CIESC Journal ›› 2022, Vol. 73 ›› Issue (2): 894-903.DOI: 10.11949/0438-1157.20211023
• Energy and environmental engineering • Previous Articles Next Articles
Li WAN1,2,3( ),Deqing LIANG1,2,3(
),Deqing LIANG1,2,3( )
)
Received:2021-07-22
Revised:2021-09-26
Online:2022-02-18
Published:2022-02-05
Contact:
Deqing LIANG
通讯作者:
梁德青
作者简介:万丽(1986—),女,博士研究生,讲师,基金资助:CLC Number:
Li WAN, Deqing LIANG. Study on a biodegradable kinetics hydrate inhibitor[J]. CIESC Journal, 2022, 73(2): 894-903.
万丽, 梁德青. 一种可生物降解水合物动力学抑制剂的研究[J]. 化工学报, 2022, 73(2): 894-903.
Add to citation manager EndNote|Ris|BibTeX
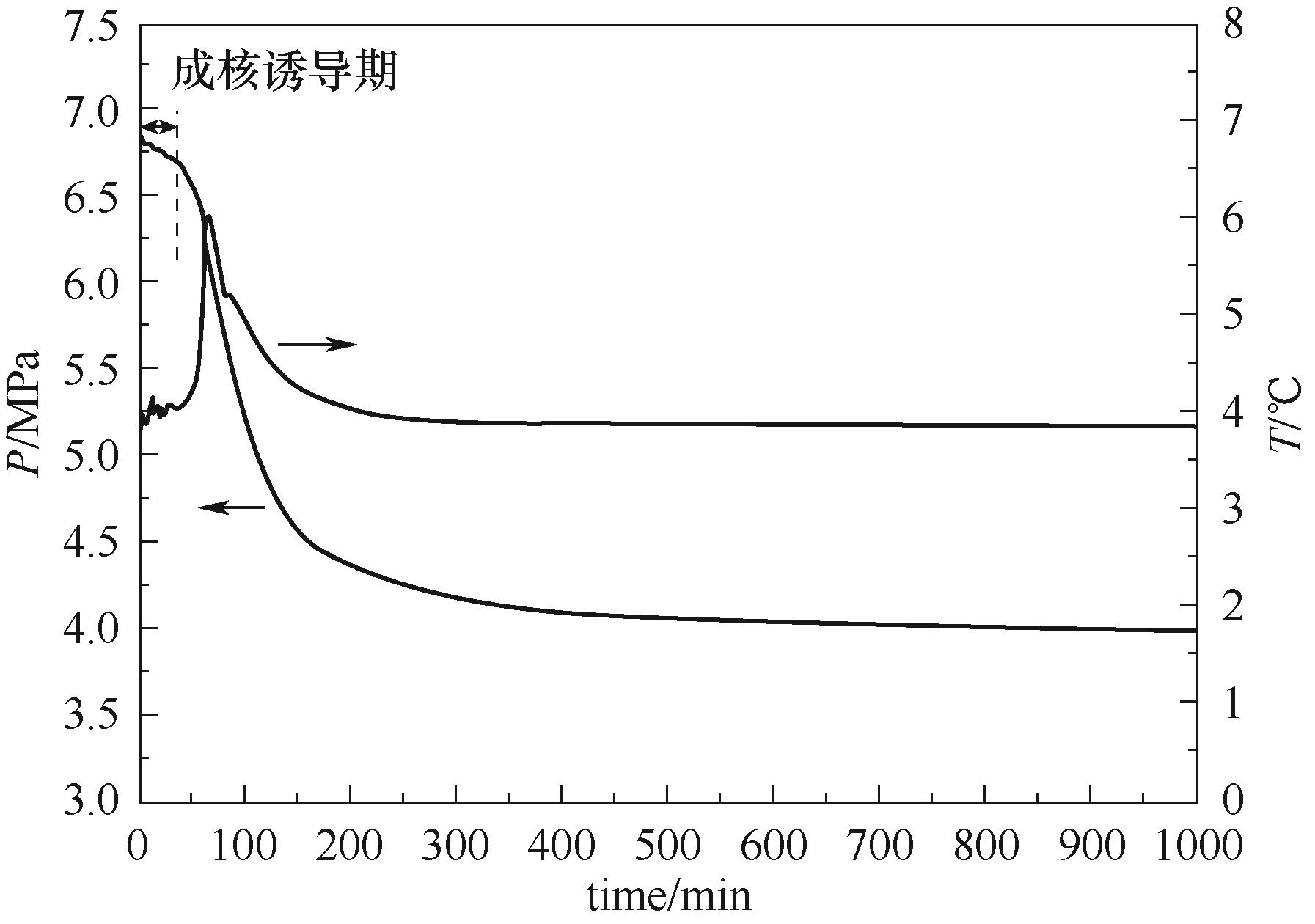
Fig.3 Typical curves of the pressure and temperature vs. time obtained by an isothermal cooling method (determined from the system adding 1% PVP at the subcooling of 6℃)
| Polymer | P0/MPa | T0/℃ | Te/℃ | Maximum subcooling/℃ |
|---|---|---|---|---|
| no additives | 9.49 | 8.44 | 13.19 | 4.75 |
| PVPK90 [0.25%(mass)] | 10.01 | 7.93 | 13.72 | 5.79 |
| PVPK90 [0.5%(mass)] | 10.44 | 7.70 | 14.13 | 6.43 |
| PVPK90 [1%(mass)] | 10.41 | 6.15 | 14.11 | 7.96 |
| PVCap [0.25%(mass)] | 9.603 | 4.44 | 13.3 | 8.86 |
| PVCap [0.5%(mass)] | 7.00 | -0.35 | 10.09 | 10.44 |
| PVCap [1%(mass)] | 8.86 | 0.70 | 12.48 | 11.78 |
| NaAlg-g-PVCap [0.25%(mass)] | 10.34 | 8.08 | 14.04 | 5.96 |
| NaAlg-g-PVCap [0.5%(mass)] | 9.29 | 7.53 | 12.97 | 5.44 |
| NaAlg-g-PVCap [1%(mass)] | 10.36 | 8.80 | 14.06 | 5.26 |
Table 1 Onset pressure (P0) and temperature (T0), related equilibrium temperature (Te), and maximum subcooling of systems with various polymers
| Polymer | P0/MPa | T0/℃ | Te/℃ | Maximum subcooling/℃ |
|---|---|---|---|---|
| no additives | 9.49 | 8.44 | 13.19 | 4.75 |
| PVPK90 [0.25%(mass)] | 10.01 | 7.93 | 13.72 | 5.79 |
| PVPK90 [0.5%(mass)] | 10.44 | 7.70 | 14.13 | 6.43 |
| PVPK90 [1%(mass)] | 10.41 | 6.15 | 14.11 | 7.96 |
| PVCap [0.25%(mass)] | 9.603 | 4.44 | 13.3 | 8.86 |
| PVCap [0.5%(mass)] | 7.00 | -0.35 | 10.09 | 10.44 |
| PVCap [1%(mass)] | 8.86 | 0.70 | 12.48 | 11.78 |
| NaAlg-g-PVCap [0.25%(mass)] | 10.34 | 8.08 | 14.04 | 5.96 |
| NaAlg-g-PVCap [0.5%(mass)] | 9.29 | 7.53 | 12.97 | 5.44 |
| NaAlg-g-PVCap [1%(mass)] | 10.36 | 8.80 | 14.06 | 5.26 |

Fig.6 The maximum subcooling of PVP, PVCap, NaAlg-g-PVCap systems with various concentrations of the inhibitors (The error bar represents the experimental deviation)
| Polymer | Rate of hydrate formation, NR30/ (mol/(s·m3)) | ||
|---|---|---|---|
| ΔTsub= 5℃ | ΔTsub= 6℃ | ΔTsub= 7.5℃ | |
| no additives | 4.3×10-7 | 5.5×10-7 | 6.9×10-7 |
| 1% PVP | 1.7×10-7 | 1.0×10-7 | 1.6×10-7 |
| 1% NaAlg-g-PVCap | 6.3×10-8 | 1.5×10-7 | 1.7×10-7 |
| 1% PVCap | 0 | 0 | 4.2×10-8 |
| 0.5%NaAlg-g-PVCap | — | — | 1.0×10-7 |
Table 2 The initial rate of hydrate formation in the presence of various polymers under different subcooling temperatures
| Polymer | Rate of hydrate formation, NR30/ (mol/(s·m3)) | ||
|---|---|---|---|
| ΔTsub= 5℃ | ΔTsub= 6℃ | ΔTsub= 7.5℃ | |
| no additives | 4.3×10-7 | 5.5×10-7 | 6.9×10-7 |
| 1% PVP | 1.7×10-7 | 1.0×10-7 | 1.6×10-7 |
| 1% NaAlg-g-PVCap | 6.3×10-8 | 1.5×10-7 | 1.7×10-7 |
| 1% PVCap | 0 | 0 | 4.2×10-8 |
| 0.5%NaAlg-g-PVCap | — | — | 1.0×10-7 |
| 聚合物 | BOD5/(mg/mg) | COD/(mg/mg) | BOD5/COD |
|---|---|---|---|
PVCap NaAlg-g-PVCap | 0.98 0.87 | 2.83 1.98 | 0.35 0.44 |
Table 3 5 d-biodegradation of the synthesized polymers
| 聚合物 | BOD5/(mg/mg) | COD/(mg/mg) | BOD5/COD |
|---|---|---|---|
PVCap NaAlg-g-PVCap | 0.98 0.87 | 2.83 1.98 | 0.35 0.44 |
| 1 | Storr M T, Taylor P C, Monfort J P, et al. Kinetic inhibitor of hydrate crystallization[J]. Journal of the American Chemical Society, 2004, 126(5): 1569-1576. |
| 2 | Sloan E D, Koh C A. Clathrate Hydrates of Natural Gas[M]. 3rd ed. California: CRC Press, 2007. |
| 3 | Sloan E D. Fundamental principles and applications of natural gas hydrates[J]. Nature, 2003, 426(6964): 353-363. |
| 4 | Kelland M A. History of the development of low dosage hydrate inhibitors[J]. Energy & Fuels, 2006, 20(3): 825-847. |
| 5 | Yasuda K, Takeya S, Sakashita M, et al. Binary ethanol-methane clathrate hydrate formation in the system CH4-C2H5OH-H2O: confirmation of structure II hydrate formation[J]. The Journal of Physical Chemistry C, 2009, 113(28): 12598-12601. |
| 6 | 樊栓狮, 王燕鸿, 郎雪梅. 天然气水合物动力学抑制技术研究进展[J]. 天然气工业, 2011, 31(12): 99-109, 132. |
| Fan S S, Wang Y H, Lang X M. Progress in the research of kinetic hydrate inhibitors[J]. Natural Gas Industry, 2011, 31(12): 99-109, 132. | |
| 7 | Carroll J J. Natural Gas Hydrates : a Guide for Engineers[M]. Gulf Professional Publishing, 2009. |
| 8 | Wan L, Liang D Q, Ding Q H, et al. Investigation into the inhibition of methane hydrate formation in the presence of hydroxy-terminated poly(N-vinylcaprolactam)[J]. Fuel, 2019, 239: 173-179. |
| 9 | Cortez-Lemus N A, Licea-Claverie A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular[J]. Progress in Polymer Science, 2016, 53: 1-51. |
| 10 | Kamal M S, Hussein I A, Sultan A S, et al. Application of various water soluble polymers in gas hydrate inhibition[J]. Renewable and Sustainable Energy Reviews, 2016, 60: 206-225. |
| 11 | Daraboina N, Ripmeester J, Walker V K, et al. Natural gas hydrate formation and decomposition in the presence of kinetic inhibitors (1): High pressure calorimetry[J]. Energy & Fuels, 2011, 25(10): 4392-4397. |
| 12 | Naeiji P, Arjomandi A, Varaminian F. Amino acids as kinetic inhibitors for tetrahydrofuran hydrate formation: experimental study and kinetic modeling[J]. Journal of Natural Gas Science and Engineering, 2014, 21: 64-70. |
| 13 | Del Villano L, Kommedal R, Kelland M A. Class of kinetic hydrate inhibitors with good biodegradability[J]. Energy & Fuels, 2008, 22(5): 3143-3149. |
| 14 | Silva B L L D, Ferraz I L, do Nascimento D F, et al. Sodium alginate polymer as a kinetic inhibitor of methane hydrate formation[J]. Journal of Materials Research and Technology, 2021, 12: 1999-2010. |
| 15 | Perfeldt C M, Chua P C, Daraboina N, et al. Inhibition of gas hydrate nucleation and growth: efficacy of an antifreeze protein from the longhorn beetle rhagium mordax[J]. Energy & Fuels, 2014, 28(6): 3666-3672. |
| 16 | Davies P L, Baardsnes J, Kuiper M J, et al. Structure and function of antifreeze proteins[J]. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 2002, 357(1423): 927-35. |
| 17 | Alireza Bagherzadeh S, Alavi S, Ripmeester J A, et al. Why ice-binding type Ⅰ antifreeze protein acts as a gas hydrate crystal inhibitor[J]. Physical Chemistry Chemical Physics, 2015, 17(15): 9984-9990. |
| 18 | Sa J H, Lee B R, Park D H, et al. Amino acids as natural inhibitors for hydrate formation in CO2 sequestration[J]. Environmental Science & Technology, 2011, 45(13): 5885-5891. |
| 19 | Bavoh C B, Partoon B, Lal B, et al. Methane hydrate-liquid-vapour-equilibrium phase condition measurements in the presence of natural amino acids[J]. Journal of Natural Gas Science and Engineering, 2017, 37: 425-434. |
| 20 | Bavoh C B, Nashed O, Khan M S, et al. The impact of amino acids on methane hydrate phase boundary and formation kinetics[J]. The Journal of Chemical Thermodynamics, 2018, 117: 48-53. |
| 21 | Roosta H, Dashti A, Mazloumi S H, et al. Inhibition properties of new amino acids for prevention of hydrate formation in carbon dioxide-water system: experimental and modeling investigations[J]. Journal of Molecular Liquids, 2016, 215: 656-663. |
| 22 | Sa J H, Kwak G H, Han K, et al. Inhibition of methane and natural gas hydrate formation by altering the structure of water with amino acids[J]. Scientific Reports, 2016, 6: 31582. |
| 23 | Pal S, Mal D, Singh R P. Cationic starch: an effective flocculating agent[J]. Carbohydrate Polymers, 2005, 59(4): 417-423. |
| 24 | Lee J D, Wu H J, Englezos P. Cationic starches as gas hydrate kinetic inhibitors[J]. Chemical Engineering Science, 2007, 62(23): 6548-6555. |
| 25 | Talaghat M R. Experimental investigation of double gas hydrate formation in the presence of modified starch as a kinetic inhibitor in a flow mini-loop apparatus[J]. The Canadian Journal of Chemical Engineering, 2012, 90(2): 429-436. |
| 26 | Roosta H, Dashti A, Hossein Mazloumi S, et al. Inhibition and promotion effects of modified HECs and modified starches on the growth rate of hydrate in methane-propane-water system[J]. Journal of Molecular Liquids, 2017, 243: 553-563. |
| 27 | Xu Y J, Yang M L, Yang X X. Chitosan as green kinetic inhibitors for gas hydrate formation[J]. Journal of Natural Gas Chemistry, 2010, 19(4): 431-435. |
| 28 | Xu S, Fan S, Fang S, et al. Pectin as an extraordinary natural kinetic hydrate inhibitor[R]. Scientific Reports, 2016: 23220. |
| 29 | Wan L, Zhang N, Liang D Q. Inhibition effects of polysaccharides for gas hydrate formation in methane-water system[J]. Journal of Molecular Liquids, 2019, 292: 111435. |
| 30 | 蔡云升. 冰淇淋生产中的稳定剂、乳化剂及复合乳化稳定剂[J]. 冷饮与速冻食品工业, 2002(3): 1-6. |
| Cai Y S. The Stabilizers, the emulsifiers, the mixed emulsifier-stabilizers in production of ice cream[J]. Beverage & Fast Frozen Food Industry, 2002(3): 1-6. | |
| 31 | 周诗岽, 李青岭, 李乐, 等. 新型天然气水合物动力学抑制剂的制备及性能[J]. 石油化工, 2017, 46(4): 467-470. |
| Zhou S D, Li Q L, Li L, et al. Synthesis and properties of novel kinetic inhibit ors for natural gas hydrate[J]. Petrochemical Technology, 2017, 46(4): 467-470. | |
| 32 | 刘婷婷, 胡耀强, 高明星, 等. 组合型水合物抑制剂的评价及应用[J]. 石油与天然气化工, 2019, 48(5): 39-41, 55. |
| Liu T T, Hu Y Q, Gao M X, et al. Evaluation and application of a combined natural gas hydrate inhibitor[J]. Chemical Engineering of Oil & Gas, 2019, 48(5): 39-41, 55. | |
| 33 | Sa J H, Kwak G H, Lee B R, et al. Hydrophobic amino acids as a new class of kinetic inhibitors for gas hydrate formation[R]. Scientific Reports, 2013: 2428. |
| 34 | Kelland M A. A review of kinetic hydrate inhibitors from an environmental perspective[J]. Energy & Fuels, 2018, 32(12): 12001-12012. |
| 35 | Arslan-Alaton I, Akmehmet Balcioglu I. Biodegradability assessment of ozonated raw and biotreated pharmaceutical wastewater[J]. Archives of Environmental Contamination and Toxicology, 2002, 43(4): 425-431. |
| 36 | He Q, Yao K, Sun D H, et al. Biodegradability of tannin-containing wastewater from leather industry[J]. Biodegradation, 2007, 18(4): 465-472. |
| 37 | Reuschenbach P, Pagga U, Strotmann U. A critical comparison of respirometric biodegradation tests based on OECD 301 and related test methods[J]. Water Research, 2003, 37(7): 1571-1582. |
| 38 | Sloan E D, Subramanian S, Matthews P N, et al. Quantifying hydrate formation and kinetic inhibition[J]. Industrial & Engineering Chemistry Research, 1998, 37(8): 3124-3132. |
| 39 | Seo S D, Paik H J, Lim D H, et al. Effects of poly(N-vinylcaprolactam) molecular weight and molecular weight distribution on methane hydrate formation[J]. Energy & Fuels, 2017, 31(6): 6358-6363. |
| 40 | Posteraro D, Ivall J, Maric M, et al. New insights into the effect of polyvinylpyrrolidone (PVP) concentration on methane hydrate growth (2): Liquid phase methane mole fraction[J]. Chemical Engineering Science, 2015, 126: 91-98. |
| 41 | Veluswamy H P, Ang W J, Zhao D, et al. Influence of cationic and non-ionic surfactants on the kinetics of mixed hydrogen/tetrahydrofuran hydrates[J]. Chemical Engineering Science, 2015, 132: 186-199. |
| 42 | Veluswamy H P, Kumar A, Kumar R, et al. An innovative approach to enhance methane hydrate formation kinetics with leucine for energy storage application[J]. Applied Energy, 2017, 188: 190-199. |
| 43 | Veluswamy H P, Lee P Y, Premasinghe K, et al. Effect of biofriendly amino acids on the kinetics of methane hydrate formation and dissociation[J]. Industrial & Engineering Chemistry Research, 2017, 56(21): 6145-6154. |
| 44 | Kang S P, Shin J Y, Lim J S, et al. Experimental measurement of the induction time of natural gas hydrate and its prediction with polymeric kinetic inhibitor[J]. Chemical Engineering Science, 2014, 116: 817-823. |
| 45 | Lederhos J P, Long J P, Sum A, et al. Effective kinetic inhibitors for natural gas hydrates[J]. Chemical Engineering Science, 1996, 51(8): 1221-1229. |
| 46 | Sharifi H, Ripmeester J, Walker V K, et al. Kinetic inhibition of natural gas hydrates in saline solutions and heptane[J]. Fuel, 2014, 117: 109-117. |
| [1] | Lei WANG, Lei WANG, Yunlong BAI, Liuliu HE. Preparation of SA lithium ion sieve membrane and its adsorptive properties [J]. CIESC Journal, 2023, 74(5): 2046-2056. |
| [2] | Zhen LONG, Jinhang WANG, Yong HE, Deqing LIANG. Characteristics study on hydrates formation from gas mixture under ionic liquid together with kinetic hydrate inhibitors [J]. CIESC Journal, 2023, 74(4): 1703-1711. |
| [3] | Xiqiang ZHAO, Jian ZHANG, Shuang SUN, Wenlong WANG, Yanpeng MAO, Jing SUN, Jinglong LIU, Zhanlong SONG. Study on the performance of biochar modified microspheres to remove inorganic phosphorus from chemical wastewater [J]. CIESC Journal, 2022, 73(5): 2158-2173. |
| [4] | Zhihao WANG, Xin SONG, Yaran YIN, Xianming ZHANG. Regulation of gelation rate on the morphology of helical fibers during microfluidic spinning [J]. CIESC Journal, 2022, 73(11): 5158-5166. |
| [5] | Zhenlin ZHU, Songlin WANG, Bingxue JIANG, Jiaxu LI, Wei DENG, Haiqiang WU, Xuan YANG, Pingwei LIU, Wenjun WANG. Study on biodegradation of polyesters and their evaluation methods [J]. CIESC Journal, 2022, 73(1): 110-121. |
| [6] | NA Shasha, LI Weixing, XING Weihong. Development of inorganic nano particles modified sodium alginate hybrid membranes for pervaporation [J]. CIESC Journal, 2016, 67(9): 3730-3737. |
| [7] | GAO Yan, CHEN Yao, NI Jinlei, TONG Shaoping, MA Chun'an. A simple method for evaluating biodegradability of pre-ozonized water [J]. CIESC Journal, 2014, 65(6): 2323-2328. |
| [8] | LI Yu, LIU Yuanyuan, LI Shuai, LIANG Gang, ZHANG Yanan, HU Qingxi. Gel fraction and swelling degree of hollow alginate fiber fabricated by direct writing and crosslinking [J]. CIESC Journal, 2014, 65(12): 5090-5096. |
| [9] | WU Jie, DING Shijie, CHEN Jing, JIANG Jinlong, WANG Junjun. Preparation and sustained release properties of acidified-attapulgite/alginate composite material [J]. CIESC Journal, 2014, 65(11): 4627-4632. |
| [10] | LIU Yingjie1,JIA Xiaoqiang1,2,3,WEN Jianping1,2,3,BAN Rui1. Recent Advances in polyhydroxyalkanoates production by mixed cultures [J]. Chemical Industry and Engineering Progree, 2014, 33(10): 2729-2734. |
| [11] | ZHANG Rui1,2,LU Ning1,ZHU Qing1,SU Tianxiang1,WANG Jianjian1. Preparation and properties of biodegradable poly(3-hydroxybutyrate- co-4-hydroxybutyrate)/ layered α-zirconium phosphate nanocomposites [J]. Chemical Industry and Engineering Progree, 2014, 33(10): 2716-2721. |
| [12] | WU Wenguo1,2,LIU Wei1,WANG Shibin1,2,LIU Yuangang1,2,CHEN Aizheng1,2. Drug loading and release of poly-L-arginine/calcium alginate microcapsules [J]. Chemical Industry and Engineering Progree, 2014, 33(05): 1271-1275. |
| [13] | WU Huiling,ZHANG Shuping. Progress in the application of sodium alginate/nanomaterial composites [J]. Chemical Industry and Engineering Progree, 2014, 33(04): 954-959. |
| [14] | ZHU Wenhui, WANG Xingrun, DONG Liangfei, WANG Qi, HE Jie. Characteristics of removing Cr(Ⅵ)of Fe-Cu bimetal PRB coated by sodium alginate [J]. CIESC Journal, 2013, 64(9): 3373-3380. |
| [15] | GAO Zhen, WU Changyong, ZHOU Yuexi, SONG Jiamei, LIU Mingguo, CHANG Lijun. Effect of pre-ozonation on biological effluent of petrochemical wastewater treatment plant [J]. CIESC Journal, 2013, 64(9): 3390-3395. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||