化工学报 ›› 2021, Vol. 72 ›› Issue (8): 4371-4380.DOI: 10.11949/0438-1157.20201787
赵林洲1( ),郑燕娥1,2(
),郑燕娥1,2( ),李孔斋2,3,王亚明1,蒋丽红1,范浩熙1,王雅静1,祝星2,3,魏永刚2,3
),李孔斋2,3,王亚明1,蒋丽红1,范浩熙1,王雅静1,祝星2,3,魏永刚2,3
收稿日期:2020-12-09
修回日期:2021-04-02
出版日期:2021-08-05
发布日期:2021-08-05
通讯作者:
郑燕娥
作者简介:赵林洲(1994—),男,硕士研究生,基金资助:
Linzhou ZHAO1( ),Yan'e ZHENG1,2(
),Yan'e ZHENG1,2( ),Kongzhai Li2,3,Yaming WANG1,Lihong JIANG1,Haoxi FAN1,Yajing WANG1,Xing ZHU2,3,Yonggang WEI2,3
),Kongzhai Li2,3,Yaming WANG1,Lihong JIANG1,Haoxi FAN1,Yajing WANG1,Xing ZHU2,3,Yonggang WEI2,3
Received:2020-12-09
Revised:2021-04-02
Online:2021-08-05
Published:2021-08-05
Contact:
Yan'e ZHENG
摘要:
化学链甲烷重整耦合CO2还原技术既能生产合成气还可以还原CO2生成CO。采用共沉淀法制备不同Ce/Ni摩尔比的系列Ce1-xNixOy(x = 0,0.2,0.4,0.6,0.8,1)氧载体。通过XRD、BET、XPS及CH4-TPR等表征对氧载体的理化性质进行了研究。系统考察了Ce1-xNixOy氧载体在化学链甲烷重整耦合CO2还原反应中的反应性能。与单一金属氧化物NiO和CeO2相比,Ce1-xNixOy复合氧载体在该反应中具有更高的活性和热稳定性。在甲烷部分氧化阶段,Ce0.2Ni0.8Oy和Ce0.4Ni0.6Oy氧载体具有较高的CH4转化率。经历了20次redox循环实验,Ce0.2Ni0.8Oy氧载体的CO2转化率几乎保持不变,表明Ce0.2Ni0.8Oy氧载体具有较高的热稳定性。
中图分类号:
赵林洲, 郑燕娥, 李孔斋, 王亚明, 蒋丽红, 范浩熙, 王雅静, 祝星, 魏永刚. Ce1-xNixOy氧载体在化学链甲烷重整耦合CO2还原中的应用[J]. 化工学报, 2021, 72(8): 4371-4380.
Linzhou ZHAO, Yan'e ZHENG, Kongzhai Li, Yaming WANG, Lihong JIANG, Haoxi FAN, Yajing WANG, Xing ZHU, Yonggang WEI. Application of Ce1-xNixOy oxygen carriers in chemical-looping reforming of methane coupled with CO2 reduction[J]. CIESC Journal, 2021, 72(8): 4371-4380.

图2 Ce1-xNixOy氧载体的N2吸附-脱附曲线(a)和孔径分布图(b)
Fig.2 Nitrogen adsorption-desorption isotherms (a) and pore-size distributions (b) of Ce1-xNixOy oxygen carriers
| 样品 | 孔体积/(cm3/g) | 平均孔径/nm | 比表面积/(m2/g) |
|---|---|---|---|
| NiO | 0.031 | 10.725 | 11.45 |
| Ce0.2Ni0.8Oy | 0.120 | 16.092 | 29.59 |
| Ce0.4Ni0.6Oy | 0.079 | 13.857 | 22.74 |
| Ce0.6Ni0.4Oy | 0.070 | 13.726 | 20.25 |
| Ce0.8Ni0.2Oy | 0.054 | 9.989 | 21.53 |
| CeO2 | 0.056 | 16.015 | 13.97 |
表1 氧载体的孔体积、平均孔径和比表面积
Table 1 The pore volumes, average pore diameter and BET surface areas of oxygen carriers
| 样品 | 孔体积/(cm3/g) | 平均孔径/nm | 比表面积/(m2/g) |
|---|---|---|---|
| NiO | 0.031 | 10.725 | 11.45 |
| Ce0.2Ni0.8Oy | 0.120 | 16.092 | 29.59 |
| Ce0.4Ni0.6Oy | 0.079 | 13.857 | 22.74 |
| Ce0.6Ni0.4Oy | 0.070 | 13.726 | 20.25 |
| Ce0.8Ni0.2Oy | 0.054 | 9.989 | 21.53 |
| CeO2 | 0.056 | 16.015 | 13.97 |
| 氧载体 | O百分比 | Osurf/Olatt | Ce百分比 | Ce3+/ Ce4+ | Ni百分比 | Ni2+/Ni3+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OⅠ | OⅡ | OⅢ | Ce3+ | Ce4+ | Ni2+ | Ni3+ | ||||
| Ce0.2Ni0.8Oy | 32.27 | 34.87 | 32.86 | 2.04 | 15.89 | 84.11 | 0.19 | 35.96 | 64.04 | 0.56 |
| Ce0.4Ni0.6Oy | 12.75 | 20.47 | 66.79 | 0.50 | 21.91 | 78.09 | 0.28 | 29.85 | 70.15 | 0.42 |
表2 Ce0.2Ni0.8Oy和Ce0.4Ni0.6Oy氧载体的相关XPS参数
Table 2 The relevant XPS parameters of Ce0.2Ni0.8Oyand Ce0.4Ni0.6Oy oxygen carriers
| 氧载体 | O百分比 | Osurf/Olatt | Ce百分比 | Ce3+/ Ce4+ | Ni百分比 | Ni2+/Ni3+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OⅠ | OⅡ | OⅢ | Ce3+ | Ce4+ | Ni2+ | Ni3+ | ||||
| Ce0.2Ni0.8Oy | 32.27 | 34.87 | 32.86 | 2.04 | 15.89 | 84.11 | 0.19 | 35.96 | 64.04 | 0.56 |
| Ce0.4Ni0.6Oy | 12.75 | 20.47 | 66.79 | 0.50 | 21.91 | 78.09 | 0.28 | 29.85 | 70.15 | 0.42 |
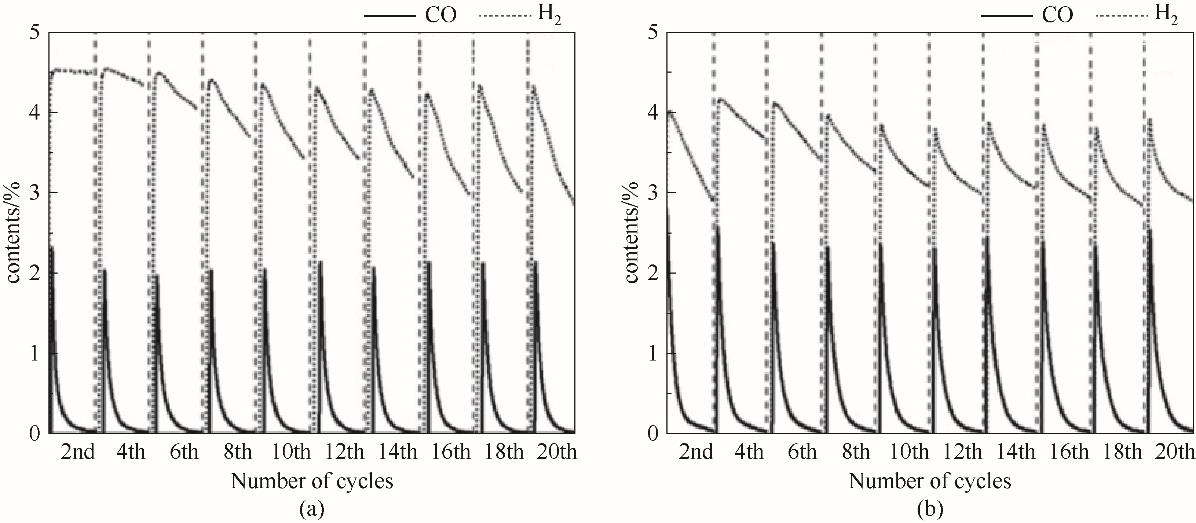
图7 Ce0.2Ni0.8Oy(a)和Ce0.4Ni0.6Oy(b)氧载体在20次redox循环中CH4氧化阶段H2和CO含量变化
Fig.7 H2 and CO contents change of Ce0.2Ni0.8Oy(a) and Ce0.4Ni0.6Oy(b) oxygen carriers in CH4 oxidation stage in 20 redox cycles
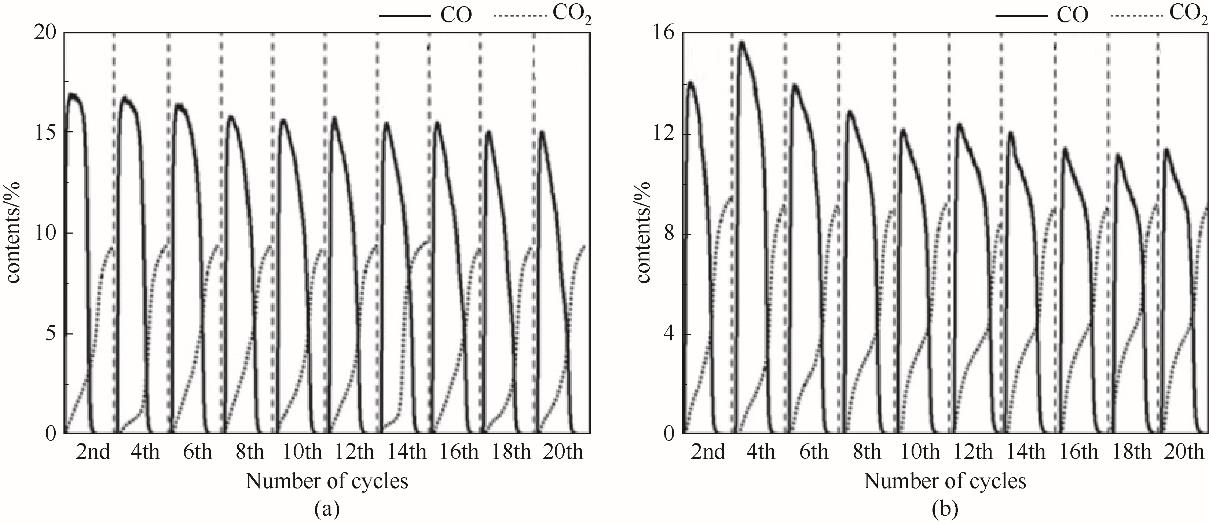
图8 Ce0.2Ni0.8Oy(a)和Ce0.4Ni0.6Oy(b)氧载体在20次redox循环中CO2还原阶段CO含量变化
Fig.8 CO contents change of Ce0.2Ni0.8Oy(a) and Ce0.4Ni0.6Oy(b) oxygen carriers in CO2 reduction stage in 20 redox cycles
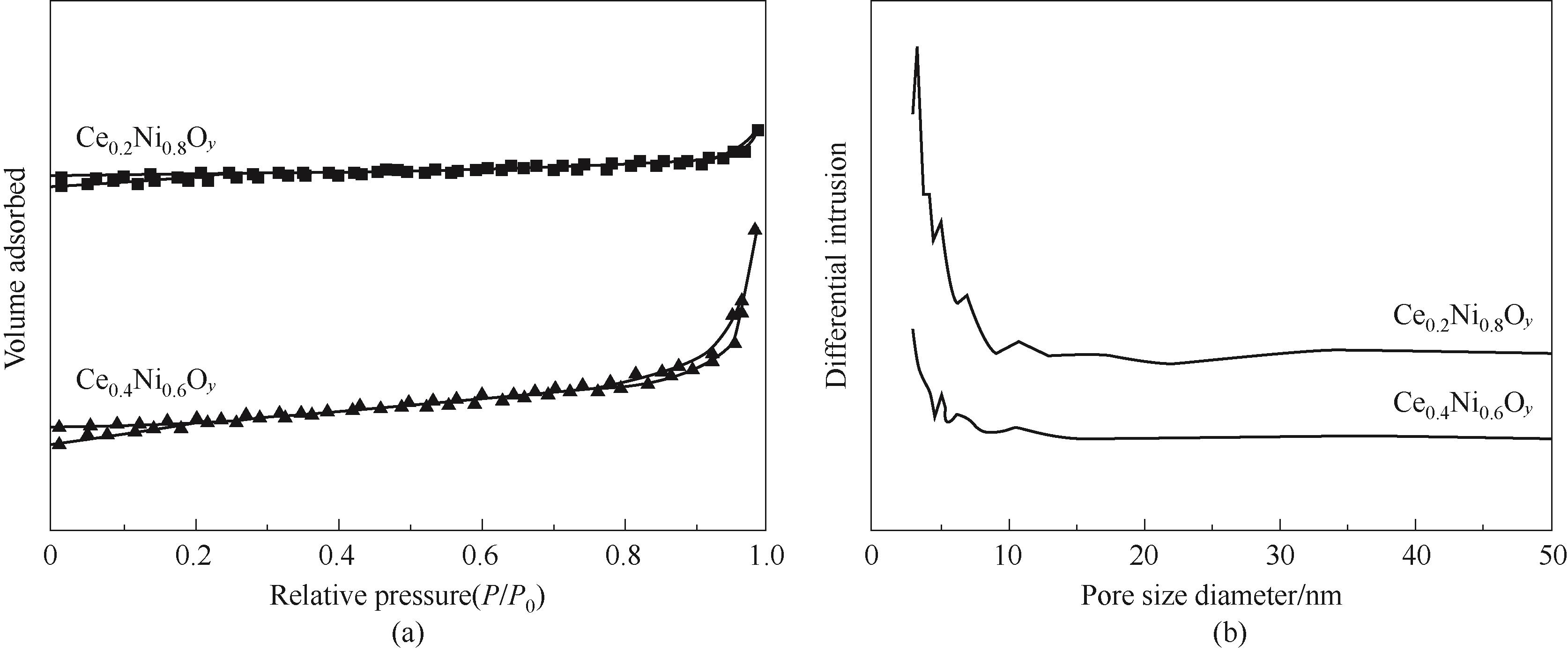
图10 20次redox循环后Ce0.2Ni0.8Oy和Ce0.4Ni0.6Oy氧载体的N2吸附-解吸曲线(a)和孔径分布图(b)
Fig.10 Nitrogen adsorption-desorption isotherms (a) and pore-size distributions (b) of Ce0.2Ni0.8Oy and Ce0.4Ni0.6Oy oxygen carriers after 20 redox cycles
| 样品 | 孔体积/(cm3/g) | 平均孔径/nm | 比表面积/(m2/g) |
|---|---|---|---|
| Ce0.2Ni0.8Oy | 0.038 | 11.525 | 10.09 |
| Ce0.4Ni0.6Oy | 0.028 | 11.531 | 7.24 |
表3 经过20次redox循环后Ce0.2Ni0.8Oy和Ce0.4Ni0.6Oy氧载体的孔体积、平均孔径和比表面积
Table 3 The pore volumes, average pore diameter and BET surface areas of Ce0.2Ni0.8Oy and Ce0.4Ni0.6Oy oxygen carriers after 20 redox cycles
| 样品 | 孔体积/(cm3/g) | 平均孔径/nm | 比表面积/(m2/g) |
|---|---|---|---|
| Ce0.2Ni0.8Oy | 0.038 | 11.525 | 10.09 |
| Ce0.4Ni0.6Oy | 0.028 | 11.531 | 7.24 |
| 氧载体 | 氧百分比 | Osurf/Olatt | Ce百分比 | Ce3+/Ce4+ | Ni百分比 | Ni2+/Ni3+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OⅠ | OⅡ | OⅢ | Ce3+ | Ce4+ | Ni2+ | Ni3+ | ||||
| Ce0.2Ni0.8Oy | 23.45 | 39.03 | 37.53 | 1.66 | 10.65 | 89.35 | 0.12 | 39.28 | 60.72 | 0.65 |
| Ce0.4Ni0.6Oy | 14.35 | 27.01 | 58.64 | 0.70 | 7.89 | 92.11 | 0.08 | 34.28 | 65.72 | 0.52 |
表4 经过20次redox循环的Ce0.2Ni0.8Oy和Ce0.4Ni0.6Oy氧载体的相关XPS参数
Table 4 The relevant XPS parameters of Ce0.2Ni0.8Oy and Ce0.4Ni0.6Oy oxygen carriers after 20 redox cycles
| 氧载体 | 氧百分比 | Osurf/Olatt | Ce百分比 | Ce3+/Ce4+ | Ni百分比 | Ni2+/Ni3+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OⅠ | OⅡ | OⅢ | Ce3+ | Ce4+ | Ni2+ | Ni3+ | ||||
| Ce0.2Ni0.8Oy | 23.45 | 39.03 | 37.53 | 1.66 | 10.65 | 89.35 | 0.12 | 39.28 | 60.72 | 0.65 |
| Ce0.4Ni0.6Oy | 14.35 | 27.01 | 58.64 | 0.70 | 7.89 | 92.11 | 0.08 | 34.28 | 65.72 | 0.52 |
| 1 | Liu L P, Ryu B, Sun Z L, et al. Monitoring and research on environmental impacts related to marine natural gas hydrates: review and future perspective[J]. Journal of Natural Gas Science and Engineering, 2019, 65: 82-107. |
| 2 | Wei C, Wang M H. Spatial distribution of greenhouse gases (CO2 and CH4) on expressways in the megacity Shanghai, China[J]. Environmental Science and Pollution Research, 2020, 27(25): 31143-31152. |
| 3 | Niu J T, Ran J Y, Chen D. Understanding the mechanism of CO2 reforming of methane to syngas on Ni@Pt surface compared with Ni (1 1 1) and Pt (1 1 1)[J]. Applied Surface Science, 2020, 513: 145840-145873. |
| 4 | Liu C C, Chen Y, Zhao Y X, et al. Nano-ZSM-5-supported cobalt for the production of liquid fuel in Fischer-Tropsch synthesis: effect of preparation method and reaction temperature[J]. Fuel, 2020, 263: 116619. |
| 5 | Angeli S D, Monteleone G, Giaconia A, et al. State-of-the-art catalysts for CH4 steam reforming at low temperature[J]. International Journal of Hydrogen Energy, 2014, 39(5): 1979-1997. |
| 6 | Abdullah B, Abd Ghani N A, Vo D V N. Recent advances in dry reforming of methane over Ni-based catalysts[J]. Journal of Cleaner Production, 2017, 162: 170-185. |
| 7 | Christian Enger B, Lødeng R, Holmen A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts[J]. Applied Catalysis A: General, 2008, 346(1/2): 1-27. |
| 8 | Sheu E J, Mokheimer E M A, Ghoniem A F. A review of solar methane reforming systems[J]. International Journal of Hydrogen Energy, 2015, 40(38): 12929-12955. |
| 9 | Mikheyev P A, Demyanov A V, Kochetov I V, et al. Ozone and oxygen atoms production in a dielectric barrier discharge in pure oxygen and O2/CH4 mixtures. Modeling and experiment[J]. Plasma Sources Science and Technology, 2020, 29(1): 015012. |
| 10 | Carrillo A J, Kim K J, Hood Z D, et al. La0.6Sr0.4Cr0.8Co0.2O3 perovskite decorated with exsolved Co nanoparticles for stable CO2 splitting and syngas production[J]. ACS Applied Energy Materials, 2020, 3(5): 4569-4579. |
| 11 | Yang Z Y, Zheng Y E, Li K Z, et al. Chemical-looping reforming of methane over La-Mn-Fe-O oxygen carriers: effect of calcination temperature[J]. Chemical Engineering Science, 2021, 229: 116085. |
| 12 | Wang Y J, Zheng Y E, Wang Y H, et al. Syngas production modified by oxygen vacancies over CeO2-ZrO2-CuO oxygen carrier via chemical looping reforming of methane[J]. Applied Surface Science, 2019, 481: 151-160. |
| 13 | Pérez-Vega R, Abad A, Izquierdo M T, et al. Evaluation of Mn-Fe mixed oxide doped with TiO2 for the combustion with CO2 capture by chemical looping assisted by oxygen uncoupling[J]. Applied Energy, 2019, 237: 822-835. |
| 14 | Wu Y J, Luo C H, Su Q Q. Study of NH3 removal based on chemical-looping combustion[J]. Industrial & Engineering Chemistry Research, 2019, 58(12): 5054-5063. |
| 15 | Liu R, Pei C L, Zhang X H, et al. Chemical looping partial oxidation over FeWOx/SiO2 catalysts[J]. Chinese Journal of Catalysis, 2020, 41(7): 1140-1151. |
| 16 | Forero C R, Gayán P, García-Labiano F, et al. High temperature behaviour of a CuO/γ-Al2O3 oxygen carrier for chemical-looping combustion[J]. International Journal of Greenhouse Gas Control, 2011, 5(4): 659-667. |
| 17 | Zhu M, Song Y H, Chen S Y, et al. Chemical looping dry reforming of methane with hydrogen generation on Fe2O3/Al2O3 oxygen carrier[J]. Chemical Engineering Journal, 2019, 368: 812-823. |
| 18 | Cao Y, He B S, Tong W X, et al. On the kinetics of Mn2O3/ZrO2 oxygen carrier for chemical looping air separation[J]. Chemical Engineering and Processing - Process Intensification, 2019, 136: 82-91. |
| 19 | Zheng Y E, Wei Y G, Li K Z, et al. Chemical-looping steam methane reforming over macroporous CeO2-ZrO2 solid solution: effect of calcination temperature[J]. International Journal of Hydrogen Energy, 2014, 39(25): 13361-13368. |
| 20 | Liu Y K, Long Y H, Tang Y Q, et al. Effect of preparation method on the structural characteristics of NiO-ZrO2 oxygen carriers for chemical-looping combustion[J]. Chemical Research in Chinese Universities, 2019, 35(6): 1024-1031. |
| 21 | Zheng Y E, Li K Z, Wang H, et al. Structure dependence and reaction mechanism of CO oxidation: a model study on macroporous CeO2 and CeO2-ZrO2 catalysts[J]. Journal of Catalysis, 2016, 344: 365-377. |
| 22 | Zheng Y E, Li K Z, Wang H, et al. Enhanced activity of CeO2-ZrO2 solid solutions for chemical-looping reforming of methane via tuning the macroporous structure[J]. Energy & Fuels, 2016, 30(1): 638-647. |
| 23 | Atzori L, Cutrufello M G, Meloni D, et al. Highly active NiO-CeO2 catalysts for synthetic natural gas production by CO2 methanation[J]. Catalysis Today, 2018, 299: 183-192. |
| 24 | Zhou G L, Liu H R, Cui K K, et al. Role of surface Ni and Ce species of Ni/CeO2 catalyst in CO2 methanation[J]. Applied Surface Science, 2016, 383: 248-252. |
| 25 | Hosseini S Y, Khosravi-Nikou M R, Shariati A. Kinetic study of the reduction step for chemical looping steam methane reforming by CeO2-Fe2O3 oxygen carriers[J]. Chemical Engineering & Technology, 2020, 43(3): 540-552. |
| 26 | Nurbaisyatul E S, Azhan H, Kasim A, et al. Effect of CeO2 nanoparticle on the structural and electrical properties of BSCCO-2223 high temperature superconductor[J]. Solid State Phenomena, 2020, 307: 104-109. |
| 27 | Lu C Q, Deng R R, Xu R D, et al. Design of hybrid oxygen carriers with CeO2 particles on MnCo2O4 microspheres for chemical looping combustion[J]. Chemical Engineering Journal, 2021, 404: 126554. |
| 28 | Ma S W, Chen S Y, Ge H J, et al. Synergistic effects of Zr and Sm co-doped Fe2O3/CeO2 oxygen carrier for chemical looping hydrogen generation[J]. Energy & Fuels, 2020, 34(8): 10256-10267. |
| 29 | Zheng Z M, Luo L X, Chen S B, et al. Activating Fe2O3 using K2CO3-containing ethanol solution for corn stalk chemical looping gasification to produce hydrogen[J]. International Journal of Hydrogen Energy, 2020, 45(41): 21004-21013. |
| 30 | Lim H S, Kang D, Lee J W. Phase transition of Fe2O3-NiO to NiFe2O4 in perovskite catalytic particles for enhanced methane chemical looping reforming-decomposition with CO2 conversion[J]. Applied Catalysis B: Environmental, 2017, 202: 175-183. |
| 31 | Isarapakdeetham S, Kim-Lohsoontorn P, Wongsakulphasatch S, et al. Hydrogen production via chemical looping steam reforming of ethanol by Ni-based oxygen carriers supported on CeO2 and La2O3 promoted Al2O3[J]. International Journal of Hydrogen Energy, 2020, 45(3): 1477-1491. |
| 32 | Medrano J A, Hamers H P, Williams G, et al. NiO/CaAl2O4 as active oxygen carrier for low temperature chemical looping applications[J]. Applied Energy, 2015, 158: 86-96. |
| 33 | Li T Y, Jayathilake R S, Taylor D D, et al. Structural studies of the perovskite series La1-xSrxCoO3-δ during chemical looping with methane[J]. Chemical Communications, 2019, 55(34): 4929-4932. |
| 34 | Löfberg A, Guerrero-Caballero J, Kane T, et al. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production[J]. Applied Catalysis B: Environmental, 2017, 212: 159-174. |
| 35 | Shan W J, Fleys M, Lapicque F, et al. Syngas production from partial oxidation of methane over Ce1-XNiXOY catalysts prepared by complexation-combustion method[J]. Applied Catalysis A: General, 2006, 311: 24-33. |
| 36 | Nematollahi B, Rezaei M, Lay E N. Preparation of highly active and stable NiO-CeO2 nanocatalysts for CO selective methanation[J]. International Journal of Hydrogen Energy, 2015, 40(27): 8539-8547. |
| 37 | Che W, Wei M R, Sang Z S, et al. Perovskite LaNiO3-δ oxide as an anion-intercalated pseudocapacitor electrode[J]. Journal of Alloys and Compounds, 2018, 731: 381-388. |
| [1] | 周小文, 杜杰, 张战国, 许光文. 基于甲烷脉冲法的Fe2O3-Al2O3载氧体还原特性研究[J]. 化工学报, 2023, 74(6): 2611-2623. |
| [2] | 葛泽峰, 吴雨青, 曾名迅, 查振婷, 马宇娜, 侯增辉, 张会岩. 灰化学成分对生物质气化特性的影响规律[J]. 化工学报, 2023, 74(5): 2136-2146. |
| [3] | 王峰, 张顺鑫, 余方博, 刘亚, 郭烈锦. 光催化CO2还原制碳氢燃料系统优化策略研究[J]. 化工学报, 2023, 74(1): 29-44. |
| [4] | 李鑫, 曾少娟, 彭奎霖, 袁磊, 张香平. CO2电催化还原制合成气研究进展及趋势[J]. 化工学报, 2023, 74(1): 313-329. |
| [5] | 孙嘉辰, 裴春雷, 陈赛, 赵志坚, 何盛宝, 巩金龙. 化学链低碳烷烃氧化脱氢技术进展[J]. 化工学报, 2023, 74(1): 205-223. |
| [6] | 袁妮妮, 郭拓, 白红存, 何育荣, 袁永宁, 马晶晶, 郭庆杰. 化学链燃烧过程Fe2O3/Al2O3载氧体表面CH4反应:ReaxFF-MD模拟[J]. 化工学报, 2022, 73(9): 4054-4061. |
| [7] | 王悦琳, 晁伟, 蓝晓程, 莫志朋, 佟淑环, 王铁峰. 合成气生物发酵法制乙醇的研究进展[J]. 化工学报, 2022, 73(8): 3448-3460. |
| [8] | 刘振宇. 煤地下气化低效的化学反应工程根源:滞留层及通道中的传质与反应[J]. 化工学报, 2022, 73(8): 3299-3306. |
| [9] | 朱莲峰, 王超, 张梦娟, 刘方正, 贾鑫, 安萍, 许光文, 韩振南. 水蒸气/氧流化床两段煤气化制备低焦油合成气工艺实验[J]. 化工学报, 2022, 73(8): 3720-3730. |
| [10] | 王佳铭, 阮雪华, 贺高红. 面向不同工业二氧化碳分离体系的膜材料研究进展[J]. 化工学报, 2022, 73(8): 3417-3432. |
| [11] | 周乐, 沈程凯, 吴超, 侯北平, 宋执环. 深度融合特征提取网络及其在化工过程软测量中的应用[J]. 化工学报, 2022, 73(7): 3156-3165. |
| [12] | 孟博, 刘艳萍, 蒋新科, 韩一帆. Fe5C2-MnO x 尺度调控及催化合成气制烯烃性能研究[J]. 化工学报, 2022, 73(6): 2677-2689. |
| [13] | 袁聪, 蒲舸, 高杰, 贾帅辉. 改性BaFe2O4载氧体生物质化学链气化特性[J]. 化工学报, 2022, 73(3): 1359-1368. |
| [14] | 王淋, 付乾, 肖帅, 李卓, 李俊, 张亮, 朱恂, 廖强. 高效可见光响应微生物/光电化学耦合人工光合作用系统[J]. 化工学报, 2022, 73(2): 887-893. |
| [15] | 王保文, 张港, 刘同庆, 李炜光, 王梦家, 林德顺, 马晶晶. CeO2/CuFe2O4氧载体CH4化学链重整耦合CO2热催化还原研究[J]. 化工学报, 2022, 73(12): 5414-5426. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号