CIESC Journal ›› 2025, Vol. 76 ›› Issue (10): 5336-5350.DOI: 10.11949/0438-1157.20250409
• Energy and environmental engineering • Previous Articles Next Articles
Shangfei SONG1( ), Yunchao LI1, Wenyu WU1, Yumo ZHU1, Qingyun LIAO2, Najia LIAO3, Bohui SHI1(
), Yunchao LI1, Wenyu WU1, Yumo ZHU1, Qingyun LIAO2, Najia LIAO3, Bohui SHI1( ), Jing GONG1
), Jing GONG1
Received:2025-04-17
Revised:2025-07-22
Online:2025-11-25
Published:2025-10-25
Contact:
Bohui SHI
宋尚飞1( ), 李匀超1, 吴文宇1, 朱羽墨1, 廖清云2, 廖那伽3, 史博会1(
), 李匀超1, 吴文宇1, 朱羽墨1, 廖清云2, 廖那伽3, 史博会1( ), 宫敬1
), 宫敬1
通讯作者:
史博会
作者简介:宋尚飞(1993—),男,博士,副教授,song.sf@cup.edu.cn
基金资助:CLC Number:
Shangfei SONG, Yunchao LI, Wenyu WU, Yumo ZHU, Qingyun LIAO, Najia LIAO, Bohui SHI, Jing GONG. Influence mechanism of free guest molecules in liquid phase on decomposition kinetics of CO₂-CH₄ hydrates[J]. CIESC Journal, 2025, 76(10): 5336-5350.
宋尚飞, 李匀超, 吴文宇, 朱羽墨, 廖清云, 廖那伽, 史博会, 宫敬. 液相游离客体分子对CO2-CH4水合物分解动力学的影响机理[J]. 化工学报, 2025, 76(10): 5336-5350.
Add to citation manager EndNote|Ris|BibTeX
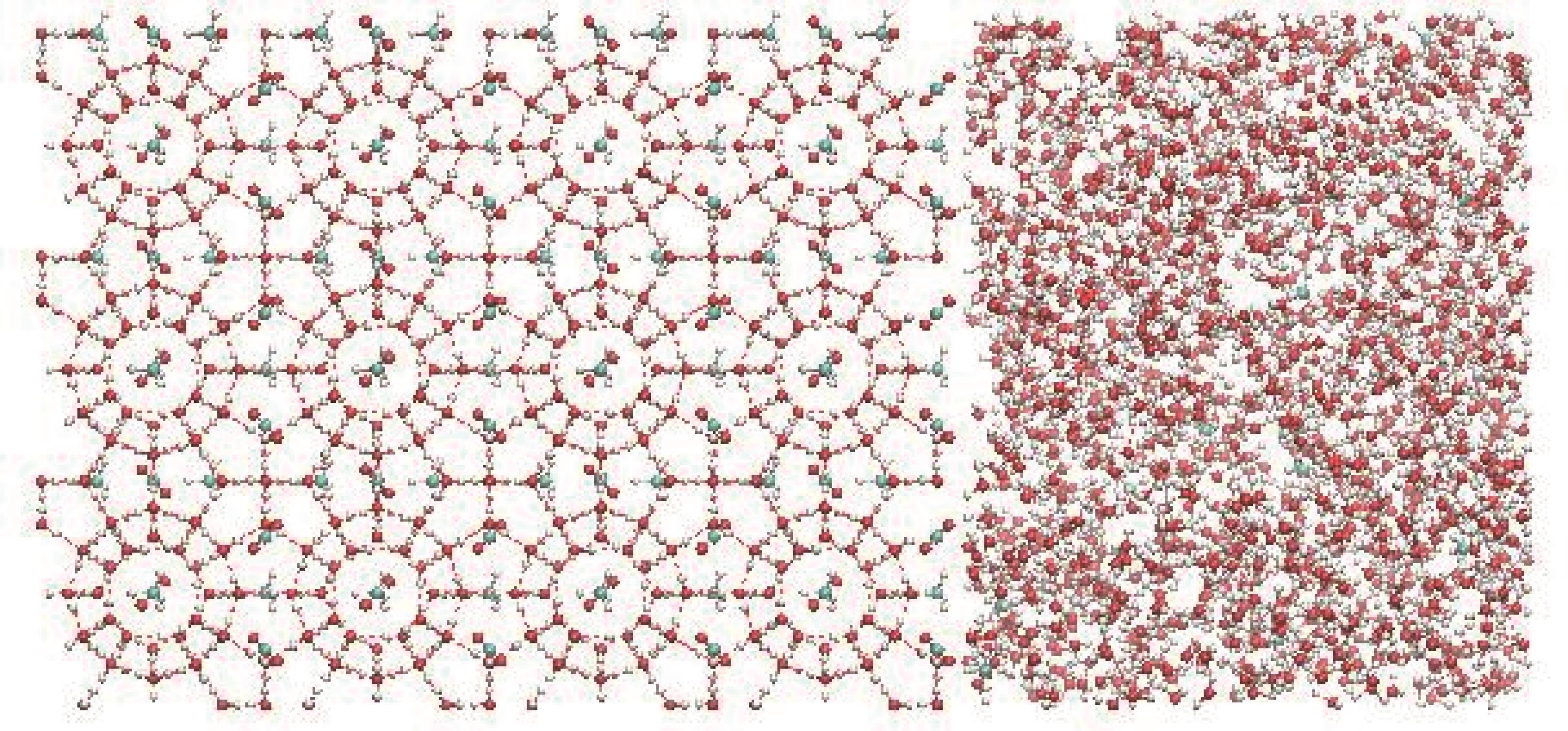
Fig.2 CO2-CH4 hydrate system with a molecular quantity ratio of 1∶1 (red spheres represent O atom, cyan spheres represent C atom, and white spheres represent H atom)
| 编号 | 模拟编号 | 液相中客体分子种类 | 液相中客体分子摩尔分数/% | 液相中客体分子数量 |
|---|---|---|---|---|
| 1 | 1-0 | — | 0 | 0 |
| 2 | M1-1 | CH4 | 1 | 15 |
| 3 | M1-5 | CH4 | 5 | 75 |
| 4 | M1-10 | CH4 | 10 | 150 |
| 5 | M1-12 | CH4 | 12 | 180 |
| 6 | M1-15 | CH4 | 15 | 225 |
| 7 | C1-1 | CO2 | 1 | 15 |
| 8 | C1-5 | CO2 | 5 | 75 |
| 9 | C1-10 | CO2 | 10 | 150 |
| 10 | C1-12 | CO2 | 12 | 180 |
| 11 | C1-15 | CO2 | 15 | 225 |
Table 1 Parameters of simulation systems for CO₂-CH₄ hydrate decomposition kinetic at 280 K
| 编号 | 模拟编号 | 液相中客体分子种类 | 液相中客体分子摩尔分数/% | 液相中客体分子数量 |
|---|---|---|---|---|
| 1 | 1-0 | — | 0 | 0 |
| 2 | M1-1 | CH4 | 1 | 15 |
| 3 | M1-5 | CH4 | 5 | 75 |
| 4 | M1-10 | CH4 | 10 | 150 |
| 5 | M1-12 | CH4 | 12 | 180 |
| 6 | M1-15 | CH4 | 15 | 225 |
| 7 | C1-1 | CO2 | 1 | 15 |
| 8 | C1-5 | CO2 | 5 | 75 |
| 9 | C1-10 | CO2 | 10 | 150 |
| 10 | C1-12 | CO2 | 12 | 180 |
| 11 | C1-15 | CO2 | 15 | 225 |
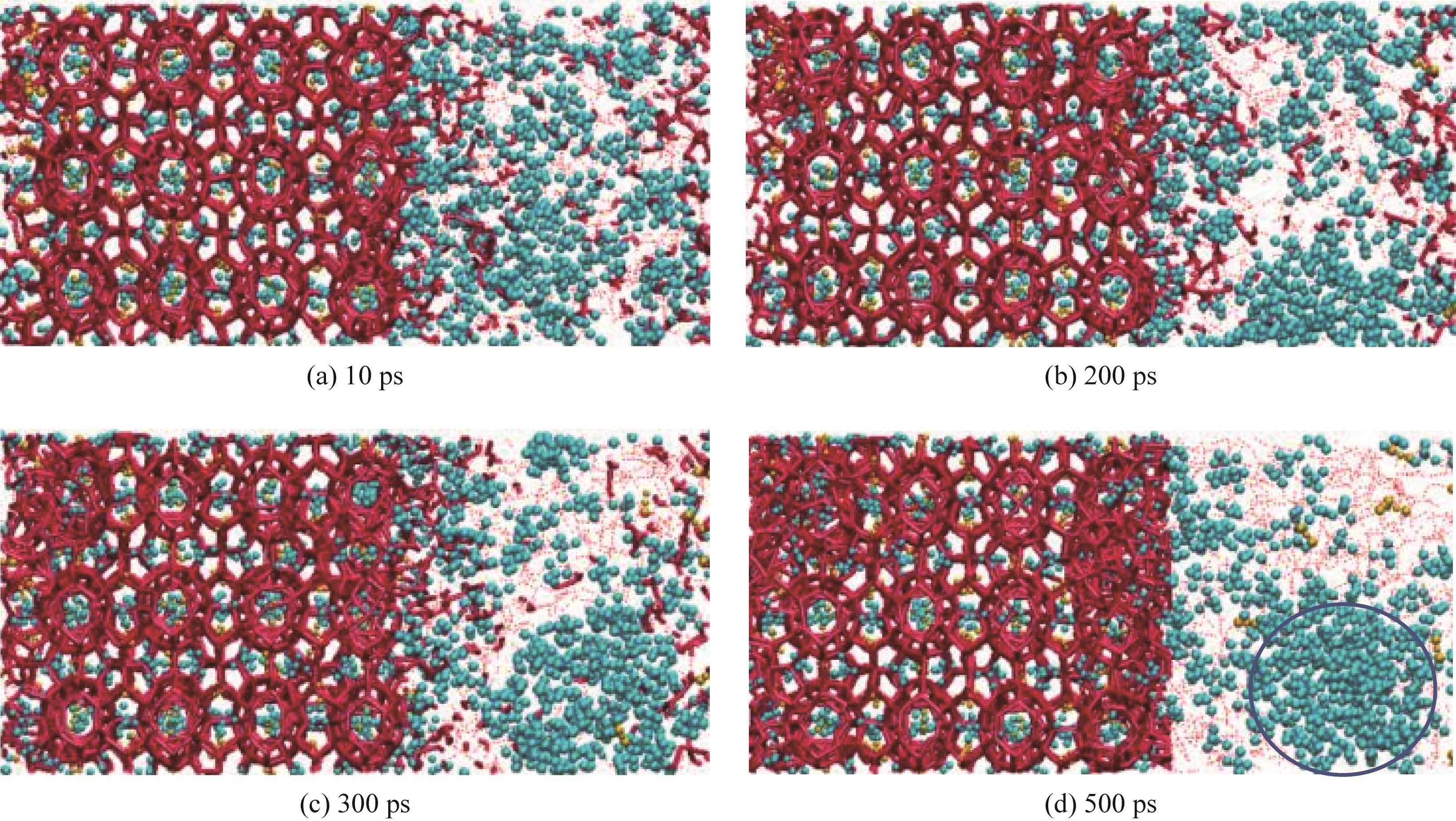
Fig.3 Simulated snapshot of hydrate decomposition process at different time points (10—500 ps) of M2-12(blue spheres represent methane molecules, yellow spheres represent carbon dioxide molecules, bold solid red lines indicate the structural framework formed by water molecules in the hydrate, and dashed red lines denote hydrogen bonds formed by water molecules in the liquid phase,the blue circle is a nanobubble,all simulation snapshots adopt the same representation scheme )
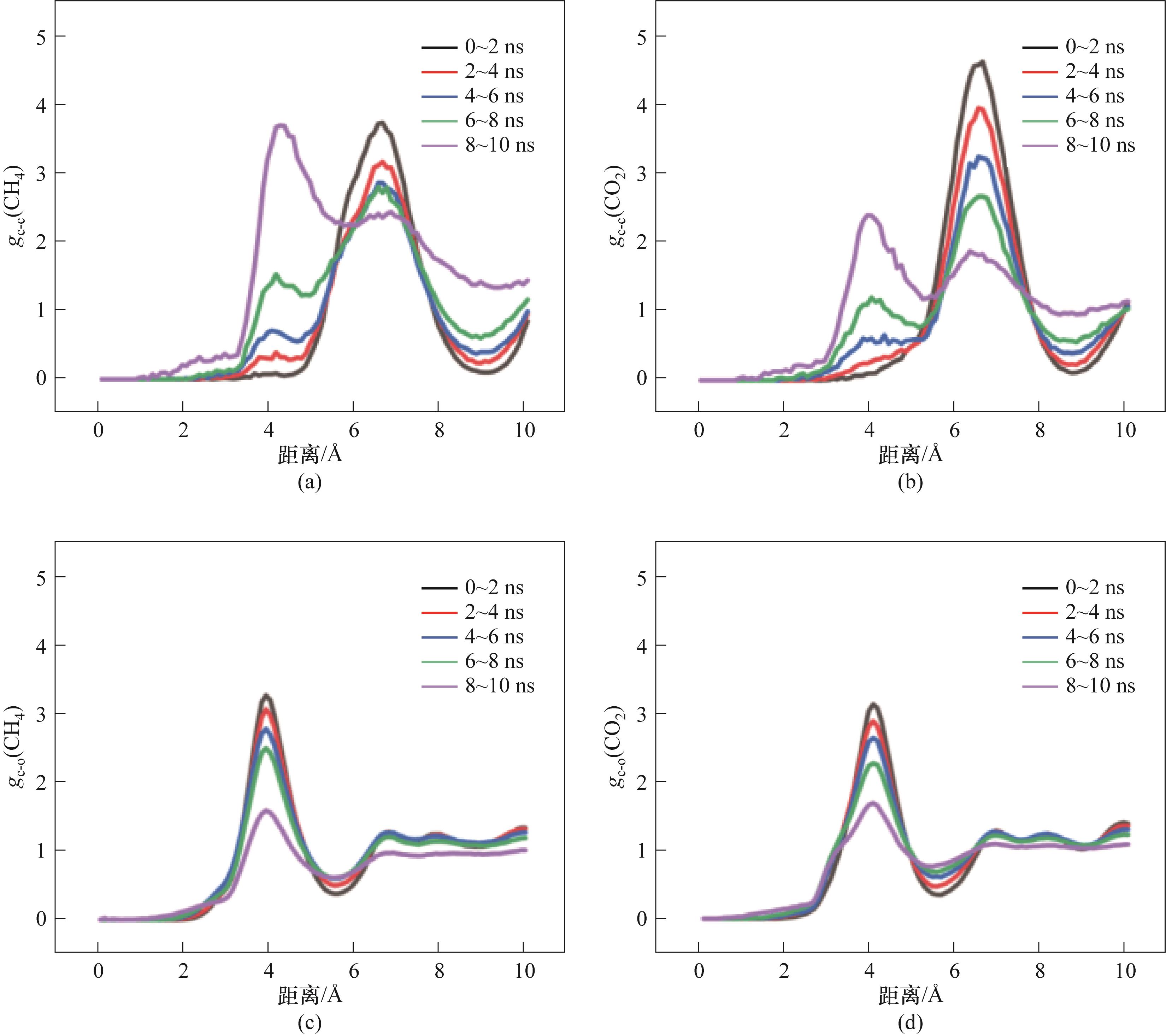
Fig.15 RDF of M2-1:(a) RDF between CH4 molecules; (b) RDF between CO2 molecules; (c) RDF between CH4 molecules and H2O molecules; (d) RDF between CO2 molecules and H2O molecules
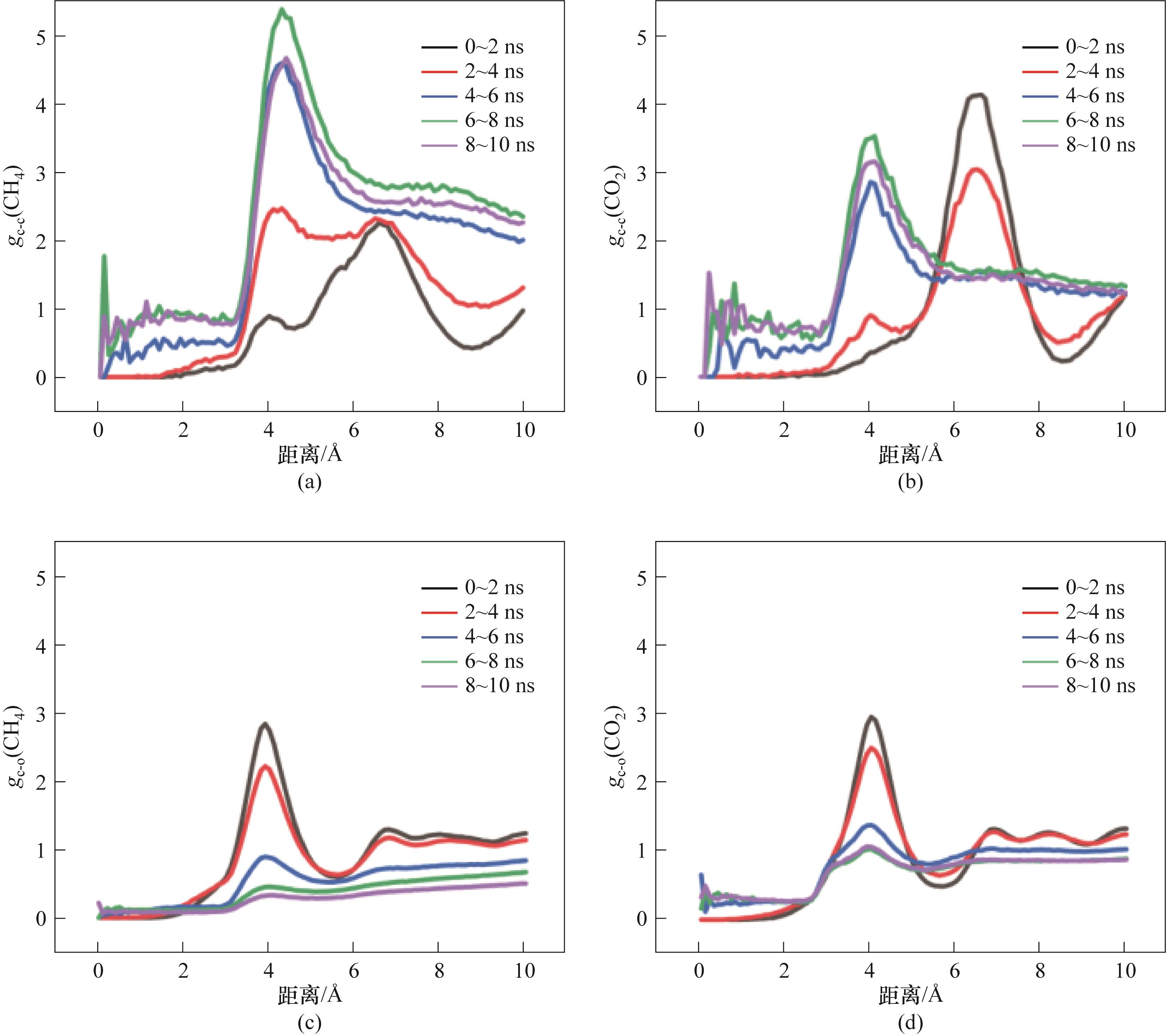
Fig.16 RDF of M2-5:(a) RDF between CH4 molecules; (b) RDF between CO2 molecules; (c) RDF between CH4 molecules and H2O molecules; (d) RDF between CO2 molecules and H2O molecules
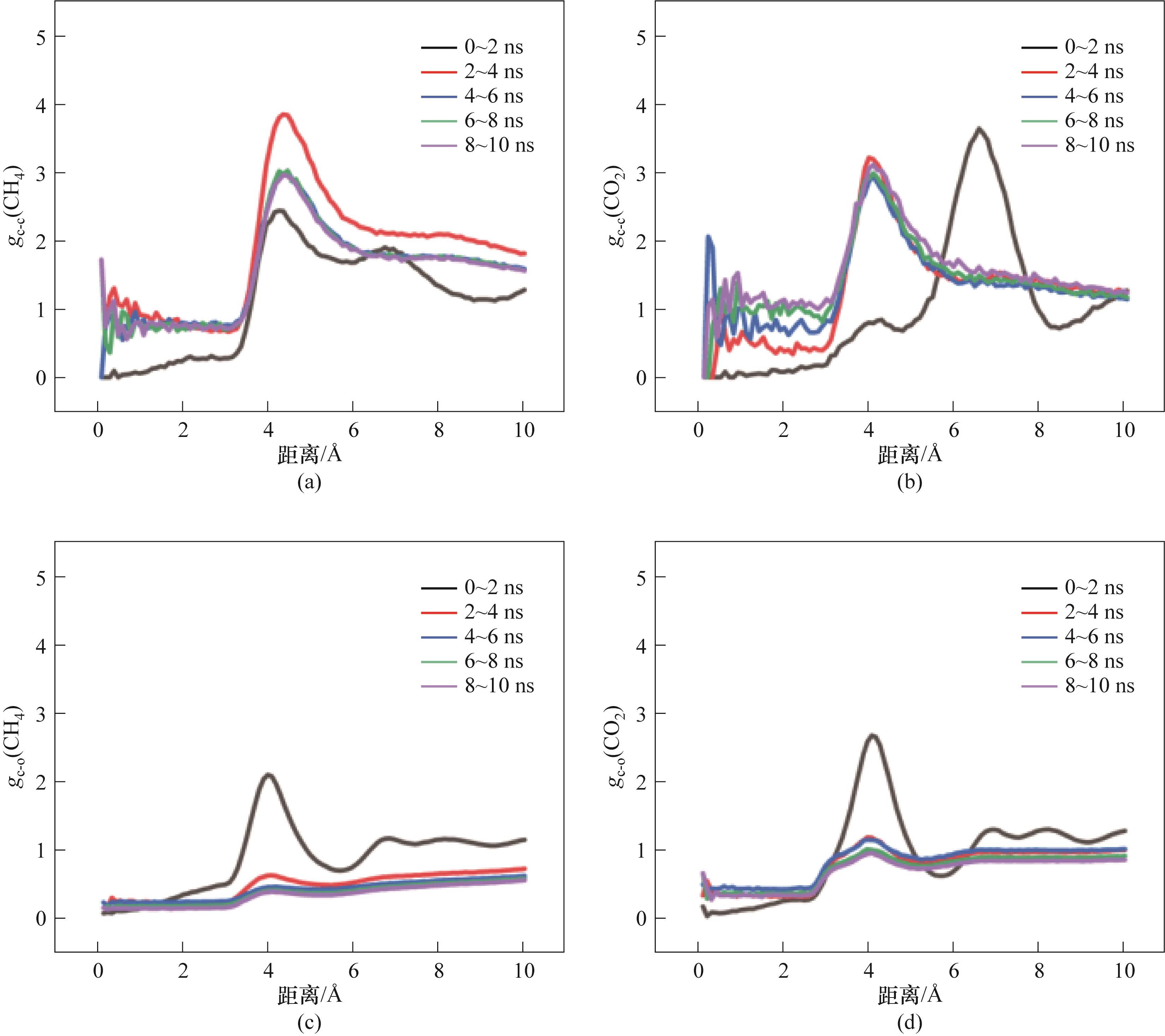
Fig.17 RDF of M2-10:(a) RDF between CH4 molecules; (b) RDF between CO2 molecules; (c) RDF between CH4 molecules and H2O molecules; (d) RDF between CO2 molecules and H2O molecules
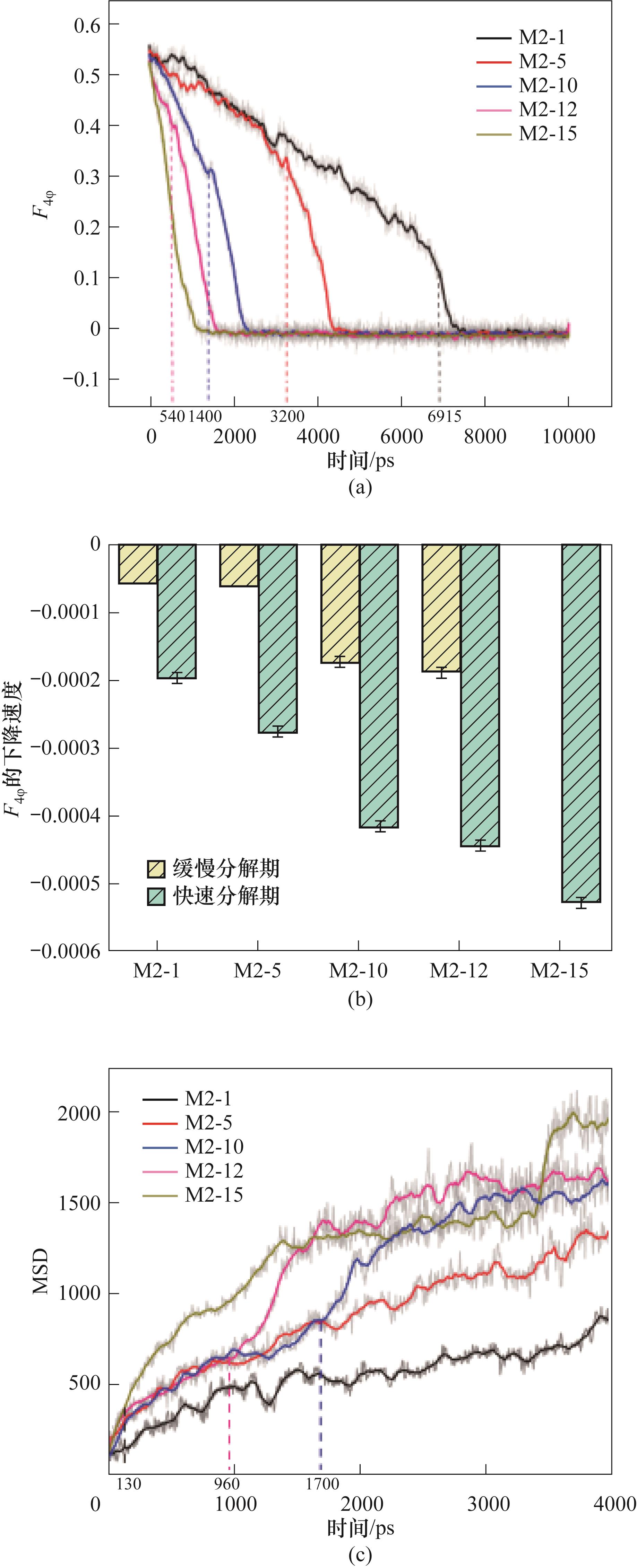
Fig.18 (a) F4φ value during the decomposition of CO2-CH4 hydrate at 290 K and 0.5 MPa; (b) The decomposition rate of CO2-CH4 hydrate characterized by the decreasing rate of F4φ value; (c) MSD during the decomposition of CO2-CH4 hydrate at 290 K and 0.5 MPa
| 模拟编号 | 液相中客体 分子摩尔 分数/% | 纳米气泡形成时间/ps | 纳米气泡内分子数达到50个的时刻/ps | 纳米气泡内分子数达到100个的时刻/ps |
|---|---|---|---|---|
| M2-1 | 1 | 5750 | 7075 | 7335 |
| M2-5 | 5 | 1245 | 3210 | 4225 |
| M2-10 | 10 | 215 | 740 | 1405 |
| M2-12 | 12 | 0 | 270 | 640 |
| M2-15 | 15 | 0 | 55 | 325 |
Table 2 The nucleation time of nanobubbles and the time required for the accumulation of guest molecules to 50 and 100
| 模拟编号 | 液相中客体 分子摩尔 分数/% | 纳米气泡形成时间/ps | 纳米气泡内分子数达到50个的时刻/ps | 纳米气泡内分子数达到100个的时刻/ps |
|---|---|---|---|---|
| M2-1 | 1 | 5750 | 7075 | 7335 |
| M2-5 | 5 | 1245 | 3210 | 4225 |
| M2-10 | 10 | 215 | 740 | 1405 |
| M2-12 | 12 | 0 | 270 | 640 |
| M2-15 | 15 | 0 | 55 | 325 |
| [1] | 欧芬兰, 于彦江, 寇贝贝, 等. 水合物藏的类型、特点及开发方法探讨[J]. 海洋地质与第四纪地质, 2022, 42(1): 194-213. |
| Ou F L, Yu Y J, Kou B B, et al. Gas hydrate reservoir types, characteristics and development methods[J]. Marine Geology & Quaternary Geology, 2022, 42(1): 194-213. | |
| [2] | Goel N. In situ methane hydrate dissociation with carbon dioxide sequestration: current knowledge and issues[J]. Journal of Petroleum Science and Engineering, 2006, 51(3/4): 169-184. |
| [3] | Koh D Y, Kang H, Lee J W, et al. Energy-efficient natural gas hydrate production using gas exchange[J]. Applied Energy, 2016, 162: 114-130. |
| [4] | 陈烨. 天然气水合物降压试采井筒多相流动规律及保障技术研究[D]. 大庆: 东北石油大学, 2020. |
| Chen Y. Study on multiphase flow law and guarantee technology of natural gas hydrate depressurization test production wellbore[D]. Daqing: Northeast Petroleum University, 2020. | |
| [5] | Wu G Z, Tian L Q, Chen D Y, et al. CO2 and CH4 hydrates: replacement or cogrowth?[J]. The Journal of Physical Chemistry C, 2019, 123(22): 13401-13409. |
| [6] | Geng C Y, Wen H, Zhou H. Molecular simulation of the potential of methane reoccupation during the replacement of methane hydrate by CO2 [J]. Journal of Physical Chemistry A, 2009, 113(18): 5463-5469. |
| [7] | Li Z D, Gan B C, Li Z, et al. Kinetic mechanisms of methane hydrate replacement and carbon dioxide hydrate reorganization[J]. Chemical Engineering Journal, 2023, 477: 146973. |
| [8] | Guo P, Song Y L, Liu H, et al. Molecular dynamic simulation study on replacement of methane hydrates with carbon dioxide under different temperatures, pressures, and concentrations of ethylene glycol[J]. ACS Omega, 2024, 9(17): 19031-19042. |
| [9] | Castellani B, Gambelli A M, Nicolini A, et al. Energy and environmental analysis of membrane-based CH4-CO2 replacement processes in natural gas hydrates [J]. Energies, 2019, 12(5): 850. |
| [10] | Li J, Wang Z L. Fluctuation-dissipation analysis of nonequilibrium thermal transport at the hydrate dissociation interface[J]. Physical Chemistry Chemical Physics, 2019, 21(42): 23492-23500. |
| [11] | Liu Y, Zhao J J, Xu J C. Dissociation mechanism of carbon dioxide hydrate by molecular dynamic simulation and ab initio calculation[J]. Computational and Theoretical Chemistry, 2012, 991: 165-173. |
| [12] | Li J, Liang Z J, Wang Z L, et al. Molecular dynamics simulation of decomposition of methane hydrate and interfacial characteristics in nanostructure region[J]. International Journal of Thermophysics, 2020, 41(2): 13. |
| [13] | Ma Y, Gao Q, Guan J, et al. Experimental study on the formation and dissociation characteristics of mixed CO2 + CH4 hydrates in quartz sand[J]. Energy & Fuels, 2023, 37(17): 12934-12945. |
| [14] | Maini B B, Bishnoi P R. Experimental investigation of hydrate formation behaviour of a natural gas bubble in a simulated deep sea environment[J]. Chemical Engineering Science, 1981, 36(1): 183-189. |
| [15] | Yi L Z, Liang D Q, Liang S, et al. Molecular dynamics study of CH4-CO2 mixed hydrate dissociation[J]. Asia-Pacific Journal of Chemical Engineering, 2015, 10(6): 823-832. |
| [16] | Hei Y X, Liu Z L, Shi D, et al. Molecular dynamics simulations to evaluate the decomposition properties of methane hydrate under different thermodynamic conditions[J]. Computational and Theoretical Chemistry, 2024, 1236: 114585. |
| [17] | Ouyang Q, Pandey J S, von Solms N. Critical parameters influencing mixed CH4/CO2 hydrates dissociation during multistep depressurization[J]. Fuel, 2022, 320: 123985. |
| [18] | Ripmeester J A, Alireza S, Hosseini B, et al. Fundamentals of methane hydrate decomposition[C]//Canadian Unconventional Resources and International Petroleum Conference. Calgary, Alberta, Canada: SPE, 2010: SPE-138112-MS. |
| [19] | Bagherzadeh S A, Alavi S, Ripmeester J, et al. Formation of methane nano-bubbles during hydrate decomposition and their effect on hydrate growth[J]. The Journal of Chemical Physics, 2015, 142(21): 214701. |
| [20] | Bagherzadeh S A, Englezos P, Alavi S, et al. Molecular simulation of non-equilibrium methane hydrate decomposition process[J]. The Journal of Chemical Thermodynamics, 2012, 44(1): 13-19. |
| [21] | Li Y C, Song S F, Liao N J, et al. Molecular-level study on decomposition kinetics of CO2–CH4 hydrates[J]. Energy & Fuels, 2024, 38(14): 12875-12887. |
| [22] | Plimpton S. Fast parallel algorithms for short-range molecular dynamics[J]. Journal of Computational Physics, 1995, 117(1): 1-19. |
| [23] | Vega C, Abascal J L F. Simulating water with rigid non-polarizable models: a general perspective[J]. Physical Chemistry Chemical Physics, 2011, 13(44): 19663-19688. |
| [24] | Potoff J J, Siepmann J I. Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen[J]. AIChE Journal, 2001, 47(7): 1676-1682. |
| [25] | Harris J G, Yung K H. Carbon dioxide's liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model[J]. The Journal of Physical Chemistry, 1995, 99(31): 12021-12024. |
| [26] | Cygan R T, Romanov V N, Myshakin E M. Molecular simulation of carbon dioxide capture by montmorillonite using an accurate and flexible force field[J]. The Journal of Physical Chemistry C, 2012, 116(24): 13079-13091. |
| [27] | Lu Y, Sun L J, Guan D W, et al. Molecular behavior of CO2 hydrate growth in the presence of dissolvable ionic organics[J]. Chemical Engineering Journal, 2022, 428: 131176. |
| [28] | Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method[J]. Journal of Applied Physics, 1981, 52(12): 7182-7190. |
| [29] | Nosé S. A molecular dynamics method for simulations in the canonical ensemble[J]. Molecular Physics, 1984, 52(2): 255-268. |
| [30] | Errington J R, Debenedetti P G. Relationship between structural order and the anomalies of liquid water[J]. Nature, 2001, 409(6818): 318-321. |
| [31] | Zhou Y B, Huang M Y, Tian F L, et al. Einstein-Stokes relation for small bubbles at the nanoscale[J]. The Journal of Chemical Physics, 2024, 160(5): 054109. |
| [32] | English N J, Johnson J K, Taylor C E. Molecular-dynamics simulations of methane hydrate dissociation[J]. The Journal of Chemical Physics, 2005, 123(24): 244503. |
| [1] |
Jichao GUO, Xiaoxiao XU, Yunlong SUN.
Airflow simulation and optimization based on |
| [2] | Fanchen KONG, Shuo ZHANG, Mingsheng TANG, Huiming ZOU, Zhouhang HU, Changqing TIAN. Simulation of gas bearings in carbon dioxide linear compressors [J]. CIESC Journal, 2025, 76(S1): 281-288. |
| [3] | Ting HE, Kai ZHANG, Wensheng LIN, Liqiong CHEN, Jiafu CHEN. Research on integrated process of cryogenic CO2 removal under supercritical pressure and liquefaction for biogas [J]. CIESC Journal, 2025, 76(S1): 418-425. |
| [4] | Jianmin ZHANG, Meigui HE, Wanxin JIA, Jing ZHAO, Wanqin JIN. Poly(ethylene oxide)/crown ether blend membrane and performance for CO2 separation [J]. CIESC Journal, 2025, 76(9): 4862-4871. |
| [5] | Yifei WANG, Yuxing LI, Xin OUYANG, Xuefeng ZHAO, Lan MENG, Qihui HU, Buze YIN, Yaqi GUO. Numerical calculation of CO2 pipeline fracture propagation based on crack tip decompression characteristics [J]. CIESC Journal, 2025, 76(9): 4683-4693. |
| [6] | Xianghai LI, Delin LAI, Gang KONG, Jian ZHOU. Molecular dynamics simulations on synergistic underwater oleophobicity mechanism of dual-biomimic surfaces [J]. CIESC Journal, 2025, 76(9): 4551-4562. |
| [7] | Guoxiang HU, Yikui ZHU, Hua LONG, Xiaowen LIU, Qingang XIONG. Study on the underlying mechanism of choline chloride-lactic acid molar ratio influencing alkali lignin solubility in choline chloride-lactic acid deep eutectic solvents [J]. CIESC Journal, 2025, 76(9): 4449-4461. |
| [8] | Zheng GAO, Hui WANG, Zhiguo QU. Data-driven high-throughput screening of anion-pillared metal-organic frameworks for hydrogen storage [J]. CIESC Journal, 2025, 76(8): 4259-4272. |
| [9] | Yuntao ZHOU, Lifeng CUI, Jie ZHANG, Fuhong YU, Xingang LI, Ye TIAN. Ga2O3 modified CuCeO catalysts for CO2 hydrogenation to methanol [J]. CIESC Journal, 2025, 76(8): 4042-4051. |
| [10] | Jiahao LIN, Fangzhong FU, Haohui YE, Jin HU, Mingcan YAO, Helin FAN, Xu WANG, Ruixiang WANG, Zhifeng XU. Effect of NdF3 content on local structure and transport properties of NdF3-LiF molten salt [J]. CIESC Journal, 2025, 76(8): 3834-3841. |
| [11] | Xiaoling WANG, Shaoqing WANG, Yungang ZHAO, Fangzhe CHANG, Ruifeng MU. Mechanism of organic Ca transformation during coal hydropyrolysis: insights from ReaxFF molecular dynamics simulations [J]. CIESC Journal, 2025, 76(8): 4297-4309. |
| [12] | Liang QIAO, Shang LI, Xinliang LIU, Ming WANG, Pei ZHANG, Yingfei HOU. Synthesis and molecular simulation of terpolymer viscosity reducer for heavy oil [J]. CIESC Journal, 2025, 76(7): 3686-3695. |
| [13] | Zeming DONG, Juwei LOU, Nan WANG, Liangqi CHEN, Jiangfeng WANG, Pan ZHAO. Research on thermodynamic properties of supercritical compressed carbon dioxide energy storage system with waste heat recovery [J]. CIESC Journal, 2025, 76(7): 3477-3486. |
| [14] | Zhenning FAN, Haining LIANG, Maoli FANG, Yifan HE, Shuai YU, Xingqing YAN, Jiaran AN, Fanfan QIAO, Jianliang YU. Research and comparison of throttling and venting characteristics of CO2 pipelines in different phase states [J]. CIESC Journal, 2025, 76(7): 3742-3751. |
| [15] | Xuerui LU, Guoyan ZHOU, Qi FANG, Mengzheng YU, Xiucheng ZHANG, Shandong TU. Numerical study on the carbon deposition effect in external reformer of solid oxide fuel cells [J]. CIESC Journal, 2025, 76(7): 3295-3304. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||