化工学报 ›› 2020, Vol. 71 ›› Issue (11): 4990-4998.DOI: 10.11949/0438-1157.20200810
收稿日期:2020-06-22
修回日期:2020-09-10
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
刘勇
作者简介:吴岳峰(1995—),男,硕士研究生,基金资助:
Yuefeng WU( ),Yongfang QU,Dahuan LI,Miaojun SU,Yong LIU(
),Yongfang QU,Dahuan LI,Miaojun SU,Yong LIU( )
)
Received:2020-06-22
Revised:2020-09-10
Online:2020-11-05
Published:2020-11-05
Contact:
Yong LIU
摘要:
以氨基功能化的聚离子液体为载体,通过离子交换和水热还原,制备出负载型催化剂PVAD/MoO2/Ag,考察了其催化苯乙烯氧化的性能。采用FT-IR、TG、XRD、SEM和BET对其所制备的催化剂进行表征。苯乙烯氧化性能研究结果表明,PVAD/MoO2/Ag4%表现出最佳的催化活性,在乙腈为溶剂,温度为90℃,氧气压力为0.8 MPa的条件下,反应8 h,苯乙烯的转化率为49.9%,苯甲醛的选择性达到74.4%。并且催化剂重复使用5次,活性没有明显下降,表现出良好的重复利用性。
中图分类号:
吴岳峰,曲永芳,李大欢,苏苗军,刘勇. 聚离子液体载MoO2/Ag催化分子氧氧化苯乙烯的研究[J]. 化工学报, 2020, 71(11): 4990-4998.
Yuefeng WU,Yongfang QU,Dahuan LI,Miaojun SU,Yong LIU. Study on oxidation of styrene with molecular oxygen catalyzed by MoO2/Ag on polyionic liquid[J]. CIESC Journal, 2020, 71(11): 4990-4998.
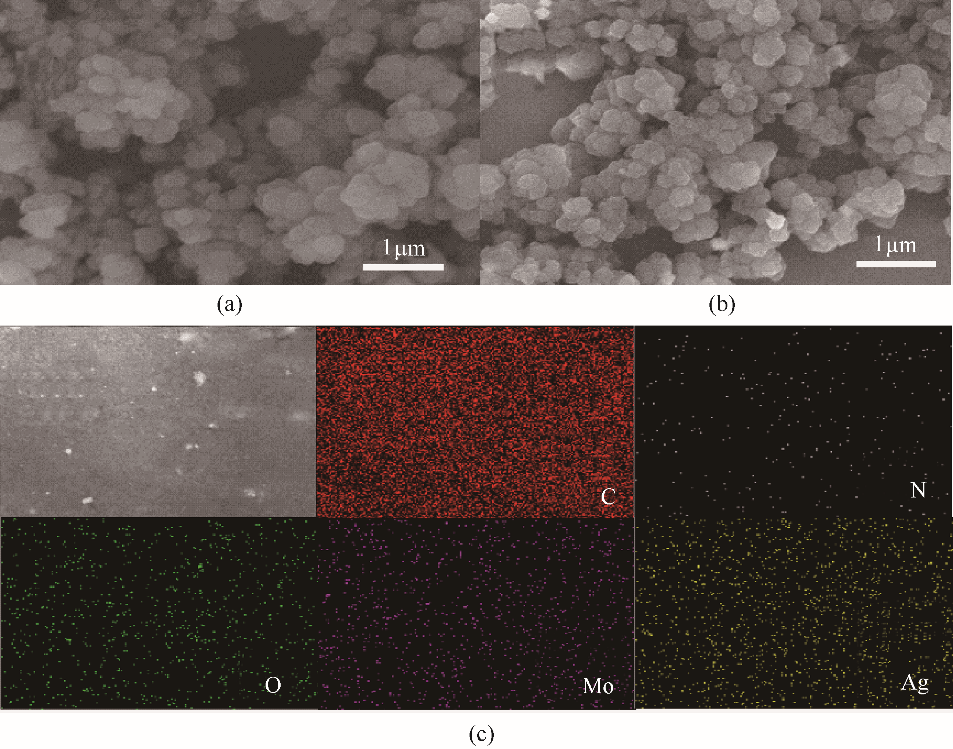
图4 PVAD(a)和 PVAD/MoO2/Ag4%(b)的扫描电镜图片及元素分布图(c)
Fig.4 SEM images of PVAD (a) and PVAD/MoO2/Ag4% (b) and elemental (C, N, O, Mo and Ag) mapping images of PVAD/MoO2/Ag4%(c)
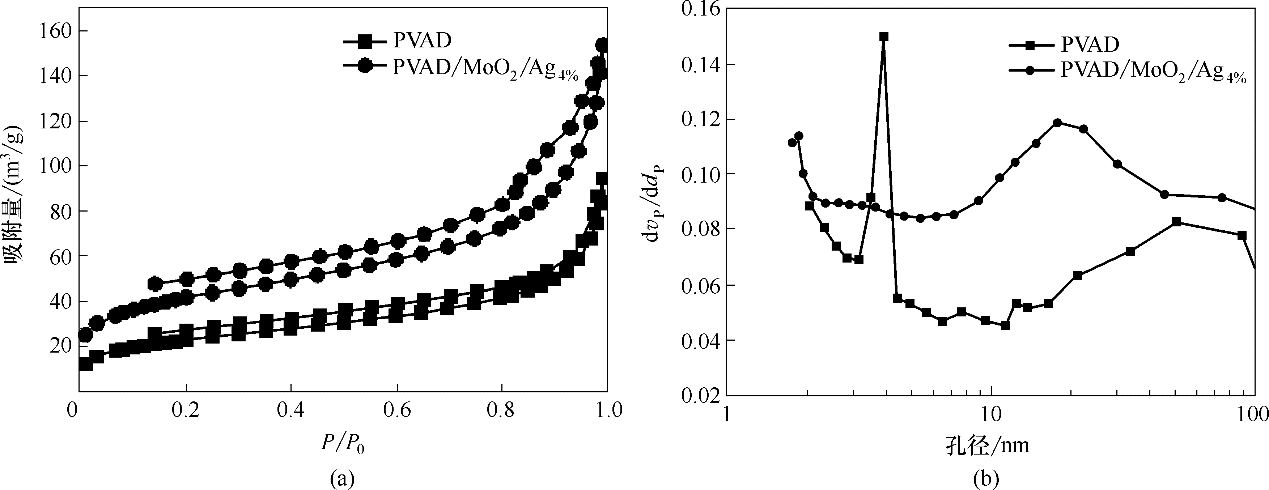
图6 PVAD和PVAD/MoO2/Ag4%的N2吸/脱附曲线(a)及孔径分布图(b)
Fig.6 Isothermal N2 adsorption/desorption curve (a) and pore size distribution diagram (b) of PVAD and PVAD/MoO2/Ag4%
| 催化剂 | SBET /(m2/g) | vp/(cm3/g) | dp/nm |
|---|---|---|---|
| PVAD | 86.6 | 0.13 | 9.43 |
| PVAD/MoO2/Ag4% | 141.8 | 0.21 | 8.79 |
表1 催化剂的结构特性
Table 1 Structural properties of catalysts
| 催化剂 | SBET /(m2/g) | vp/(cm3/g) | dp/nm |
|---|---|---|---|
| PVAD | 86.6 | 0.13 | 9.43 |
| PVAD/MoO2/Ag4% | 141.8 | 0.21 | 8.79 |
| 溶剂 | 转化率/% | 选择性/% | |
|---|---|---|---|
| 苯甲醛 | 苯乙烯氧化物 | ||
| MeCN | 34.0 | 84.4 | 3.2 |
| DMF | 23.1 | 12.2 | 10.8 |
| DCM | 4.19 | 100 | 0 |
| PhMe | 1.4 | 100 | 0 |
| EtOH | 12.5 | 78.8 | 5.4 |
表2 溶剂对苯乙烯的氧化结果的影响
Table 2 Effects of solvents on the results of catalytic oxidation of styrene
| 溶剂 | 转化率/% | 选择性/% | |
|---|---|---|---|
| 苯甲醛 | 苯乙烯氧化物 | ||
| MeCN | 34.0 | 84.4 | 3.2 |
| DMF | 23.1 | 12.2 | 10.8 |
| DCM | 4.19 | 100 | 0 |
| PhMe | 1.4 | 100 | 0 |
| EtOH | 12.5 | 78.8 | 5.4 |
| 催化剂 | 转化率/% | 选择性/% | |
|---|---|---|---|
| 苯甲醛 | 苯乙烯 氧化物 | ||
PVAD/MoO2 PVAD/MoO2/Ag2% | 16.7 27.3 | 83.9 84.9 | 2.3 2.8 |
| PVAD/MoO2/Ag4% | 34.0 | 84.4 | 3.2 |
| PVAD/MoO2/Ag7% | 24.0 | 84.7 | 3.0 |
| PVAD/MoO2/Ag10% | 19.0 | 83.1 | 2.7 |
表3 Ag负载量对催化剂活性的影响
Table 3 Effect of Ag loading on catalyst activity
| 催化剂 | 转化率/% | 选择性/% | |
|---|---|---|---|
| 苯甲醛 | 苯乙烯 氧化物 | ||
PVAD/MoO2 PVAD/MoO2/Ag2% | 16.7 27.3 | 83.9 84.9 | 2.3 2.8 |
| PVAD/MoO2/Ag4% | 34.0 | 84.4 | 3.2 |
| PVAD/MoO2/Ag7% | 24.0 | 84.7 | 3.0 |
| PVAD/MoO2/Ag10% | 19.0 | 83.1 | 2.7 |
| 催化剂 | 氧化剂 | 温度/℃ | 时间/h | 转化率/% | 选择性/% | 文献 |
|---|---|---|---|---|---|---|
| PVAD/MoO2/Ag | O2 | 90 | 8 | 49.9 | 74.4 | this work |
| PVAD/MoO2/Ag | O2 | 80 | 8 | 34.0 | 84.4 | this work |
| MOF-74(Cu-30/Co-70) | O2 | 80 | 20 | 30.4 | 43 | [ |
| Au NPs@XAD-4-G3.0 PAMAM | O2 | 80 | 24 | 80 | 48 | [ |
| SO42--Fe-V/ZrO2 | H2O2 | 80 | 4 | 62.3 | 74.0 | [ |
| H3PW12O40/SBA-15 | H2O2 | 70 | 24 | 22.6 | 100.0 | [ |
| Mg0.5Cu0.5Fe2O4 | H2O2 | 80 | 6 | 21.2 | 75.2 | [ |
| Ce0.95Zr0.05O2 | TBHP | 80 | 12 | 70.2 | 31.3 | [ |
| ZSM-5(60) | TBHP | 65 | 6 | 45.3 | 87.2 | [ |
表4 不同催化剂催化氧化苯乙烯的结果
Table 4 Results of the catalytic oxidation of styrene with different catalysts
| 催化剂 | 氧化剂 | 温度/℃ | 时间/h | 转化率/% | 选择性/% | 文献 |
|---|---|---|---|---|---|---|
| PVAD/MoO2/Ag | O2 | 90 | 8 | 49.9 | 74.4 | this work |
| PVAD/MoO2/Ag | O2 | 80 | 8 | 34.0 | 84.4 | this work |
| MOF-74(Cu-30/Co-70) | O2 | 80 | 20 | 30.4 | 43 | [ |
| Au NPs@XAD-4-G3.0 PAMAM | O2 | 80 | 24 | 80 | 48 | [ |
| SO42--Fe-V/ZrO2 | H2O2 | 80 | 4 | 62.3 | 74.0 | [ |
| H3PW12O40/SBA-15 | H2O2 | 70 | 24 | 22.6 | 100.0 | [ |
| Mg0.5Cu0.5Fe2O4 | H2O2 | 80 | 6 | 21.2 | 75.2 | [ |
| Ce0.95Zr0.05O2 | TBHP | 80 | 12 | 70.2 | 31.3 | [ |
| ZSM-5(60) | TBHP | 65 | 6 | 45.3 | 87.2 | [ |
| 1 | Zhou T, Zhang J, Ma Y Y, et al. A bicadmium-substituted polyoxometalate network based on a vanadosilicate cluste for the selective oxidation of styrene to benzaldehyde[J]. Inorganic Chemistry, 2020, 59(9): 5803-5807. |
| 2 | Das D R, Kalita P, Talukdar A K. Ti/Cr incorporated mesoporous MCM-48 for oxidation of styrene to benzaldehyde[J]. Journal of Porous Materials, 2020, 27: 893-903. |
| 3 | Clara S, Liliana B P. Studies on styrene selective oxidation to benzaldehyde catalyzed by Cr-ZSM-5: reaction parameters effects and kinetics[J]. Applied Catalysis A: General, 2011, 400: 117-121. |
| 4 | Lu C, Meng Y N, Zhou A D, et al. The synergistic effect of benzyl benzoate on the selective oxidation of toluene to benzaldehyde[J]. Chemical Engineering Research and Design, 2018, 141: 181-186. |
| 5 | Mahmoud N, Mojtaba B, Hirbod K. Preparation, characterization and catalytic activity of CoFe2O4 nanoparticles as a magnetically recoverable catalyst for selective oxidation of benzylalcohol to benzaldehyde and reduction of organic dyes[J]. Journal of Colloid and Interface Science, 2016, 465: 271-278. |
| 6 | Cheng D G, Chong M B, Chen F Q, et al. XPS characterization of CeO2 catalyst for hydrogenation of benzoic acid to benzaldehyde[J]. Catalysis Letters, 2008, 120(1/2): 82-85. |
| 7 | Lv J G, Shen Y, Peng L M, et al. Exclusively selective oxidation of toluene to benzaldehyde on ceria nanocubes by molecular oxygen[J]. Chemical Communications, 2010, 46(32): 5909-5911. |
| 8 | Liu J Y, Wang Z H, Jian P M, et al. Highly selective oxidation of styrene to benzaldehyde over a tailor-made cobalt oxide encapsulated zeolite catalyst[J]. Journal of Colloid and Interface Science, 2018, 517: 144-154. |
| 9 | Li Y J, Qiu P, Zhao Y Z, et al. Fabricating surface-functionalized CsPbBr3/Cs4PbBr6 nanosheets for visible-light photocatalytic oxidation of styrene[J]. Frontiers in Chemistry, 2020, 8: 130. |
| 10 | Marchena C L, Pecchi G A, Pierella L B. Selective styrene oxidation on alkaline tantalates ATaO3 (A=Li, Na, K) as heterogeneous catalysts[J]. Catalysis Communications, 2019, 119: 28-32. |
| 11 | Thao N T, Trung H H. Selective oxidation of styrene over Mg-Co-Al hydrotalcite like-catalysts using air as oxidant[J]. Catalysis Communications, 2014, 45: 153-157. |
| 12 | Zhu X C, Shen R W, Zhang L X. Catalytic oxidation of styrene to benzaldehyde over a copper Schiff-base/SBA-15 catalyst[J]. Chinese Journal of Catalysis, 2014, 35(10): 1716-1726. |
| 13 | Li Z N, Gao Y, Zhang X X. Oxidation of styrene to benzaldehyde by p-toluenesulfonic acid using hydrogen peroxide in the presence of activated carbon[J]. Chinese Journal of Catalysis, 2015, 36(5): 721-727. |
| 14 | Liu J H, Wang F, Gu Z G, et al. Vanadium phosphorus oxide catalyst modified by silver doping for mild oxidation of styrene to benzaldehyde[J]. Chemical Engineering Journal, 2009, 151(1/2/3): 319-323. |
| 15 | Narayanan S, Vijaya J J, Sivasanker S, et al. Hierarchical ZSM-5 catalytic performance evaluated in the selective oxidation of styrene to benzaldehyde using TBHP[J]. Journal of Porous Materials, 2016, 23(3): 741-752. |
| 16 | Kohantorabi M, Gholami M R. Cyclohexene oxidation catalyzed by flower-like core-shell Fe3O4@Au/metal organic frameworks nanocomposite[J]. Materials Chemistry and Physics, 2018, 213: 472-481. |
| 17 | Feng B, Hou Z S, Wang X R, et al. Selective aerobic oxidation of styrene to benzaldehyde catalyzed by water-soluble palladium(II) complex in water[J]. Green Chemistry, 2009, 11(9): 1446-1452. |
| 18 | Rak M J, Lerro M, Moores A. Hollow iron oxide nanoshells are active and selective catalysts for the partial oxidation of styrene with molecular oxygen[J]. Chemical Communications, 2014, 50(83): 12482-12485. |
| 19 | Datta S J, Yoon K B. Co-ETS-10 and Co-AM-6 as active catalysts for the oxidation of styrene to styrene oxide and benzaldehyde using molecular oxygen[J]. Chinese Journal of Catalysis, 2015, 36(6): 897-905. |
| 20 | Chandra P, Doke D S, Umbarkar S B, et al. One-pot synthesis of ultrasmall MoO3 nanoparticles supported on SiO2, TiO2, and ZrO2 nanospheres: an efficient epoxidation catalyst[J]. Journal of Materials Chemistry A, 2014, 2(44): 19060-19066. |
| 21 | Wang J S, Li X, Zhang S F, et al. Facile synthesis of ultrasmall monodisperse “raisin-bun”-type MoO3/SiO2 nanocomposites with enhanced catalytic properties[J]. Nanoscale, 2013, 5(11): 4823. |
| 22 | Zhang Q, Zhang, M H, Wiltowski T. Adsorption and dissociation of O2 on MoO2(111) surfaces: a DFT study[J]. Physical Chemistry Chemical Physics, 2017, 19(43): 29244. |
| 23 | Antoine D, Martina E J, Ferdinand B, et al. Activation of molecular oxygen by a molybdenum complex for catalytic oxidation[J]. Dalton Transactions, 2015, 44: 20514. |
| 24 | Campbell C T. Atomic and molecular oxygen adsorption on Ag(111)[J]. Surface Science, 1985, 157: 43-60. |
| 25 | Acharyya S S, Ghosh S, Sharma S K, et al. Cetyl alcohol mediated fabrication of forest of Ag/Mn3O4 nanowhiskers catalyst for the selective oxidation of styrene with molecular oxygen[J]. RSC Advances, 2015, 5(109): 89879-89887. |
| 26 | Lee E J, Lee J, Seo Y J, et al. Direct epoxidation of propylene to propylene oxide with molecular oxygen over Ag-Mo-W/ZrO2 catalysts[J]. Catalysis Communications, 2017, 89: 156-160. |
| 27 | Qian W J, Texter J, Yan F. Frontiers in poly(ionic liquid)s: syntheses and applications[J]. Chemical Society Reviews, 2017, 46(4): 1124-1159. |
| 28 | Zhang Y F, Zhang Y Y, Chen B H. Swelling poly (ionic liquid)s: heterogeneous catalysts that are superior than homogeneous catalyst for ethylene carbonate transformation[J]. ChemistrySelect, 2017, 2(29): 9443-9449. |
| 29 | Qin L, Wang B S, Zhang Y Y, et al. Anion exchange: a novel way of preparing hierarchical porous structure in poly(ionic liquid)s[J]. Chem. Commun., 2017, 53(26): 3785-3788. |
| 30 | Li W Y, Zong Y X, Wang J K, et al. Sulfonated poly(4-vinylpyridine) heteropolyacid salts: a reusable green solid catalyst for Mannich reaction[J]. Chinese Chemical Letters, 2014, 25(4): 575-578. |
| 31 | Wilke A, Yuan J Y, Antonietti M, et al. Enhanced carbon dioxide adsorption by a mesoporous poly(ionic liquid)[J]. ACS Macro Letters, 2012, 1(8): 1028-1031. |
| 32 | Riduan S N, Zhang Y G. Imidazolium salts and their polymeric materials for biological applications[J]. Chemical Society Reviews, 2013, 42(23): 9055-9070. |
| 33 | Wu Z W, Chen C, Guo Q R, et al. Novel approach for preparation of poly (ionic liquid) catalyst with macroporous structure for biodiesel production[J]. Fuel, 2016, 184(15): 128-135. |
| 34 | Wang Q, Cai X C, Liu Y Q, et al. Pd nanoparticles encapsulated into mesoporous ionic copolymer: efficient and recyclable catalyst for the oxidation of benzyl alcohol with O2 balloon in water[J]. Applied Catalysis. B: Environmental, 2016, 189: 242-251. |
| 35 | Wang Q, Hou W, Li S, et al. Hydrophilic mesoporous poly(ionic liquid)-supported Au-Pd alloy nanoparticles towards aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid under mild conditions[J]. Green Chemistry, 2017, 19: 3820-3830. |
| 36 | Hou W, Wang Q, Guo Z J, et al. Nanobelt α-CuV2O6 with hydrophilic mesoporous poly(ionic liquid): a binary catalyst for synthesis of 2, 5-diformylfuran from fructose[J]. Catalysis Science & Technology, 2017, 7(4): 1006-1016. |
| 37 | Opre Z, Mallat T, Baiker A. Epoxidation of styrene with cobalt-hydroxyapatite and oxygen in dimethylformamide: a green technology[J]. Journal of Catalysis, 2007, 245(2): 482-486. |
| 38 | Tang Q, Wang Y, Liang J, et al. CO2+-Exchanged faujasite zeolites as efficient heterogeneous catalysts for epoxidation of styrene with molecular oxygen[J]. Chemical Communications, 2004, 10(4): 440. |
| 39 | Volovych I, Schwarze M, Nairoukh Z, et al. Sol-gel immobilized catalyst systems for tandem transformations with trans-stilbene as an intermediate[J]. Catalysis Communications, 2014, 53: 1-4. |
| 40 | Artur B, Anabela S, Elise M, et al. MoO2 nanoparticles as highly efficient olefin epoxidation catalysts[J]. Applied Catalysis A: General, 2015, 504: 399-407. |
| 41 | Fu Y, Xu L, Shen H, et al. Tunable catalytic properties of multi-metal-organic frameworks for aerobic styrene oxidation[J]. Chemical Engineering Journal, 2016, 299: 135-141. |
| 42 | Sharma A S, Shah D, Kaur H. Gold nanoparticles supported on dendrimer@resin for the efficient oxidation of styrene using elemental oxygen[J]. RSC Adv., 2015, 5(53): 42935-42941. |
| 43 | Jin W Q, Wang H X, Lu B, et al. SO42--Fe-V/ZrO2 composite for selective oxidation of styrene to benzaldehyde in H2O2 aqueous solution[J]. Industrial & Engineering Chemistry Research, 2020, 59(10): 4411-4418. |
| 44 | Sun W, Hu J. Oxidation of styrene to benzaldehyde with hydrogen peroxide in the presence of catalysts obtained by the immobilization of H3PW12O40 on SBA-15 mesoporous material[J]. Reaction Kinetics, Mechanisms and Catalysis, 2016, 119(1): 305-318. |
| 45 | Tong J, Cai X, Wang H, et al. Improvement of catalytic activity in selective oxidation of styrene with H2O2 over spinel Mg-Cu ferrite hollow spheres in water[J]. Materials Research Bulletin, 2014, 55(7): 205-211. |
| 46 | Liu X, Ding J, Lin X, et al. Zr-doped CeO2 nanorods as versatile catalyst in the epoxidation of styrene with tert-butyl hydroperoxide as the oxidant[J]. Applied Catalysis A: General, 2015, 503: 117-123. |
| 47 | Narayanan S, Vijaya J J, Sivasanker S, et al. Hierarchical ZSM-5 catalytic performance evaluated in the selective oxidation of styrene to benzaldehyde using TBHP[J]. Journal of Porous Materials, 2016, 23(3): 741-752. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [5] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [6] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [7] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [11] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [12] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [13] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [14] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [15] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号