CIESC Journal ›› 2020, Vol. 71 ›› Issue (1): 43-53.DOI: 10.11949/0438-1157.20191175
• Reviews and monographs • Previous Articles Next Articles
Xingqun PU1( ),Xiaojie JU1,2,Rui XIE1,2,Wei WANG1,2,Zhuang LIU1,2,Liangyin CHU1,2(
),Xiaojie JU1,2,Rui XIE1,2,Wei WANG1,2,Zhuang LIU1,2,Liangyin CHU1,2( )
)
Received:2019-10-11
Revised:2019-10-16
Online:2020-01-05
Published:2020-01-05
Contact:
Liangyin CHU
蒲兴群1( ),巨晓洁1,2,谢锐1,2,汪伟1,2,刘壮1,2,褚良银1,2(
),巨晓洁1,2,谢锐1,2,汪伟1,2,刘壮1,2,褚良银1,2( )
)
通讯作者:
褚良银
作者简介:蒲兴群(1994—),女,博士研究生,基金资助:CLC Number:
Xingqun PU, Xiaojie JU, Rui XIE, Wei WANG, Zhuang LIU, Liangyin CHU. Polymeric microneedle arrays for applications in transdermal drug delivery systems[J]. CIESC Journal, 2020, 71(1): 43-53.
蒲兴群, 巨晓洁, 谢锐, 汪伟, 刘壮, 褚良银. 聚合物阵列微针及其在透皮给药系统的应用[J]. 化工学报, 2020, 71(1): 43-53.
Add to citation manager EndNote|Ris|BibTeX
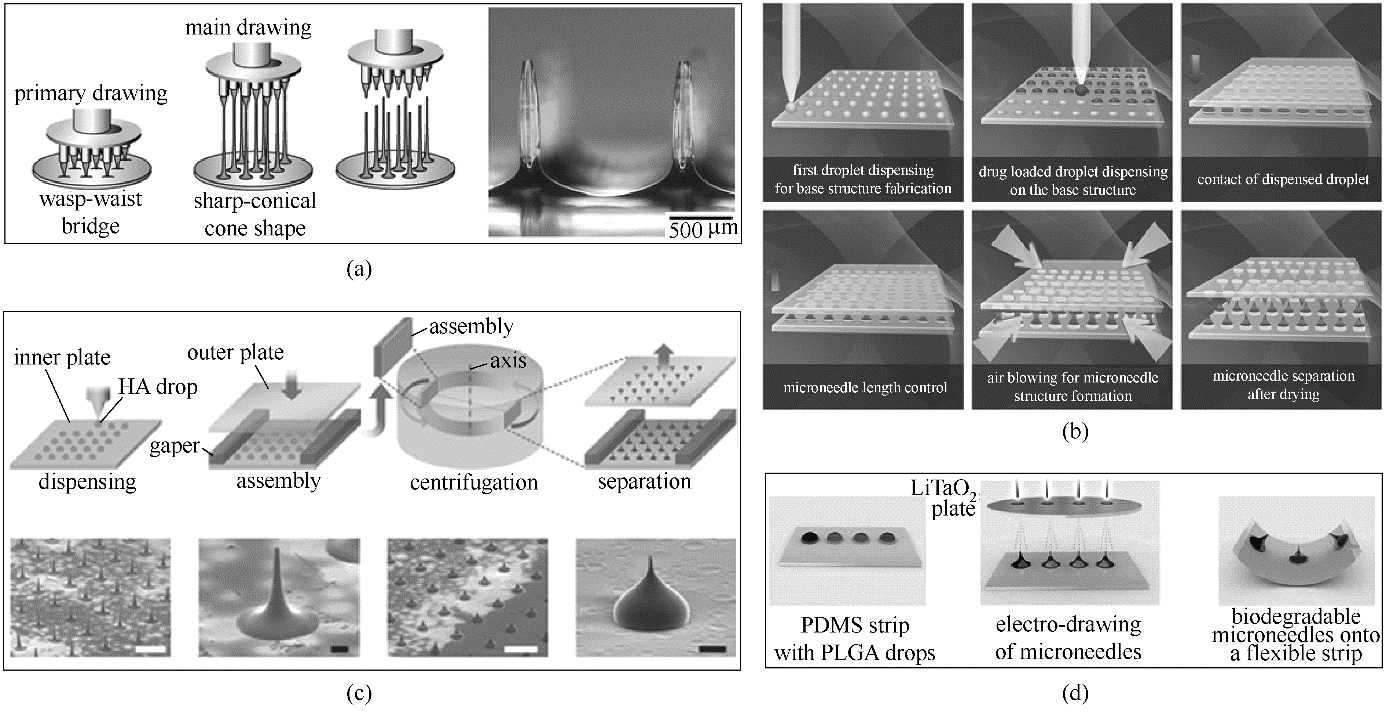
Fig.2 Schematic illustration of drawing techniques(a) drawing lithography [56]; (b) droplet-born air blowing [60]; (c) centrifugal lithography [61]; (d) electro-drawing [63]
| 1 | 刘基, 王媚, 王露, 等. 经皮给药系统研究进展[J]. 现代中医药, 2018, 38(6): 160-163. |
| Liu J, Wang M, Wang L, et al. Research progress in transdermal drug delivery system[J]. Modern Traditional Chinese Medicine, 2018, 38(6): 160-163. | |
| 2 | 张振波, 房德敏. 微针技术在经皮给药系统的研究进展[J]. 天津药学, 2018, 30(6): 44-47. |
| Zhang Z B, Fang D M. Research progress in transdermal drug delivery system of microneedle technology[J]. Tianjin Pharmacy, 2018, 30(6): 44-47. | |
| 3 | Larrañeta E, McCrudden M T C, Courtenay A J, et al. Microneedles: a new frontier in nanomedicine delivery[J]. Pharmaceutical Research, 2016, 33(5): 1055-1073. |
| 4 | 沈瑞雪, 朱壮志, 章俊云, 等. 可溶性微针在经皮给药系统中的开发进展[J]. 世界临床药物, 2017, 38(9): 638-642. |
| Shen R X, Zhu Z Z, Zhang J Y, et al. Dissolving microneedle, a novel transdermal drug delivery system[J]. World Clinical Drugs, 2017, 38(9): 638-642. | |
| 5 | Wang H L, Fan P F, Guo X S, et al. Ultrasound-mediated transdermal drug delivery of fluorescent nanoparticles and hyaluronic acid into porcine skin in vitro[J]. Chinese Physics B, 2016, 25(12): 124314. |
| 6 | Cassagne M, Laurent C, Rodrigues M, et al. Iontophoresis transcorneal delivery technique for transepithelial corneal collagen crosslinking with riboflavin in a rabbit model[J]. Investigative Ophthalmology & Visual Science, 2016, 57(2): 594-603. |
| 7 | Lambricht L, Lopes A, Kos S, et al. Clinical potential of electroporation for gene therapy and DNA vaccine delivery[J]. Expert Opinion on Drug Delivery, 2016, 13(2): 295-310. |
| 8 | Pham Q D, Björklund S, Engblom J, et al. Chemical penetration enhancers in stratum corneum-relation between molecular effects and barrier function[J]. Journal of Controlled Release, 2016, 232: 175-187. |
| 9 | Henry S, McAllister D V, Allen M G, et al. Microfabricated microneedles: a novel approach to transdermal drug delivery[J]. Journal of Pharmaceutical Sciences, 1998, 87(8): 922-925. |
| 10 | 朱凤, 金凡茂, 赵昱, 等. 微针经皮给药技术研究进展[J]. 中国生化药物杂志, 2016, 36(8): 149-152. |
| Zhu F, Jin F M, Zhao Y, et al. Research progress in transdermal deliver technology of micro needle[J]. Chinese Journal of Biochemical and Pharmaceutics, 2016, 36(8): 149-152. | |
| 11 | Hao Y, Li W, Zhou X L, et al. Microneedles-based transdermal drug delivery systems: a review[J]. Journal of Biomedical Nanotechnology, 2017, 13(12): 1581-1597. |
| 12 | Vinayakumar K B, Hegde G M, Nayak M M, et al. Fabrication and characterization of gold coated hollow silicon microneedle array for drug delivery[J]. Microelectronic Engineering, 2014, 128: 12-18. |
| 13 | Shin J H, Noh J Y, Kim K H, et al. Effect of zymosan and poly (I: C) adjuvants on responses to microneedle immunization coated with whole inactivated influenza vaccine[J]. Journal of Controlled Release, 2017, 265: 83-92. |
| 14 | Chong R H E, Gonzalez-Gonzalez E, Lara M F, et al. Gene silencing following siRNA delivery to skin via coated steel microneedles: in vitro and in vivo proof-of-concept[J]. Journal of Controlled Release, 2013, 166(3): 211-219. |
| 15 | Ruan W, Zhai Y, Yu K, et al. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment[J]. International Journal of Pharmaceutics, 2018, 553(1/2): 298-309. |
| 16 | Dharadhar S, Majumdar A, Dhoble S, et al. Microneedles for transdermal drug delivery: a systematic review[J]. Drug Development and Industrial Pharmacy, 2019, 45(2): 188-201. |
| 17 | Zhang Y, Yu J, Wang J, et al. Thrombin-responsive transcutaneous patch for auto-anticoagulant regulation[J]. Advanced Materials, 2017, 29(4): 1604043. |
| 18 | Jeong H R, Kim J Y, Kim S N, et al. Local dermal delivery of cyclosporin A, a hydrophobic and high molecular weight drug, using dissolving microneedles[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2018, 127: 237-243. |
| 19 | Kim H G, Gater D L, Kim Y C. Development of transdermal vitamin D3 (VD3) delivery system using combinations of PLGA nanoparticles and microneedles[J]. Drug Delivery and Translational Research, 2018, 8(1): 281-290. |
| 20 | Donadei A, Kraan H, Ophorst O, et al. Skin delivery of trivalent Sabin inactivated poliovirus vaccine using dissolvable microneedle patches induces neutralizing antibodies[J]. Journal of Controlled Release, 2019, 311: 96-103. |
| 21 | Ling M H, Chen M C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats[J]. Acta Biomaterialia, 2013, 9(11): 8952-8961. |
| 22 | Li Y, Liu F, Su C, et al. Biodegradable therapeutic microneedle patch for rapidly antihypertensive treatment[J]. ACS Applied Materials & Interfaces, 2019, 11(34): 30575-30584. |
| 23 | Ali A A, McCrudden C M, McCaffrey J, et al. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles[J]. Nanomedicine: Nanotechnology, Biology and Medicine, 2017, 13(3): 921-932. |
| 24 | Wei Z, Zheng S, Wang R, et al. A flexible microneedle array as low-voltage electroporation electrodes for in vivo DNA and siRNA delivery[J]. Lab on a Chip, 2014, 14(20): 4093-4102. |
| 25 | Wang C, Ye Y, Hochu G M, et al. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody[J]. Nano Letters, 2016, 16(4): 2334-2340. |
| 26 | Ye Y, Wang J, Hu Q, et al. Synergistic transcutaneous immunotherapy enhances antitumor immune responses through delivery of checkpoint inhibitors[J]. ACS Nano, 2016, 10(9): 8956-8963. |
| 27 | Esser E S, Romanyuk A A, Vassilieva E V, et al. Tetanus vaccination with a dissolving microneedle patch confers protective immune responses in pregnancy[J]. Journal of Controlled Release, 2016, 236: 47-56. |
| 28 | Sullivan S P, Koutsonanos D G, del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination[J]. Nature Medicine, 2010, 16(8): 915-920. |
| 29 | Sharma S, Hatware K, Bhadane P, et al. Recent advances in microneedle composites for biomedical applications: advanced drug delivery technologies[J]. Materials Science and Engineering: C, 2019, 103: 109717. |
| 30 | Lahiji S F, Jang Y, Ma Y, et al. Effects of dissolving microneedle fabrication parameters on the activity of encapsulated lysozyme[J]. European Journal of Pharmaceutical Sciences, 2018, 117: 290-296. |
| 31 | Leone M, Mönkäre J, Bouwstra J A, et al. Dissolving microneedle patches for dermal vaccination[J]. Pharmaceutical Research, 2017, 34(11): 2223-2240. |
| 32 | Wang Q L, Zhu D D, Chen Y, et al. A fabrication method of microneedle molds with controlled microstructures[J]. Materials Science and Engineering: C, 2016, 65: 135-142. |
| 33 | 赵笑, 李欣芳, 张鹏, 等. 聚合物微针介导经皮给药的研究[J]. 化学进展, 2017, 29(12): 1518-1525. |
| Zhao X, Li X F, Zhang P, et al. Research of polymeric microneedles for transdermal drug delivery[J]. Progress in Chemistry, 2017, 29(12): 1518-1525. | |
| 34 | McGrath M G, Vucen S, Vrdoljak A, et al. Production of dissolvable microneedles using an atomised spray process: effect of microneedle composition on skin penetration[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2014, 86(2): 200-211. |
| 35 | Lee J W, Choi S O, Felner E I, et al. Dissolving microneedle patch for transdermal delivery of human growth hormone[J].Small, 2011, 7(4): 531-539. |
| 36 | Larraneta E, Lutton R E M, Woolfson A D, et al. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development[J]. Materials Science and Engineering: RReports, 2016, 104: 1-32. |
| 37 | Chen M C, Ling M H, Lai K Y, et al. Chitosan microneedle patches for sustained transdermal delivery of macromolecules[J]. Biomacromolecules, 2012, 13(12): 4022-4031. |
| 38 | Meng W, Huang Y, Fu Y, et al. Polymer composites of boron nitride nanotubes and nanosheets[J]. Journal of Materials Chemistry C, 2014, 2(47): 10049-10061. |
| 39 | Chen J, Qiu Y, Zhang S, et al. Dissolving microneedle-based intradermal delivery of interferon-α-2b[J]. Drug Development and Industrial Pharmacy, 2016, 42(6): 890-896. |
| 40 | Di J, Yao S, Ye Y, et al. Stretch-triggered drug delivery from wearable elastomer films containing therapeutic depots[J]. ACS Nano, 2015, 9(9): 9407-9415. |
| 41 | Zheng H, Yin L, Zhang X, et al. Redox sensitive shell and core crosslinked hyaluronic acid nanocarriers for tumor-targeted drug delivery[J]. Journal of Biomedical Nanotechnology, 2016, 12(8): 1641-1653. |
| 42 | Gao Y, Sarfraz M K, Clas S D, et al. Hyaluronic acid-tocopherol succinate-based self-assembling micelles for targeted delivery of rifampicin to alveolar macrophages[J]. Journal of Biomedical Nanotechnology, 2015, 11(8): 1312-1329. |
| 43 | Yu J, Zhang Y, Ye Y, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery[J]. Proceedings of the National Academy of Sciences, 2015, 112(27): 8260-8265. |
| 44 | Park Y H, Ha S K, Choi I, et al. Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery[J]. Biotechnology and Bioprocess Engineering, 2016, 21(1): 110-118. |
| 45 | Xu H, Li X, Kong H, et al. Characterization of the uptake efficiency and cytotoxicity of tetrandrine-loaded poly(N-vinylpyrrolidone)-block-poly (ε-caprolactone)(PVP-b-PCL) nanoparticles in the A549 lung adenocarcinoma cell line[J]. Journal of Biomedical Nanotechnology, 2016, 12(8): 1699-1707. |
| 46 | McGrath M G, Vucen S, Vrdoljak A, et al. Production of dissolvable microneedles using an atomised spray process: effect of microneedle composition on skin penetration[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2014, 86(2): 200-211. |
| 47 | Nguyen H X, Bozorg B D, Kim Y, et al. Poly (vinyl alcohol) microneedles: fabrication, characterization, and application for transdermal drug delivery of doxorubicin[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2018, 129: 88-103. |
| 48 | Yang S, Feng Y, Zhang L, et al. A scalable fabrication process of polymer microneedles[J]. International Journal of Nanomedicine, 2012, 7: 1415-1422. |
| 49 | Tanpichai S, Oksman K. Crosslinked poly (vinyl alcohol) composite films with cellulose nanocrystals: mechanical and thermal properties[J]. Journal of Applied Polymer Science, 2018, 135(3): 45710. |
| 50 | DeMuth P C, Li A V, Abbink P, et al. Vaccine delivery with microneedle skin patches in nonhuman primates[J]. Nature Biotechnology, 2013, 31(12): 1082-1085. |
| 51 | Chen M C, Wang K W, Chen D H, et al. Remotely triggered release of small molecules from LaB6@ SiO2-loaded polycaprolactone microneedles[J]. Acta Biomaterialia, 2015, 13: 344-353. |
| 52 | Xin L, Cao J Q, Liu C, et al. Evaluation of rMETase-loaded stealth PLGA/liposomes modified with anti-CAGE scFV for treatment of gastric carcinoma[J]. Journal of Biomedical Nanotechnology, 2015, 11(7): 1153-1161. |
| 53 | Han M, Kim D K, Kang S H, et al. Improvement in antigen-delivery using fabrication of a grooves-embedded microneedle array[J]. Sensors and Actuators B: Chemical, 2009, 137(1): 274-280. |
| 54 | Sammoura F, Kang J J, Heo Y M, et al. Polymeric microneedle fabrication using a microinjection molding technique[J]. Microsystem Technologies, 2007, 13(5/6): 517-522. |
| 55 | Worgull M, Kolew A, Heilig M, et al. Hot embossing of high performance polymers[J]. Microsystem Technologies, 2011, 17(4): 585-592. |
| 56 | Lee K, Lee C Y, Jung H. Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose[J]. Biomaterials, 2011, 32(11): 3134-3140. |
| 57 | Lee K, Lee H C, Lee D S, et al. Drawing lithography: three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle[J]. Advanced Materials, 2010, 22(4): 483-486. |
| 58 | Zhang J, Wang Y, Jin J Y, et al. Use of drawing lithography-fabricated polyglycolic acid microneedles for transdermal delivery of itraconazole to a human basal cell carcinoma model regenerated on mice[J]. Journal of Metals, 2016, 68(4): 1128-1133. |
| 59 | Lee K, Jung H. Drawing lithography for microneedles: a review of fundamentals and biomedical applications[J]. Biomaterials, 2012, 33(30): 7309-7326. |
| 60 | Kim J D, Kim M, Yang H, et al. Droplet-born air blowing: novel dissolving microneedle fabrication[J]. Journal of Controlled Release, 2013, 170(3): 430-436. |
| 61 | Huh I, Kim S, Yang H, et al. Effects of two droplet-based dissolving microneedle manufacturing methods on the activity of encapsulated epidermal growth factor and ascorbic acid[J]. European Journal of Pharmaceutical Sciences, 2018, 114: 285-292. |
| 62 | Yang H, Kim S, Kang G, et al. Centrifugal lithography: self-shaping of polymer microstructures encapsulating biopharmaceutics by centrifuging polymer drops[J]. Advanced Healthcare Materials, 2017, 6(19): 1700326. |
| 63 | Vecchione R, Coppola S, Esposito E, et al. Electro-drawn drug-loaded biodegradable polymer microneedles as a viable route to hypodermic injection[J]. Advanced Functional Materials, 2014, 24(23): 3515-3523. |
| 64 | Anderson K B, Lockwood S Y, Martin R S, et al. A 3D printed fluidic device that enables integrated features[J]. Analytical Chemistry, 2013, 85(12): 5622-5626. |
| 65 | 杨雅丽, 童想柳, 边琼, 等. 3D 打印技术在透皮领域的研究进展[J]. 中国医药工业杂志, 2018, 49(11): 1492-1499. |
| Yang Y L, Tong X L, Bian Q, et al. Research advances of 3D printing technology in tandermal drug delivery system[J]. Chinese Journal of Pharmaceuticals, 2018, 49(11): 1492-1499. | |
| 66 | Johnson A R, Caudill C L, Tumbleston J R, et al. Single-step fabrication of computationally designed microneedles by continuous liquid interface production[J]. Public Library of Science One, 2016, 11(9): e0162518. |
| 67 | Bhatnagar S, Bankar N G, Kulkarni M V, et al. Dissolvable microneedle patch containing doxorubicin and docetaxel is effective in 4T1 xenografted breast cancer mouse model[J]. International Journal of Pharmaceutics, 2019, 556: 263-275. |
| 68 | Pei P, Yang F, Liu J, et al. Composite-dissolving microneedle patches for chemotherapy and photothermal therapy in superficial tumor treatment[J]. Biomaterials Science, 2018, 6(6): 1414-1423. |
| 69 | Hao Y, Chen Y W, Lei M Y, et al. Near-infrared responsive PEGylated gold nanorod and doxorubicin loaded dissolvable hyaluronic acid microneedles for human epidermoid cancer therapy[J]. Advanced Therapeutics, 2018, 1(2): 1800008. |
| 70 | Zhao X, Li X, Zhang P, et al. Tip-loaded fast-dissolving microneedle patches for photodynamic therapy of subcutaneous tumor[J]. Journal of Controlled Release, 2018, 286: 201-209. |
| 71 | Pan J, Ruan W, Qin M, et al. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles[J]. Scientific Reports, 2018, 8(1): 1117. |
| 72 | GhavamiNejad A, Li J, Lu B, et al. Glucose-responsive composite microneedle patch for hypoglycemia-triggered delivery of native glucagon[J]. Advanced Materials, 2019, 31(30): 1901051. |
| 73 | Liu S, Jin M, Quan Y, et al. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin[J]. Journal of Controlled Release, 2012, 161(3): 933-941. |
| 74 | Yu W, Jiang G, Zhang Y, et al. Polymer microneedles fabricated from alginate and hyaluronate for transdermal delivery of insulin[J]. Materials Science and Engineering: C, 2017, 80: 187-196. |
| 75 | Chen M C, Ling M H, Kusuma S J. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin[J]. Acta Biomaterialia, 2015, 24: 106-116. |
| 76 | Ye Y, Yu J, Wang C, et al. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery[J]. Advanced Materials, 2016, 28(16): 3115-3121. |
| 77 | Hu X, Yu J, Qian C, et al. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery[J]. ACS Nano, 2017, 11(1): 613-620. |
| 78 | Yu J, Qian C, Zhang Y, et al. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery[J]. Nano Letters, 2017, 17(2): 733-739. |
| 79 | Tong Z, Zhou J, Zhong J, et al. Glucose-and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats[J]. ACS Applied Materials & Interfaces, 2018, 10(23): 20014-20024. |
| 80 | Zhang Y, Wang J, Yu J, et al. Bioresponsive microneedles with a sheath structure for H2O2 and pH cascade-triggered insulin delivery[J]. Small, 2018, 14(14): 1704181. |
| 81 | Nagao K, Ginhoux F, Leitner W W, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions[J]. Proceedings of the National Academy of Sciences, 2009, 106(9): 3312-3317. |
| 82 | Pearton M, Pirri D, Kang S M, et al. Host responses in human skin after conventional intradermal injection or microneedle administration of virus-like-particle influenza vaccine[J]. Advanced Healthcare Materials, 2013, 2(10): 1401-1410. |
| 83 | Moon S, Wang Y, Edens C, et al. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch[J]. Vaccine, 2013, 31(34): 3396-3402. |
| 84 | Liao J F, Lee J C, Lin C K, et al. Self-assembly DNA polyplex vaccine inside dissolving microneedles for high-potency intradermal vaccination[J]. Theranostics, 2017, 7(10): 2593-2605. |
| 85 | Chen Y H, Lai K Y, Chiu Y H, et al. Implantable microneedles with an immune-boosting function for effective intradermal influenza vaccination[J]. Acta Biomaterialia, 2019, 97: 230-238. |
| 86 | Tang J, Wang J, Huang K, et al. Cardiac cell-integrated microneedle patch for treating myocardial infarction[J]. Science Advances, 2018, 4(11): eaat9365. |
| 87 | Zhang Y, Liu Q, Yu J, et al. Locally induced adipose tissue browning by microneedle patch for obesity treatment[J]. ACS Nano, 2017, 11(9): 9223-9230. |
| [1] | Junkun TAN, Yudong LIU, Shichao GENG, Bing CHEN, Mingwei TONG. Test and numerical simulation of freezing and rewarming performance of vacuum probe [J]. CIESC Journal, 2020, 71(4): 1440-1449. |
| [2] |
JIANG Guoqiang, ZHU Dequan, ZAN Jia, DING Fuxin.
Transdermal drug delivery by electroporation: The effects of surfactants on pathway lifetime and drug transport [J]. , 2007, 15(3): 397-402. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||