钙/羧甲基壳聚糖(CA/CMCS)复合微球,并通过中心旋转组合设计(CCRD)系统优化了微球双水相微流控制备过程的处方参数及制备工艺。对微球形貌及固定化酶的酶学性质的研究结果显示,酶被成功负载于微球内部,固定化后其热稳定性与pH稳定性显著提高,重复使用性和储存稳定性也明显优于游离酶,同时在催化动力学方面表现出更优的性能。进一步地,制备的固定化β-半乳糖苷酶(β-Gal)/葡萄糖氧化酶(GOD)/辣根过氧化物酶(HRP)三酶体系的复合微球能够高效催化多酶级联反应,且提高底物浓度可显著提升反应速率;此外,制备的固定化超氧化物歧化酶(SOD)/过氧化氢酶(CAT)双酶体系的复合微球表现出优异的活性氧清除能力,在重复使用8次后清除率仍可维持在60%以上,显示出良好的操作稳定性与应用前景。上述研究表明,利用双水相微流控技术制备的复合固定化酶微球表现出了用于生物催化应用的优势与潜力。
中图分类号:
杜福泰, 辛欢, 郑惠元, 王伟江, 马庆明. 双水相微流控制备复合固定化酶微球及其生物催化应用研究[J]. 化工学报, DOI: 10.11949/0438-1157.20251118.
Futai DU, Huan XIN, Huiyuan ZHENG, Weijiang WANG, Qingming MA. Microfluidic aqueous two-phase system-based fabrication of immobilized enzyme composite microspheres for biocatalytic applications[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251118.
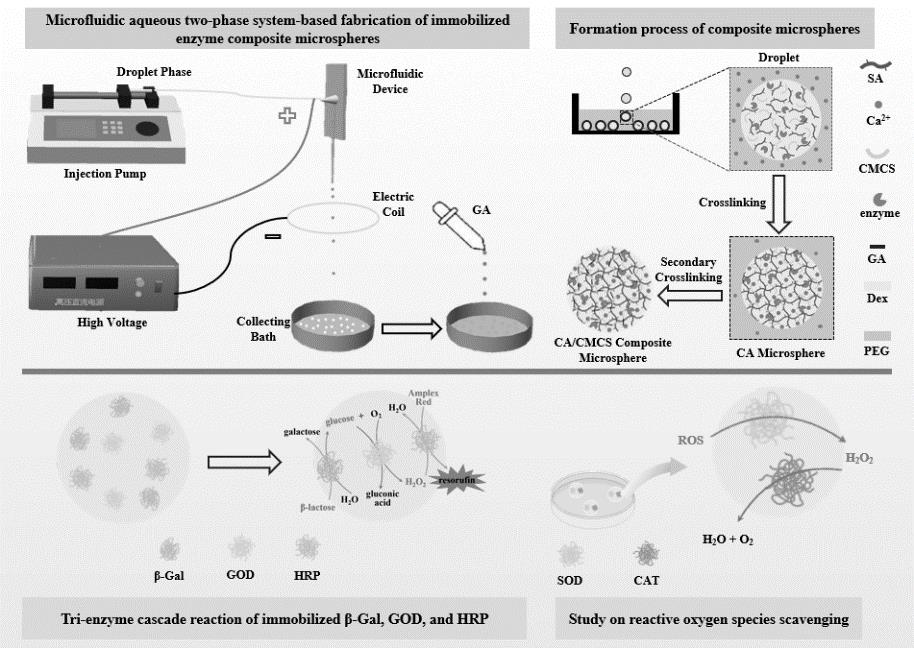
图1 双水相微流控制备复合固定化酶微球及应用
Fig.1 Microfluidic aqueous two-phase system-based fabrication of immobilized enzyme composite microspheres and their applications
| 试剂/处理 | 空白对照 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| 丙酮酸标准溶液/mL | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| PBS(0.2 M,pH 7.4) | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 |
| 各试管摇匀后,置于37℃恒温磁力搅拌器,保温10 min | ||||||
| 2,4-二硝基苯肼/mL | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| 各试管摇匀后,置于37℃恒温磁力搅拌器,保温20 min | ||||||
| 0.4 M NaOH/mL | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| 充分摇匀后,立即在520 nm波长下比色 | ||||||
表1 丙酮酸标准曲线制作表
Table 1 Preparation of the standard curve for pyruvate
| 试剂/处理 | 空白对照 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| 丙酮酸标准溶液/mL | 0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| PBS(0.2 M,pH 7.4) | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 | 0.1 |
| 各试管摇匀后,置于37℃恒温磁力搅拌器,保温10 min | ||||||
| 2,4-二硝基苯肼/mL | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| 各试管摇匀后,置于37℃恒温磁力搅拌器,保温20 min | ||||||
| 0.4 M NaOH/mL | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| 充分摇匀后,立即在520 nm波长下比色 | ||||||
| 水平 | 实验因素 | |||||
|---|---|---|---|---|---|---|
| 海藻酸钠浓度/% | 羧甲基壳聚糖浓度/% | 氯化钙浓度/% | 引发时间/min | 戊二醛浓度/% | 交联时间/h | |
| 1 | 0.6 | 0.2 | 0.5 | 10 | 0.1 | 1 |
| 2 | 0.9 | 0.3 | 1.0 | 20 | 0.3 | 2 |
| 3 | 1.2 | 0.4 | 1.5 | 30 | 0.5 | 4 |
| 4 | 1.5 | 0.5 | 2.0 | 40 | 0.75 | 6 |
| 5 | 1.8 | 0.6 | 2.5 | 50 | 1.0 | 8 |
表2 处方优化的单因素实验设计表
Table 2 Single-factor experimental design table for prescription optimization
| 水平 | 实验因素 | |||||
|---|---|---|---|---|---|---|
| 海藻酸钠浓度/% | 羧甲基壳聚糖浓度/% | 氯化钙浓度/% | 引发时间/min | 戊二醛浓度/% | 交联时间/h | |
| 1 | 0.6 | 0.2 | 0.5 | 10 | 0.1 | 1 |
| 2 | 0.9 | 0.3 | 1.0 | 20 | 0.3 | 2 |
| 3 | 1.2 | 0.4 | 1.5 | 30 | 0.5 | 4 |
| 4 | 1.5 | 0.5 | 2.0 | 40 | 0.75 | 6 |
| 5 | 1.8 | 0.6 | 2.5 | 50 | 1.0 | 8 |
| 水平 | 实验因素 | |
|---|---|---|
| 流速/(mL/h) | 电压/kV | |
| 1 | 10 | 5 |
| 2 | 15 | 7 |
| 3 | 20 | 9 |
| 4 | 25 | 11 |
| 5 | 30 | 13 |
表3 工艺优化的单因素实验设计表
Table 3 Single-factor experimental design table for process optimization
| 水平 | 实验因素 | |
|---|---|---|
| 流速/(mL/h) | 电压/kV | |
| 1 | 10 | 5 |
| 2 | 15 | 7 |
| 3 | 20 | 9 |
| 4 | 25 | 11 |
| 5 | 30 | 13 |
| 离心管编号 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| PBS(0.05M,pH7.8) | 2.0 | 2.0 | 2.0 | 2.0 |
EDTA-Na2 (1 μmol/mL) | 0.2 | 0.2 | 0.2 | 0.2 |
L-甲硫氨酸 (0.13 mmol/mL) | 0.2 | 0.2 | 0.2 | 0.2 |
| NBT(0.75 μmol/mL) | 0.2 | 0.2 | 0.2 | 0.2 |
| 纯水 | 0.1 | 0.1 | 0.08 | 0.08 |
| 核黄素(2 μmol/mL) | 0.3 | 0.3 | 0.3 | 0.3 |
| 酶液 | 0 | 0 | 0.02 | 0.02 |
| 总体积 | 3.0 | 3.0 | 3.0 | 3.0 |
表4 SOD活力测定实验加样方法
Table 4 Sample addition method for SOD activity assay experiment
| 离心管编号 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| PBS(0.05M,pH7.8) | 2.0 | 2.0 | 2.0 | 2.0 |
EDTA-Na2 (1 μmol/mL) | 0.2 | 0.2 | 0.2 | 0.2 |
L-甲硫氨酸 (0.13 mmol/mL) | 0.2 | 0.2 | 0.2 | 0.2 |
| NBT(0.75 μmol/mL) | 0.2 | 0.2 | 0.2 | 0.2 |
| 纯水 | 0.1 | 0.1 | 0.08 | 0.08 |
| 核黄素(2 μmol/mL) | 0.3 | 0.3 | 0.3 | 0.3 |
| 酶液 | 0 | 0 | 0.02 | 0.02 |
| 总体积 | 3.0 | 3.0 | 3.0 | 3.0 |
| 离心管编号 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
PBS (0.05M,pH7.8) | 2.0 | 2.0 | 2.0 | 2.0 |
| 酶液 | 0 | 0 | 0.08 | 0.08 |
| H2O2(0.05M) | 0.7 | 0.7 | 0.7 | 0.7 |
| 纯水 | 0.3 | 0.3 | 0.22 | 0.22 |
| 总体积 | 3.0 | 3.0 | 3.0 | 3.0 |
表5 CAT活力测定实验加样方法
Table 5 Sample addition method for CAT activity assay experiment
| 离心管编号 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
PBS (0.05M,pH7.8) | 2.0 | 2.0 | 2.0 | 2.0 |
| 酶液 | 0 | 0 | 0.08 | 0.08 |
| H2O2(0.05M) | 0.7 | 0.7 | 0.7 | 0.7 |
| 纯水 | 0.3 | 0.3 | 0.22 | 0.22 |
| 总体积 | 3.0 | 3.0 | 3.0 | 3.0 |
| 分组 | 样品 |
|---|---|
| 空白组 | 0.2 mL ABTS•+ + 0.4 mL PBS |
| 对照组 | 0.2 mL ABTS•+ + 0.2 mL邻苯三酚 + 0.2 mL PBS |
| SOD组 | 0.2 mL ABTS•+ + 0.2 mL邻苯三酚 + 0.1 mL SOD + 0.1 mL PBS |
| SOD/CAT组 | 0.2 mL ABTS•+ + 0.2 mL邻苯三酚 + 0.2 mL SOD/CAT |
| 固定化SOD/CAT组 | 0.2 mL ABTS•+ + 0.2 mL邻苯三酚 + 当量的固定化SOD/CAT + 0.2 mL PBS |
表6 SOD/CAT体系清除活性氧实验的加样方法
Table 6 Sample addition method for the experiment of eliminating reactive oxygen species using the SOD/CAT system
| 分组 | 样品 |
|---|---|
| 空白组 | 0.2 mL ABTS•+ + 0.4 mL PBS |
| 对照组 | 0.2 mL ABTS•+ + 0.2 mL邻苯三酚 + 0.2 mL PBS |
| SOD组 | 0.2 mL ABTS•+ + 0.2 mL邻苯三酚 + 0.1 mL SOD + 0.1 mL PBS |
| SOD/CAT组 | 0.2 mL ABTS•+ + 0.2 mL邻苯三酚 + 0.2 mL SOD/CAT |
| 固定化SOD/CAT组 | 0.2 mL ABTS•+ + 0.2 mL邻苯三酚 + 当量的固定化SOD/CAT + 0.2 mL PBS |
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 9788.26 | 27 | 362.53 | 29.41 | < 0.0001 | significant |
| A-海藻酸钠浓度 | 865.4 | 1 | 865.4 | 70.19 | < 0.0001 | |
| B-羧甲基壳聚糖浓度 | 1.27 | 1 | 1.27 | 0.1028 | 0.7516 | |
| C-氯化钙浓度 | 464.58 | 1 | 464.58 | 37.68 | < 0.0001 | |
| D-引发时间 | 19.61 | 1 | 19.61 | 1.59 | 0.2204 | |
| E-戊二醛浓度 | 0.5046 | 1 | 0.5046 | 0.0409 | 0.8415 | |
| F-交联时间 | 7.2 | 1 | 7.2 | 0.5844 | 0.4527 | |
| AB | 18.79 | 1 | 18.79 | 1.52 | 0.23 | |
| AC | 117.96 | 1 | 117.96 | 9.57 | 0.0053 | |
| AD | 0.0024 | 1 | 0.0024 | 0.0002 | 0.9889 | |
| AE | 0.0015 | 1 | 0.0015 | 0.0001 | 0.9913 | |
| AF | 0.02 | 1 | 0.02 | 0.0016 | 0.9682 | |
| BC | 14.28 | 1 | 14.28 | 1.16 | 0.2934 | |
| BD | 16.97 | 1 | 16.97 | 1.38 | 0.2533 | |
| BE | 0.0512 | 1 | 0.0512 | 0.0042 | 0.9492 | |
| BF | 0.0528 | 1 | 0.0528 | 0.0043 | 0.9484 | |
| CD | 5.36 | 1 | 5.36 | 0.435 | 0.5164 | |
| CE | 0.0024 | 1 | 0.0024 | 0.0002 | 0.9889 | |
| CF | 0.2278 | 1 | 0.2278 | 0.0185 | 0.8931 | |
| DE | 1.07 | 1 | 1.07 | 0.0864 | 0.7715 | |
| DF | 0.001 | 1 | 0.001 | 0.0001 | 0.9929 | |
| EF | 5.41 | 1 | 5.41 | 0.439 | 0.5145 | |
| A² | 3983.5 | 1 | 3983.5 | 323.11 | < 0.0001 | |
| B² | 627.56 | 1 | 627.56 | 50.9 | < 0.0001 | |
| C² | 3384.32 | 1 | 3384.32 | 274.51 | < 0.0001 | |
| D² | 1359.03 | 1 | 1359.03 | 110.23 | < 0.0001 | |
| E² | 258.46 | 1 | 258.46 | 20.96 | 0.0001 | |
| F² | 621.71 | 1 | 621.71 | 50.43 | < 0.0001 | |
| Residual | 271.23 | 22 | 12.33 | |||
| Lack of Fit | 246.07 | 15 | 18.08 | 5.69 | 0.0846 | not significant |
| Pure Error | 55.82 | 7 | 12.59 | |||
| Cor Total | 10059.49 | 49 | ||||
| 确定系数R2=0.9730 | 确定校正系数Radj2=0.9399 | |||||
| 变异系数C.V.%=5.40 | 信噪比=22.1826 | |||||
表7 以酶相对活性(处方优化)为响应值的CCRD实验模型的方差分析结果
Table 7 ANOVA results of CCRD experimental models with relative activity(formulation optimization) as the response value
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 9788.26 | 27 | 362.53 | 29.41 | < 0.0001 | significant |
| A-海藻酸钠浓度 | 865.4 | 1 | 865.4 | 70.19 | < 0.0001 | |
| B-羧甲基壳聚糖浓度 | 1.27 | 1 | 1.27 | 0.1028 | 0.7516 | |
| C-氯化钙浓度 | 464.58 | 1 | 464.58 | 37.68 | < 0.0001 | |
| D-引发时间 | 19.61 | 1 | 19.61 | 1.59 | 0.2204 | |
| E-戊二醛浓度 | 0.5046 | 1 | 0.5046 | 0.0409 | 0.8415 | |
| F-交联时间 | 7.2 | 1 | 7.2 | 0.5844 | 0.4527 | |
| AB | 18.79 | 1 | 18.79 | 1.52 | 0.23 | |
| AC | 117.96 | 1 | 117.96 | 9.57 | 0.0053 | |
| AD | 0.0024 | 1 | 0.0024 | 0.0002 | 0.9889 | |
| AE | 0.0015 | 1 | 0.0015 | 0.0001 | 0.9913 | |
| AF | 0.02 | 1 | 0.02 | 0.0016 | 0.9682 | |
| BC | 14.28 | 1 | 14.28 | 1.16 | 0.2934 | |
| BD | 16.97 | 1 | 16.97 | 1.38 | 0.2533 | |
| BE | 0.0512 | 1 | 0.0512 | 0.0042 | 0.9492 | |
| BF | 0.0528 | 1 | 0.0528 | 0.0043 | 0.9484 | |
| CD | 5.36 | 1 | 5.36 | 0.435 | 0.5164 | |
| CE | 0.0024 | 1 | 0.0024 | 0.0002 | 0.9889 | |
| CF | 0.2278 | 1 | 0.2278 | 0.0185 | 0.8931 | |
| DE | 1.07 | 1 | 1.07 | 0.0864 | 0.7715 | |
| DF | 0.001 | 1 | 0.001 | 0.0001 | 0.9929 | |
| EF | 5.41 | 1 | 5.41 | 0.439 | 0.5145 | |
| A² | 3983.5 | 1 | 3983.5 | 323.11 | < 0.0001 | |
| B² | 627.56 | 1 | 627.56 | 50.9 | < 0.0001 | |
| C² | 3384.32 | 1 | 3384.32 | 274.51 | < 0.0001 | |
| D² | 1359.03 | 1 | 1359.03 | 110.23 | < 0.0001 | |
| E² | 258.46 | 1 | 258.46 | 20.96 | 0.0001 | |
| F² | 621.71 | 1 | 621.71 | 50.43 | < 0.0001 | |
| Residual | 271.23 | 22 | 12.33 | |||
| Lack of Fit | 246.07 | 15 | 18.08 | 5.69 | 0.0846 | not significant |
| Pure Error | 55.82 | 7 | 12.59 | |||
| Cor Total | 10059.49 | 49 | ||||
| 确定系数R2=0.9730 | 确定校正系数Radj2=0.9399 | |||||
| 变异系数C.V.%=5.40 | 信噪比=22.1826 | |||||
| SA浓度/% | CMCS浓度/% | CaCl2浓度/% | 引发时间/min | 戊二醛浓度/% | 交联时间/h | 相对酶活性/% |
|---|---|---|---|---|---|---|
| 1.185 | 0.400 | 1.506 | 30.000 | 0.282 | 4.000 | 92.730 |
表8 CA/CMCS微球的最优制备处方
Table 8 The optimal prescription for the fabrication of CA/CMCS microspheres
| SA浓度/% | CMCS浓度/% | CaCl2浓度/% | 引发时间/min | 戊二醛浓度/% | 交联时间/h | 相对酶活性/% |
|---|---|---|---|---|---|---|
| 1.185 | 0.400 | 1.506 | 30.000 | 0.282 | 4.000 | 92.730 |
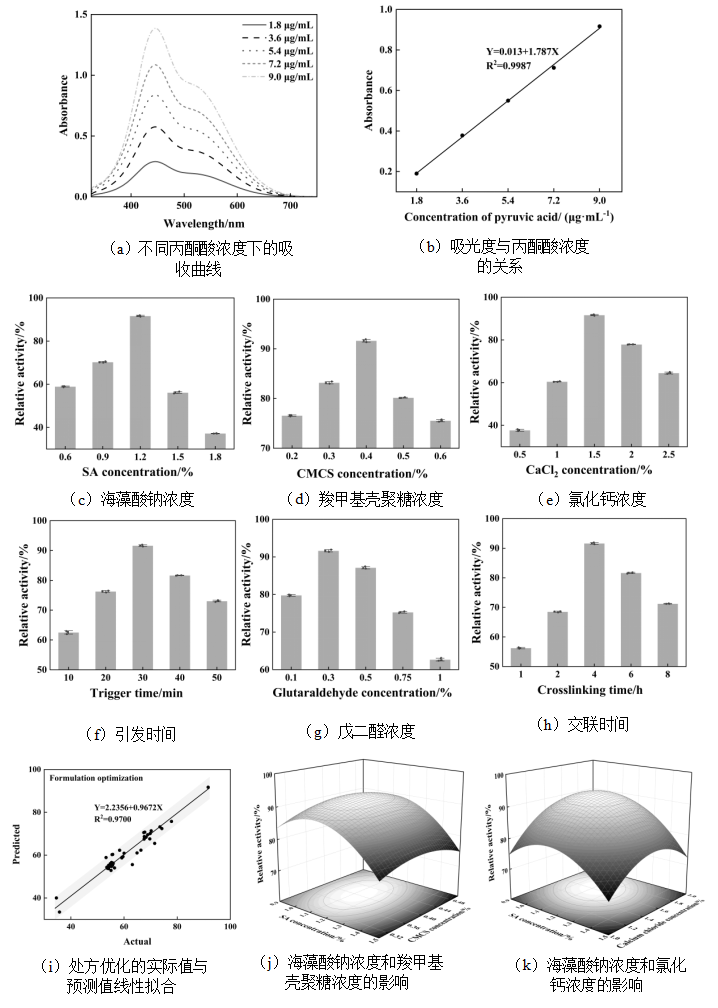
图2 模型反应的定量分析以及制备负载LOX复合微球的处方优化
Fig.2 Quantitative analysis of model response and formulation optimization for the fabrication of LOX-loaded composite microspheres
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 31.50 | 5 | 6.30 | 272.07 | < 0.0001 | significant |
| A-Flow rate | 0.0842 | 1 | 0.0842 | 3.64 | 0.0982 | |
| B-Voltage | 2.57 | 1 | 2.57 | 111.08 | < 0.0001 | |
| AB | 0.0001 | 1 | 0.0001 | 0.0043 | 0.9494 | |
| A² | 5.64 | 1 | 5.64 | 243.67 | < 0.0001 | |
| B² | 25.86 | 1 | 25.86 | 1116.84 | < 0.0001 | |
| Residual | 0.1621 | 7 | 0.0232 | |||
| Lack of Fit | 0.1247 | 3 | 0.0540 | 3.82 | 0.0930 | not significant |
| Pure Error | 0.0425 | 4 | 0.0108 | |||
| Cor Total | 31.66 | 12 | ||||
| 确定系数R2=0.9949 | 确定校正系数Radj2=0.9912 | |||||
| 变异系数C.V.%=0.1659 | 信噪比=47.2140 | |||||
表9 以酶相对活性(工艺优化)为响应值的CCRD实验模型的方差分析结果
Table 9 ANOVA results of CCRD experimental models with relative activity(process optimization) as the response value
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 31.50 | 5 | 6.30 | 272.07 | < 0.0001 | significant |
| A-Flow rate | 0.0842 | 1 | 0.0842 | 3.64 | 0.0982 | |
| B-Voltage | 2.57 | 1 | 2.57 | 111.08 | < 0.0001 | |
| AB | 0.0001 | 1 | 0.0001 | 0.0043 | 0.9494 | |
| A² | 5.64 | 1 | 5.64 | 243.67 | < 0.0001 | |
| B² | 25.86 | 1 | 25.86 | 1116.84 | < 0.0001 | |
| Residual | 0.1621 | 7 | 0.0232 | |||
| Lack of Fit | 0.1247 | 3 | 0.0540 | 3.82 | 0.0930 | not significant |
| Pure Error | 0.0425 | 4 | 0.0108 | |||
| Cor Total | 31.66 | 12 | ||||
| 确定系数R2=0.9949 | 确定校正系数Radj2=0.9912 | |||||
| 变异系数C.V.%=0.1659 | 信噪比=47.2140 | |||||
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 10362.51 | 5 | 2072.50 | 222.49 | < 0.0001 | significant |
| A-Flow rate | 419.73 | 1 | 419.73 | 45.06 | 0.0003 | |
| B-Voltage | 9280.46 | 1 | 9280.46 | 996.31 | < 0.0001 | |
| AB | 12.60 | 1 | 12.60 | 1.35 | 0.2829 | |
| A² | 3.85 | 1 | 3.85 | 0.4131 | 0.5409 | |
| B² | 622.06 | 1 | 622.06 | 66.78 | < 0.0001 | |
| Residual | 65.20 | 7 | 9.31 | |||
| Lack of Fit | 46.78 | 3 | 21.73 | 4.33 | 0.0975 | not significant |
| Pure Error | 18.42 | 4 | 4.46 | |||
| Cor Total | 10427.72 | 12 | ||||
| 确定系数R2=0.9937 | 确定校正系数Radj2=0.9893 | |||||
| 变异系数C.V.%=0.9657 | 信噪比=47.2140 | |||||
表10 以粒径为响应值的CCRD实验模型的方差分析结果
Table 10 ANOVA results of CCRD experimental models with diameter as the response value
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 10362.51 | 5 | 2072.50 | 222.49 | < 0.0001 | significant |
| A-Flow rate | 419.73 | 1 | 419.73 | 45.06 | 0.0003 | |
| B-Voltage | 9280.46 | 1 | 9280.46 | 996.31 | < 0.0001 | |
| AB | 12.60 | 1 | 12.60 | 1.35 | 0.2829 | |
| A² | 3.85 | 1 | 3.85 | 0.4131 | 0.5409 | |
| B² | 622.06 | 1 | 622.06 | 66.78 | < 0.0001 | |
| Residual | 65.20 | 7 | 9.31 | |||
| Lack of Fit | 46.78 | 3 | 21.73 | 4.33 | 0.0975 | not significant |
| Pure Error | 18.42 | 4 | 4.46 | |||
| Cor Total | 10427.72 | 12 | ||||
| 确定系数R2=0.9937 | 确定校正系数Radj2=0.9893 | |||||
| 变异系数C.V.%=0.9657 | 信噪比=47.2140 | |||||
| 流速/mL/h-1 | 电压/kV | 相对酶活性/% | 直径/μm |
|---|---|---|---|
| 26.666 | 7.782 | 93.477 | 337.627 |
表11 CA/CMCS微球的最优工艺
Table 11 The optimal process for CA/CMCS microspheres
| 流速/mL/h-1 | 电压/kV | 相对酶活性/% | 直径/μm |
|---|---|---|---|
| 26.666 | 7.782 | 93.477 | 337.627 |
| Enzyme form | Km/(μg/mL) | Vm/[μg/(min·mL)] |
|---|---|---|
| Free enzyme | 1.807 | 1.819 |
| Immobilized enzyme | 1.433 | 1.759 |
表12 游离酶的本征动力学参数与固定化酶的表观动力学参数
Table 12 Intrinsic kinetic parameters of free enzyme and apparent kinetic parameters of immobilized enzyme
| Enzyme form | Km/(μg/mL) | Vm/[μg/(min·mL)] |
|---|---|---|
| Free enzyme | 1.807 | 1.819 |
| Immobilized enzyme | 1.433 | 1.759 |
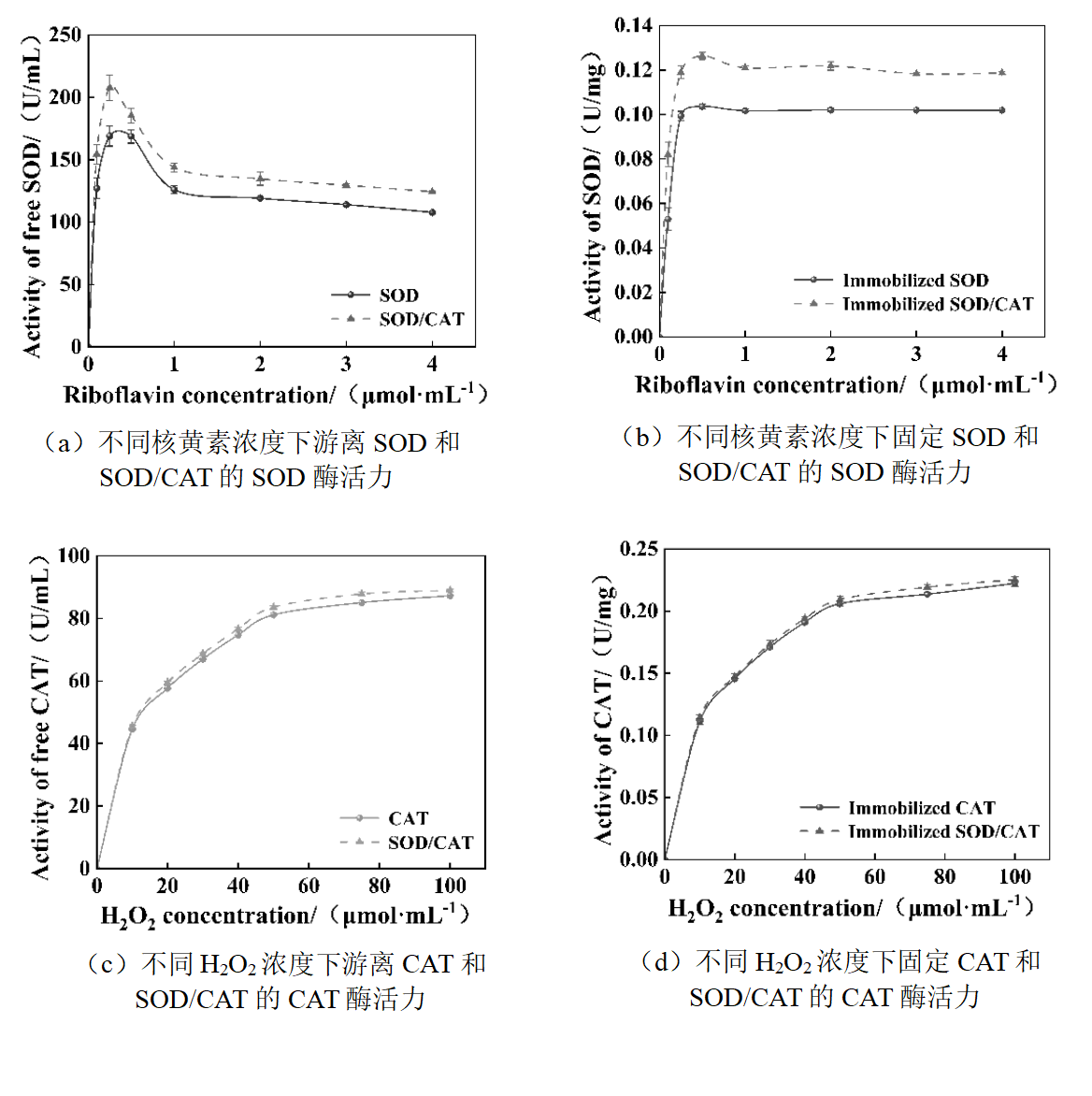
图7 不同底物浓度下单酶和双酶体系在游离和固定条件下的酶活力研究
Fig.7 Enzyme activities of single-enzyme and double-enzyme systems under free and immobilized conditions at different substrate
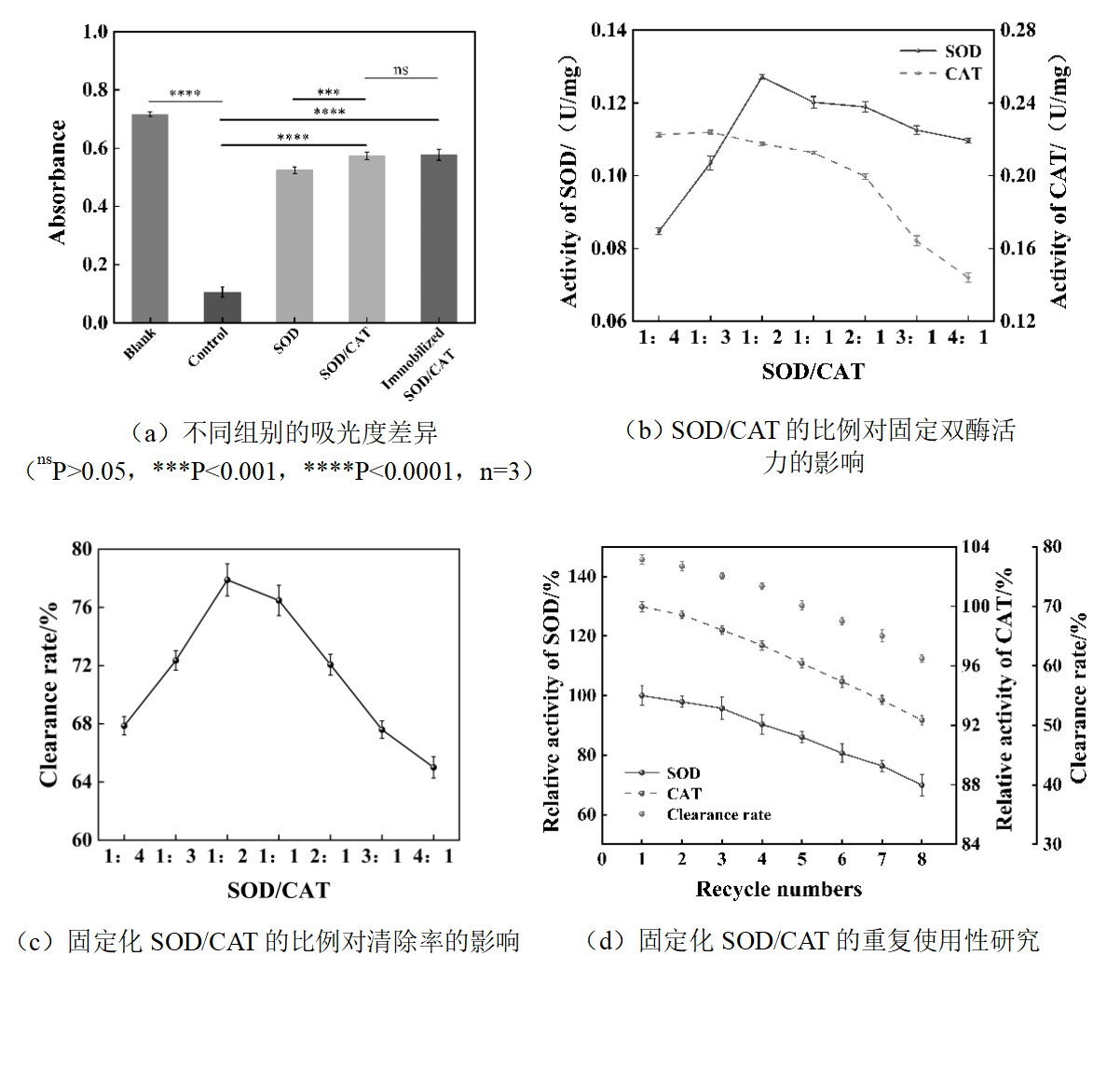
图8 固定化SOD/CAT双酶体系的复合微球对活性氧的清除及重复使用性研究
Fig.8 The removal of reactive oxygen species and reusability of immobilized SOD/CAT dual-enzyme system composite microspheres
| [1] | Xiao Y P, Wu J, Chen P H, et al. Biocatalytic cascade reactions for management of diseases[J]. Chemical Society Reviews, 2025, 54(7): 3247-3271. |
| [2] | 宋伟, 王金辉, 胡贵鹏, 等. 多酶级联催化合成(R)-β-酪氨酸[J]. 化工学报, 2022, 73(1): 352-361. |
| Song W, Wang J H, Hu G P, et al. Cascade catalysis for the synthesis of (R)-β-tyrosine[J]. CIESC Journal, 2022, 73(1): 352-361. | |
| [3] | Wang Y X, Zhao Q C, Haag R, et al. Biocatalytic synthesis using self-assembled polymeric nano- and microreactors[J]. Angewandte Chemie International Edition, 2022, 61(52): e202213974. |
| [4] | Li X Y, Zhang Y, He A, et al. Biohybrid catalysis in biomedicine[J]. Coordination Chemistry Reviews, 2025, 545: 217003. |
| [5] | Pyser J B, Chakrabarty S, Romero E O, et al. State-of-the-art biocatalysis[J]. ACS Central Science, 2021, 7(7): 1105-1116. |
| [6] | Vasudhevan P, Zhang R Y, Ma H, et al. Biocatalytic enzymes in food packaging, biomedical, and biotechnological applications: a comprehensive review[J]. International Journal of Biological Macromolecules, 2025, 300: 140069. |
| [7] | Liu X R, Qiu M Y, Zhang Y Y, et al. Enzyme immobilization based on reticular framework materials: Strategy, food applications, and prospect[J]. Advances in Colloid and Interface Science, 2025, 344: 103589. |
| [8] | Ran L, Lu Y, Chen L, et al. Design, synthesis, and application of immobilized enzymes on artificial porous materials[J]. Advanced Science, 2025, 12(20): 2500345. |
| [9] | Xu C Z, Tong S S, Sun L Q, et al. Cellulase immobilization to enhance enzymatic hydrolysis of lignocellulosic biomass: an all-inclusive review[J]. Carbohydrate Polymers, 2023, 321: 121319. |
| [10] | Zdarta J, Meyer A S, Jesionowski T, et al. Developments in support materials for immobilization of oxidoreductases: a comprehensive review[J]. Advances in Colloid and Interface Science, 2018, 258: 1-20. |
| [11] | Xing M Y, Chen Y, Li B Q, et al. Highly efficient removal of patulin using immobilized enzymes of Pseudomonas aeruginosa TF-06 entrapped in calcium alginate beads[J]. Food Chemistry, 2022, 377: 131973. |
| [12] | Ouyang J, Pu S J, Wang J Z, et al. Enzymatic hydrolysate of geniposide directly acts as cross-linking agent for enzyme immobilization[J]. Process Biochemistry, 2020, 99: 187-195. |
| [13] | Li C, Zhang G F, Liu N, et al. Preparation and properties of Rhizopus oryzae lipase immobilized using an adsorption-crosslinking method[J]. International Journal of Food Properties, 2016, 19(8): 1776-1785. |
| [14] | Wang F, Xu H, Wang M M, et al. Application of immobilized enzymes in juice clarification[J]. Foods, 2023, 12(23): 4258. |
| [15] | Guo S, Wang S, Meng J, et al. Immobilized enzyme for screening and identification of anti-diabetic components from natural products by ligand fishing[J]. Critical Reviews in Biotechnology, 2023, 43(2): 242-257. |
| [16] | 严昕怡, 大村拓, 朱江煜. 酶固定化新策略及其应用研究进展[J]. 化学通报(中英文), 2025, 88(7): 754-760. |
| Yan X Y, Omura T, Zhu J Y. Research progress in new strategies and applications of enzyme immobilization[J]. Chemistry, 2025, 88(7): 754-760. | |
| [17] | Lu J W, Nie M F, Li Y R, et al. Design of composite nanosupports and applications thereof in enzyme immobilization: a review[J]. Colloids and Surfaces B: Biointerfaces, 2022, 217: 112602. |
| [18] | Du F T, Xin H, Zheng H Y, et al. Aqueous liquid–liquid phase separation (AqLLPS) droplet microreactors for biocatalysis[J]. Green Chemistry, 2025, 27(28): 8448-8466. |
| [19] | Zhang J H, Chen J L, Sha Y, et al. Water-mediated active conformational transitions of lipase on organic solvent interfaces[J]. International Journal of Biological Macromolecules, 2024, 277: 134056. |
| [20] | Zhang N N, Bittner J P, Fiedler M, et al. Unraveling alcohol dehydrogenase catalysis in organic–aqueous biphasic systems combining experiments and molecular dynamics simulations[J]. ACS Catalysis, 2022, 12(15): 9171-9180. |
| [21] | Yuan H, Li F, Jia L F, et al. Bacteria-inspired aqueous-in-aqueous compartmentalization by in situ interfacial biomineralization[J]. Small Methods, 2023, 7(2): 2201309. |
| [22] | 贾露凡, 王艺颖, 董钰漫, 等. 微流控双水相贴壁液滴流动强化酶促反应研究[J]. 化工学报, 2023, 74(3): 1239-1246. |
| Jia L F, Wang Y Y, Dong Y M, et al. Aqueous two-phase system based adherent droplet microfluidics for enhanced enzymatic reaction[J]. CIESC Journal, 2023, 74(3): 1239-1246. | |
| [23] | [23] He D Q, Cao D Z, Ben C X, et al. Ionic liquid gel microspheres as an emerging platform for constructing liquid compartment microreactors[J]. Green Chemistry, 2022, 24(15): 5952-5964. |
| [24] | 马敬, 李磊, 邓小康, 等. 双水相微流控技术研究进展[J]. 科学通报, 2025, 70(12): 1799-1818. |
| Ma J, Li L, Deng X K, et al. Advances in aqueous two-phase microfluidic technology[J]. Chinese Science Bulletin, 2025, 70(12): 1799-1818. | |
| [25] | 潘大伟, 汪伟, 谢锐, 等. 微流控乳液模板法构建功能微颗粒过程中介尺度结构定向调控的研究进展[J]. 化工学报, 2022, 73(6): 2306-2317. |
| Pan D W, Wang W, Xie R, et al. Progress on regulation of meso-scale structures for microfluidic emulsion-template synthesis of functional microparticles[J]. CIESC Journal, 2022, 73(6): 2306-2317. | |
| [26] | 黄心童, 耿宇昊, 刘恒源, 等. 微流控制备新型功能纳米粒子研究进展[J]. 化工学报, 2023, 74(1): 355-364. |
| Huang X T, Geng Y H, Liu H Y, et al. Research progress on new functional nanoparticles prepared by microfluidic technology[J]. CIESC Journal, 2023, 74(1): 355-364. | |
| [27] | 鲍博, 赵双良, 徐建鸿. 基于微纳流控技术的流体相态特性研究进展[J]. 化工学报, 2018, 69(11): 4530-4541. |
| Bao B, Zhao S L, Xu J H. Progress in studying fluid phase behaviours with micro-and nano-fluidic technology[J]. CIESC Journal, 2018, 69(11): 4530-4541. | |
| [28] | Ben C X, Zhao S J, Wu Q, et al. Hydrophobic ionic liquid gel microspheres as bi-component carriers with a liquid phase to immobilize enzymes for enhanced performance[J]. Advanced Functional Materials, 2024, 34(46): 2407913. |
| [29] | Tang Q M, Deng N J, Chen J L, et al. One-step fabrication of coconut-like capsules via competitive reactions at an all-aqueous interface for enzyme immobilization[J]. ACS Applied Materials & Interfaces, 2023, 15(8): 10621-10628. |
| [30] | Xu F L, Wang W J, Zhao W B, et al. All-aqueous microfluidic fabrication of calcium alginate/alkylated chitosan core-shell microparticles with time-sequential functions for promoting whole-stage wound healing[J]. International Journal of Biological Macromolecules, 2024, 282: 136685. |
| [31] | Zhang X Y, Zhu Y J, Xiong Z J, et al. Broad-spectrum ROS/RNS scavenging catalase-loaded microreactors for effective oral treatment of inflammatory bowel diseases[J]. Small, 2025, 21(23): 2501341. |
| [32] | Miyagawa A, Nakatani K. Kinetic detection of hydrogen peroxide in single horseradish peroxidase-concentrated silica particle using confocal fluorescence microspectroscopic measurement[J]. Talanta, 2024, 273: 125925. |
| [33] | Sun M M, Wang L L, Zhuo Y, et al. Multi-enzyme activity of MIL-101 (Fe)-derived cascade nano-enzymes for antitumor and antimicrobial therapy[J]. Small, 2024, 20(17): 2309593. |
| [34] | Kwon K, Jung J, Sahu A, et al. Nanoreactor for cascade reaction between SOD and CAT and its tissue regeneration effect[J]. Journal of Controlled Release, 2022, 344: 160-172. |
| [35] | Yasar Mahlicli F, ?en Y, Mutlu M, et al. Immobilization of superoxide dismutase/catalase onto polysulfone membranes to suppress hemodialysis-induced oxidative stress: a comparison of two immobilization methods[J]. Journal of Membrane Science, 2015, 479: 175-189. |
| [36] | Yang M, Jiang W, Pan Z Q, et al. Synthesis, characterization and SOD-like activity of histidine immobilized silica nanoparticles[J]. Journal of Inorganic and Organometallic Polymers and Materials, 2015, 25(5): 1289-1297. |
| [37] | Yan H D, Hou W J, Lei B L, et al. Ultrarobust stable ABTS radical cation prepared using Spore@Cu-TMA biocomposites for antioxidant capacity assay[J]. Talanta, 2024, 276: 126282. |
| [38] | Savéant J M. Electrochemical concerted proton and electron transfers. further insights in the reduction mechanism of superoxide ion in the presence of water and other weak acids[J]. The Journal of Physical Chemistry C, 2007, 111(7): 2819-2822. |
| [39] | Yao Y Y, Chen S X, Li H. An improved system to evaluate superoxide-scavenging effects of bioflavonoids[J]. ChemistryOpen, 2021, 10(4): 503-514. |
| [40] | Hackenhaar C R, Rosa C F, Flores E E, et al. Development of a biocomposite based on alginate/gelatin crosslinked with genipin for β-galactosidase immobilization: Performance and characteristics[J]. Carbohydrate Polymers, 2022, 291: 119483. |
| [41] | Gao Y, Sun W T, Zhang Y G, et al. All-aqueous microfluidics fabrication of multifunctional bioactive microcapsules promotes wound healing[J]. ACS Applied Materials & Interfaces, 2022, 14(43): 48426-48437. |
| [42] | Smidsr?d O, Skja?k-Br?k G. Alginate as immobilization matrix for cells[J]. Trends in Biotechnology, 1990, 8: 71-78. |
| [43] | Egbeyemi O I, Hatem W A, Kober U A, et al. Transforming the stability, encapsulation, and sustained release properties of calcium alginate beads through gel-confined coacervation[J]. Langmuir, 2024, 40(23): 11947-11958. |
| [44] | Mafakher L, Ahmadi Y, Khalili Fard J, et al. Alpha-amylase immobilization: methods and challenges[J]. Pharmaceutical Sciences, 2023, 29(2): 144-155. |
| [45] | Wang W, Liu J Y, Khan M J, et al. Magnetic macroporous chitin microsphere as a support for covalent enzyme immobilization[J]. International Journal of Biological Macromolecules, 2024, 256: 128214. |
| [46] | Naskar P, Chakraborty D, Mondal A, et al. Immobilization of α-amylase in calcium alginate-gum odina (CA-GO) beads: an easily recoverable and reusable support[J]. International Journal of Biological Macromolecules, 2024, 258: 129062. |
| [1] | 孙慧, 屈虹男, 孙甲琛, 张根林, 贾海洋, 李春. 构建多酶复合体强化异戊二烯生物合成[J]. 化工学报, 2025, 76(7): 3436-3445. |
| [2] | 李远华, 凌思棋, 封科军, 冯颖, 郭于菁, 谢世桓. 基于cMOFs的固定化脂肪酶微反应器的构筑及其扁桃酸催化应用[J]. 化工学报, 2025, 76(3): 1170-1179. |
| [3] | 张静, 元跃, 刘艳梅, 王智文, 陈涛. 生物法制备衣康酸研究进展[J]. 化工学报, 2025, 76(3): 909-921. |
| [4] | 魏攀攀, 刘怿楠, 朱春英, 付涛涛, 高习群, 马友光. 改进的T型微通道内双水相液滴的制备[J]. 化工学报, 2025, 76(2): 576-583. |
| [5] | 宋世萍, 汤晓玲, 郑仁朝. 谷胱甘肽双功能合成酶分子改造及应用[J]. 化工学报, 2024, 75(S1): 251-258. |
| [6] | 张梦婷, 王书林, 桑熙, 元兴昊, 徐刚. 人工Cu-TM1459金属酶催化不对称迈克尔加成反应[J]. 化工学报, 2024, 75(9): 3255-3265. |
| [7] | 杨子驰, 谢冰琪, 石瑞莘, 雷虹, 陈晨, 周才金, 张吉松. 套管膜式微反应器内高效安全的气液传质-反应过程研究进展[J]. 化工学报, 2024, 75(9): 3011-3027. |
| [8] | 邢登雪, 张良, 李文强, 梁建华, 秦磊, 张根林, 李春. 酵母细胞催化合成18α-甘草酸[J]. 化工学报, 2024, 75(9): 3266-3276. |
| [9] | 吴哲明, 张碧云, 郑仁朝. 腈水解酶立体选择性改造及其合成布瓦西坦[J]. 化工学报, 2024, 75(7): 2633-2643. |
| [10] | 张颂红, 赵欣怡, 楼小玲, 沈绍传, 贠军贤. 阳离子交换纳晶胶分离乳过氧化物酶的研究[J]. 化工学报, 2024, 75(7): 2574-2582. |
| [11] | 张祎琪, 谭雪松, 李吾环, 张权, 苗长林, 庄新姝. 温和条件下乙二醇苯醚高效分离回收甘蔗渣组分[J]. 化工学报, 2024, 75(6): 2274-2282. |
| [12] | 王文雅, 张玮, 楼小玲, 钟若菲, 陈冰冰, 贠军贤. 纳米纤维素嵌合型晶胶微球的多微管成形与模拟[J]. 化工学报, 2024, 75(5): 2060-2071. |
| [13] | 薛潇, 商敏静, 苏远海. 微反应器内药物连续流合成的研究进展[J]. 化工学报, 2024, 75(4): 1439-1454. |
| [14] | 刘静, 杨文博, 吕英迪, 陶胜洋. 喷雾-反溶剂结晶法制备掺杂铝粉的复合微球[J]. 化工学报, 2024, 75(4): 1724-1734. |
| [15] | 刘梦绮, 王凯, 骆广生. 基于人工智能的微分散基础研究[J]. 化工学报, 2024, 75(4): 1096-1104. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号