• •
收稿日期:2025-10-28
修回日期:2025-11-13
出版日期:2026-02-02
通讯作者:
祝星
作者简介:章菊萍(1984-),女,博士研究生, 361336043@qq.com
基金资助:
Juping ZHANG( ), Wangyixin ZHANG, Xing ZHU(
), Wangyixin ZHANG, Xing ZHU( )
)
Received:2025-10-28
Revised:2025-11-13
Online:2026-02-02
Contact:
Xing ZHU
摘要:
苯乙烯作为关键基础化工原料,传统乙苯直接脱氢工艺存在热力学平衡限制与高能耗问题,乙苯化学链氧化脱氢(CL-ODH)是极具潜力的替代技术,而高性能氧载体的开发是该技术工业化的核心瓶颈。本文以钙钛矿型 SrMnO₃(SMO)为基体,通过浸渍法负载K、Na、Li三种碱金属,旨在揭示碱金属离子半径差异对SMO晶体结构、氧物种特性及CL-ODH催化性能的调控机制。采用X射线衍射(XRD)、氢气程序升温还原(H₂-TPR)、电子顺磁共振(EPR)、X射线光电子能谱(XPS)等表征手段,结合固定床反应活性评价,系统研究碱金属负载效应。结果表明:K⁺(离子半径1.38 Å)因与Sr²⁺(1.18 Å)尺寸匹配性适宜,未破坏SMO钙钛矿主体结构,且通过诱导晶格畸变使表面 Mn³⁺/Mn⁴⁺摩尔比提升至0.84,晶格氧(Oₗₐₜₜ)与表面吸附氧(Oₐds)比例提高 78%,同时降低还原起始温度并增加总释氧量;在600℃反应条件下,K-SMO氧载体表现最优,乙苯转化率达96.5%,苯乙烯选择性为88.2%,CO₂选择性低于5%,且在20次CL-ODH循环中转化率维持99%以上,仅选择性从88.2%轻微降至85.8%。相比之下,Na⁺(1.02 Å)引发局部结构应力导致催化性能下降(转化率 89.3%、选择性 84.7%),Li⁺(0.76 Å)因严重晶格失配生成SrLiO₂杂相,致使活性大幅衰减(转化率75.1%、选择性80.5%)。K改性实现离子尺度优化SMO氧空位浓度与晶格氧活性,为设计高效稳定的CL-ODH 钙钛矿基氧载体提供了理论依据与实验支撑,同时为苯乙烯生产节能降耗提供了可行技术路径。
中图分类号:
章菊萍, 张王一心, 祝星. 碱金属改性对SrMnO3催化乙苯化学链氧化脱氢性能的影响规律研究[J]. 化工学报, DOI: 10.11949/0438-1157.20251194.
Juping ZHANG, Wangyixin ZHANG, Xing ZHU. Performance of alkali metal-loaded SrMnO₃ oxygen carriers in chemical looping oxidative dehydrogenation of ethylbenzene[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251194.
| Catalyst | Mn 2p | O 1s | |||||
|---|---|---|---|---|---|---|---|
| Mn3+ | Mn4+ | Mn3+/Mn4+ | Osur | Olatt | Olatt/Osur | ||
| SMO | 43.22 | 56.78 | 0.76 | 73.67 | 26.33 | 0.36 | |
| K-SMO | 45.64 | 54.36 | 0.84 | 60.89 | 39.11 | 0.64 | |
| Na-SMO | 29.24 | 70.76 | 0.41 | 76.37 | 23.63 | 0.31 | |
| Li-SMO | 27.03 | 72.97 | 0.37 | 79.33 | 20.67 | 0.26 | |
表1 制备的不同碱金属负载的SrMnO3的表面组成
Table 1 Surface composition of as-prepared SrMnO3 supported by different alkali metals
| Catalyst | Mn 2p | O 1s | |||||
|---|---|---|---|---|---|---|---|
| Mn3+ | Mn4+ | Mn3+/Mn4+ | Osur | Olatt | Olatt/Osur | ||
| SMO | 43.22 | 56.78 | 0.76 | 73.67 | 26.33 | 0.36 | |
| K-SMO | 45.64 | 54.36 | 0.84 | 60.89 | 39.11 | 0.64 | |
| Na-SMO | 29.24 | 70.76 | 0.41 | 76.37 | 23.63 | 0.31 | |
| Li-SMO | 27.03 | 72.97 | 0.37 | 79.33 | 20.67 | 0.26 | |
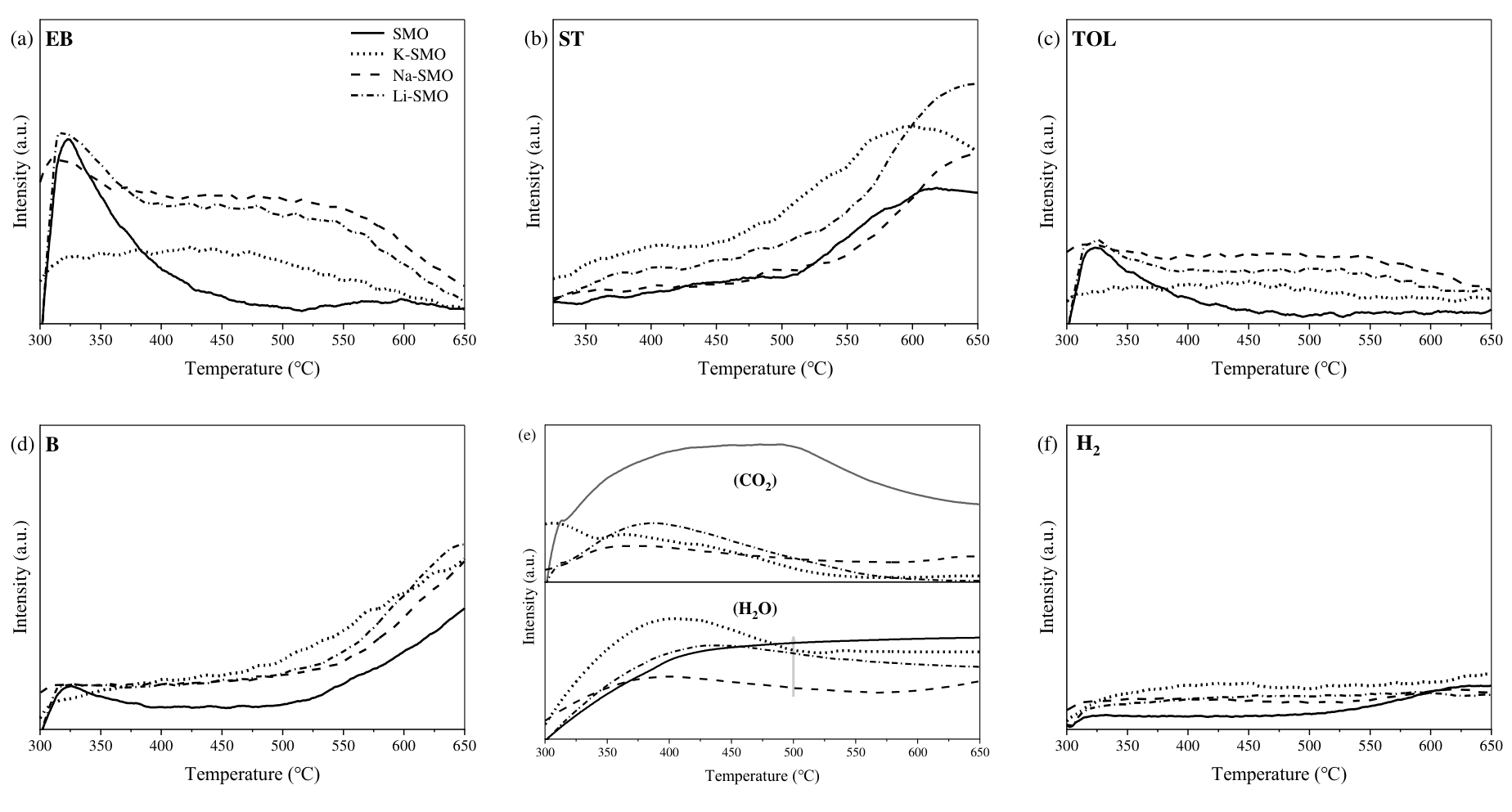
图6 不同碱金属负载的SrMnO3样品EB-TPR实验的质谱曲线(a-f)乙苯、苯乙烯、甲苯、苯、水蒸气、二氧化碳和氢气(条件:反应温度 = 300 ~ 650℃,m = 200 mg, FEB/Ar = 25 mL•min-1,EB/Ar混合气氛,持续通入Ar通过30℃乙苯鼓泡器)
Fig. 6 EB-TPR Mass Spectrum Curves of SrMnO3 supported by different alkali metals (a-f) ethylbenzene, styrene, toluene, benzene, H2O, CO2, H2 (Conditions: reaction temperature = 300 ~ 650 ℃, m = 200 mg, FEB/Ar = 25 mL•min-1, EB/Ar mixture atmosphere, containing Ar and passing an ethylbenzene bubbler at 30 ℃)

图7 不同碱金属负载的SrMnO3上乙苯恒温反应的产物分布(条件:反应温度 = 600℃,m = 500 mg,FEB/Ar = 25 mL•min-1,EB-Ar混合气氛,持续通入Ar通过30℃乙苯鼓泡器,ST为苯乙烯,TOL为甲苯,B为苯)
Fig. 7 Product distribution in the ethylbenzene isothermal reaction over SrMnO3 supported by different alkali metals (Conditions: reaction temperature = 600 ℃, m = 500 mg, FEB/Ar = 25 mL•min-1, EB-Ar mixture atmosphere, containing Ar and passing an ethylbenzene bubbler at 30 ℃, ST for styrene, TOL for toluene, B for benzene)
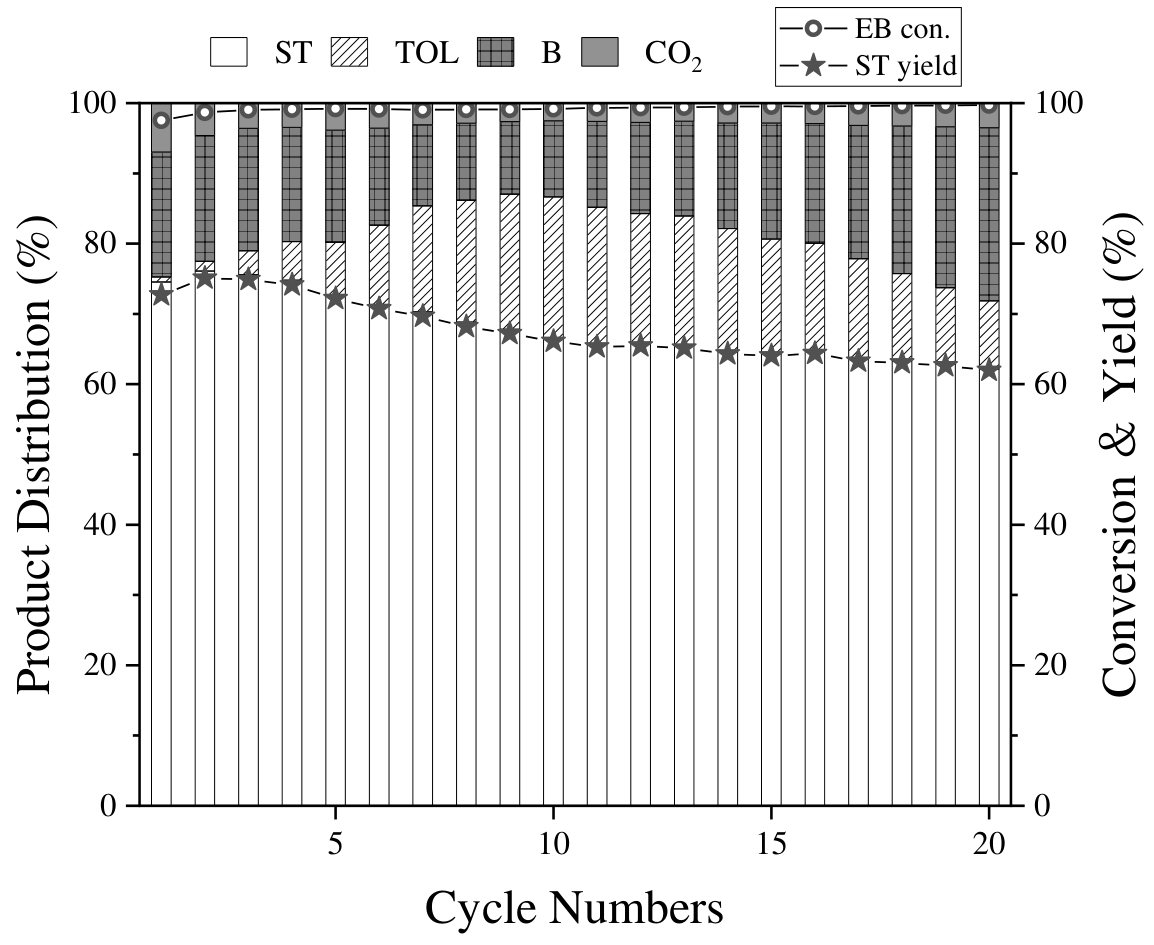
图8 K-SMO连续20 次乙苯CL-ODH反应中第15分钟的产物分布(条件:反应温度 = 600℃,m = 500 mg,FEB/Ar = 25 mL•min-1,EB-Ar混合气氛,持续通入Ar通过30℃乙苯鼓泡器,ST为苯乙烯,TOL为甲苯,B为苯)
Fig. 8 Product distribution in the 20 successive CL-ODH of ethylbenzene over K-SMO in the 15th minute (Conditions: reaction temperature = 600 ℃, m = 500 mg, FEB/Ar = 25 mL•min-1, EB-Ar mixture atmosphere, containing Ar and passing an ethylbenzene bubbler at 30 ℃, ST for styrene, TOL for toluene, B for benzene)
| [1] | Zhang Z P, Zeng T Q, Wei C L, et al. Ce-promoted Fe-K-Mg catalyst and its application in dehydrogenation of ethylbenzene[J]. Molecular Catalysis, 2023, 540: 113058. |
| [2] | 李建韬, 金月昶, 金熙俊. 苯乙烯的生产现状及工艺进展[J]. 当代化工, 2015, 44(2): 359-362. |
| Li J T, Jin Y C, Jin X J. Production status and technological progress of styrene[J]. Contemporary Chemical Industry, 2015, 44(2): 359-362. | |
| [3] | 吕鸿博. 苯乙烯生产技术和应用研究进展[J]. 石化技术, 2018, 25(2): 100. |
| Lv H B. Discussion on new progress of styrene production technology and applied research[J]. Petrochemical Industry Technology, 2018, 25(2): 100. | |
| [4] | Zhang J P, Gao W P, Yang K R, et al. Redox-activated supersaturation of ceria solid solution as a dynamic catalyst enabling low-temperature ethylbenzene oxidative dehydrogenation[J]. ACS Catalysis, 2025, 15(2): 956-966. |
| [5] | SUSlick K S. Kirk-Othmer encyclopedia of chemical technology[M]. Wiley & Sons: New York, 1998, 26: 517-541. |
| [6] | Wang W, Chen S, Pei C L, et al. Tandem propane dehydrogenation and surface oxidation catalysts for selective propylene synthesis[J]. Science, 2023, 381(6660): 886-890. |
| [7] | Luyben W L. Design and control of the styrene process[J]. Industrial & Engineering Chemistry Research, 2011, 50(3): 1231-1246. |
| [8] | Lee E H. Iron oxide catalysts for dehydrogenation of ethylbenzene in the presence of steam[J]. Catalysis Reviews, 1974, 8(1): 285-305. |
| [9] | Hirano T. Roles of potassium in potassium-promoted iron oxide catalyst for dehydrogenation of ethylbenzene[J]. Applied Catalysis, 1986, 26: 65-79. |
| [10] | 张雄福, 王金渠, 刘海鸥, 等. 沸石膜反应器乙苯脱氢反应性能[J]. 高校化学工程学报, 2001, 15(2): 121-126. |
| Zhang X F, Wang J Q, Liu H O, et al. Studies of dehydrogenation of ethylbenzene in zeolite membrane reactors[J]. Journal of Chemical Engineering of Chinese Universities, 2001, 15(2): 121-126. | |
| [11] | Cavani F, Trifirò F. Alternative processes for the production of styrene[J]. Applied Catalysis A: General, 1995, 133(2): 219-239. |
| [12] | Sivaranjani K, Verma A, Gopinath C S. Molecular oxygen-assisted oxidative dehydrogenation of ethylbenzene to styrene with nanocrystalline Ti1-xVxO2 [J]. Green Chemistry, 2012, 14(2): 461-471. |
| [13] | Guo F S, Yang P J, Pan Z M, et al. Carbon-doped BN nanosheets for the oxidative dehydrogenation of ethylbenzene[J]. Angewandte Chemie, 2017, 129(28): 8343-8347. |
| [14] | Kainthla I, Babu G V R, Bhanushali J T, et al. Development of stable MoO3/TiO2-Al2O3 catalyst for oxidative dehydrogenation of ethylbenzene to styrene using CO2 as soft oxidant[J]. Journal of CO2 Utilization, 2017, 18: 309-317. |
| [15] | Zhu X, Gao Y F, Wang X J, et al. A tailored multi-functional catalyst for ultra-efficient styrene production under a cyclic redox scheme[J]. Nature Communications, 2021, 12: 1329. |
| [16] | Nederlof C, Zarubina V, Melián-Cabrera I, et al. Oxidative dehydrogenation of ethylbenzene to styrene over alumina: effect of calcination[J]. Catalysis Science & Technology, 2013, 3(2): 519-526. |
| [17] | Zhang L, Wu Z L, Nelson N C, et al. Role of CO2 as a soft oxidant for dehydrogenation of ethylbenzene to styrene over a high-surface-area ceria catalyst[J]. ACS Catalysis, 2015, 5(11): 6426-6435. |
| [18] | Liu J C, Li F X. Mixed oxides as multi-functional reaction media for chemical looping catalysis[J]. Chemical Communications, 2023, 59(1): 10-28. |
| [19] | Madduluri V R, Rao K S R. Advantage of co embedded γ-Al2O3 catalysts over MgO and SiO2 solid oxides in the selective production of styrene monomer[J]. Catalysis Letters, 2019, 149(11): 3238-3252. |
| [20] | Zhao X, Zhou H, Sikarwar V S, et al. Biomass-based chemical looping technologies: the good, the bad and the future[J]. Energy & Environmental Science, 2017, 10(9): 1885-1910. |
| [21] | Schmitt R, Nenning A, Kraynis O, et al. A review of defect structure and chemistry in ceria and its solid solutions[J]. Chemical Society Reviews, 2020, 49(2): 554-592. |
| [22] | Venkataswamy P, Jampaiah D, Kandjani A E, et al. Transition (Mn, Fe) and rare earth (La, Pr) metal doped ceria solid solutions for high performance photocatalysis: Effect of metal doping on catalytic activity[J]. Research on Chemical Intermediates, 2018, 44(4): 2523-2543. |
| [23] | Gabra S, Marek E J, Poulston S, et al. The use of strontium ferrite perovskite as an oxygen carrier in the chemical looping epoxidation of ethylene[J]. Applied Catalysis B: Environmental, 2021, 286: 119821. |
| [24] | Zhang R J, Liu G, Huo C B, et al. Tailoring catalytic and oxygen release capability in LaFe1–xNixO3 to intensify chemical looping reactions at medium temperatures[J]. ACS Catalysis, 2024, 14(10): 7771-7787. |
| [25] | Zhou M Y, Wu J R, Lu Y G, et al. Non-metallic phosphorus-induced lattice engineering in LaFeO3 perovskite: Oxygen vacancy modulation for enhanced methane conversion in chemical looping partial oxidation[J]. Chemical Engineering Journal, 2025, 520: 165963. |
| [26] | Ding W X, Zhao K, Jiang S C, et al. Alkali-metal enhanced LaMnO3 perovskite oxides for chemical looping oxidative dehydrogenation of ethane[J]. Applied Catalysis A: General, 2021, 609: 117910. |
| [27] | Luongo G, Donat F, Bork A H, et al. Highly selective oxidative dehydrogenation of ethane to ethylene via chemical looping with oxygen uncoupling through structural engineering of the oxygen carrier[J]. Advanced Energy Materials, 2022, 12(23): 2200405. |
| [28] | Gao Y F, Haeri F, He F, et al. Alkali metal-promoted LaxSr2–xFeO4–δ redox catalysts for chemical looping oxidative dehydrogenation of ethane[J]. ACS Catalysis, 2018, 8(3): 1757-1766. |
| [29] | Wang R Y, Zhang J P, Li D F, et al. Lattice oxygen regulation of perovskites for chemical looping oxidative dehydrogenation of ethylbenzene to styrene[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(24): 8948-8957. |
| [30] | Zhang W, Zhang J P, Dai X R, et al. Oxygen-vacancies enriched SrMnO3 for chemical looping oxidative dehydrogenation of ethylbenzene[J]. Industrial & Engineering Chemistry Research, 2025, 64(17): 8722-8734. |
| [1] | 黄路生, 肖志俊, 孙亚琴, 修志龙. 苯硼酸基吸附树脂的制备及其在生物基1,3-丙二醇分离中的应用[J]. 化工学报, 2025, 76(10): 5225-5235. |
| [2] | 王保文, 张港, 刘同庆, 李炜光, 王梦家, 林德顺, 马晶晶. CeO2/CuFe2O4氧载体CH4化学链重整耦合CO2热催化还原研究[J]. 化工学报, 2022, 73(12): 5414-5426. |
| [3] | 赵林洲, 郑燕娥, 李孔斋, 王亚明, 蒋丽红, 范浩熙, 王雅静, 祝星, 魏永刚. Ce1-xNixOy氧载体在化学链甲烷重整耦合CO2还原中的应用[J]. 化工学报, 2021, 72(8): 4371-4380. |
| [4] | 刘昌会,黄文博,顾彦龙,饶中浩. 废弃聚苯乙烯塑料在环境与能源中的高值化应用进展[J]. 化工学报, 2020, 71(7): 2956-2972. |
| [5] | 吴岳峰,曲永芳,李大欢,苏苗军,刘勇. 聚离子液体载MoO2/Ag催化分子氧氧化苯乙烯的研究[J]. 化工学报, 2020, 71(11): 4990-4998. |
| [6] | 梅道锋, 赵海波, 晏水平. 基于NiO/Ca2Al2SiO7的沼气自热化学链重整制氢热分析动力学模拟[J]. 化工学报, 2019, 70(S1): 193-201. |
| [7] | 陈智豪, 廖艳芬, 莫菲, 刘桂才, 余昭胜, 马晓茜. MnFeO3和MnFe2O4氧载体在稻草化学链气化中的应用[J]. 化工学报, 2019, 70(12): 4835-4846. |
| [8] | 陈沛然, 李海航, 丁杰, 林鹏. 蔓延规则材料聚苯乙烯板的热释放速率模型[J]. 化工学报, 2018, 69(8): 3747-3753. |
| [9] | 张翱, 杨海存, 马文中, 李玉雪, 龚方红, 陶国良, 刘春林. 正反向同时引发ATRP制备凹凸棒土/聚苯乙烯杂化粒子[J]. 化工学报, 2018, 69(5): 2299-2308. |
| [10] | 郭妍婷, 尹垚骐, 黄雪, 陈曼, 冯光炷. 基于二聚脂肪酸改性苯乙烯聚酯树脂的合成及性能[J]. 化工学报, 2017, 68(S1): 266-275. |
| [11] | 李晨阳, 冯淼, 崔海峰, 曹贵平, 吕慧, 陈荣起. 蜂窝陶瓷骨架微结构修饰调控制备Pd/CNTs@CHC催化剂用于PS加氢[J]. 化工学报, 2017, 68(7): 2746-2754. |
| [12] | 李志丹, 单国荣, 潘鹏举. 搅拌速率及其改变点对高抗冲聚苯乙烯相转变的影响[J]. 化工学报, 2017, 68(2): 788-794. |
| [13] | 韩晓东, 赵志伟, 李雪华, 施晨燕. 一种氧化还原石墨烯的稳定水溶液分散体的合成方法[J]. 化工学报, 2016, 67(S1): 396-401. |
| [14] | 李雪, 赵阳, 高金津, 李微微, 王树博, 谢晓峰. 苯乙烯磺酰氯的理论模拟及用于辐照接枝法制备质子交换膜[J]. 化工学报, 2016, 67(S1): 390-395. |
| [15] | 纪晓明, 苏明旭, 汪雪, 蔡小舒. 基于超声波阻抗谱的颗粒粒径表征方法[J]. 化工学报, 2016, 67(6): 2284-2290. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号