CIESC Journal ›› 2025, Vol. 76 ›› Issue (12): 6218-6235.DOI: 10.11949/0438-1157.20250511
• Reviews and monographs • Previous Articles Next Articles
Yifan TONG1,2( ), Ningshuang ZHANG1,2, Xingpeng CAI1,2, Chengyu LI1,2, Shiyou LI1,2(
), Ningshuang ZHANG1,2, Xingpeng CAI1,2, Chengyu LI1,2, Shiyou LI1,2( )
)
Received:2025-05-09
Revised:2025-05-30
Online:2026-01-23
Published:2025-12-31
Contact:
Shiyou LI
童逸凡1,2( ), 张宁霜1,2, 蔡星鹏1,2, 李成煜1,2, 李世友1,2(
), 张宁霜1,2, 蔡星鹏1,2, 李成煜1,2, 李世友1,2( )
)
通讯作者:
李世友
作者简介:童逸凡(1999—),男,硕士研究生,tyftjzy@126.com
基金资助:CLC Number:
Yifan TONG, Ningshuang ZHANG, Xingpeng CAI, Chengyu LI, Shiyou LI. Research on modification of layered oxide cathode materials for sodium-ion battery driven by high-entropy strategy: progress, mechanism, and future[J]. CIESC Journal, 2025, 76(12): 6218-6235.
童逸凡, 张宁霜, 蔡星鹏, 李成煜, 李世友. 高熵策略驱动下的钠离子电池层状氧化物正极材料改性研究:进展、机理与展望[J]. 化工学报, 2025, 76(12): 6218-6235.
Add to citation manager EndNote|Ris|BibTeX
| 材料 | 优势 | 劣势 |
|---|---|---|
| 聚阴离子型材料[ | 结构稳定性强、工作电压高 | 电子电导率低,能量密度低,合成成本高昂 |
| 普鲁士蓝材料[ | 合成工艺简单、快速充放电能力强 | 循环寿命较短、工作温度范围较窄 |
| 过渡金属氧化物材料[ | 合成工艺简单、可逆比容量高、储钠能力强 | 充放电过程中易发生不可逆相变、空气稳定性差 |
Table 1 Summary of advantages and disadvantages of mainstream SIB cathode materials
| 材料 | 优势 | 劣势 |
|---|---|---|
| 聚阴离子型材料[ | 结构稳定性强、工作电压高 | 电子电导率低,能量密度低,合成成本高昂 |
| 普鲁士蓝材料[ | 合成工艺简单、快速充放电能力强 | 循环寿命较短、工作温度范围较窄 |
| 过渡金属氧化物材料[ | 合成工艺简单、可逆比容量高、储钠能力强 | 充放电过程中易发生不可逆相变、空气稳定性差 |
| 正极材料 | 放电比容量/(mAh·g-1) | 电压范围/V | 循环稳定性 | 倍率性能/(mAh·g-1) | 文献 |
|---|---|---|---|---|---|
| Na0.83Li0.1Ni0.25Co0.2Mn0.15Ti0.15Sn0.15O2-δ | 109.4 | 2.0~4.2 | 87.2%/200次循环/2.0C | 83.3 /10C | [ |
| NaCu0.1Ni0.25Co0.15Mn0.35Li0.05Ti0.05Sn0.05O2 | 144.5 | 2.2~4.4 | 90.1%/100次循环/1.0C | 76.7/5.0C | [ |
| NaNi0.3Cu0.1Fe0.2Mn0.3Ti0.1O2 | 141.5 | 2.0~4.0 | 85%/500次循环/1.0C | 120/5.0C | [ |
| NaNi0.2Fe0.2Mn0.35Cu0.05Zn0.1Sn0.1O2 | 128 | 2.0~4.0 | 87%/500次循环/3.0C | 64.3/2.0C | [ |
| Na2/3Li1/6Fe1/6Co1/6Ni1/6Mn1/3O2 | 171.2 | 2.0~4.5 | 90%/30次/0.3C | 78.2/10C | [ |
| [Na0.67Zn0.05]Ni0.22Cu0.06Mn0.66Ti0.01O2 | 146.1 | 2.0~4.3 | 92.7%/100次循环/1.0C | 91.54/10C | [ |
| Na0.67Mn0.6Cu0.08Ni0.09Fe0.18Ti0.05O2 | 150.3 | 2.0~4.5 | 100%/500次循环/10C | 62.5/10C | [ |
| Na[FeCoNiTi]1/6Mn1/4Zn1/12O2 | 127.3 | 2.0~4.1 | 88%/1000次循环/1.0C | 30.7/10C | [ |
| Na0.9Ni0.2Fe0.2Co0.2Mn0.2Ti0.15Cu0.05O2 | 117.8 | 2.2~4.1 | 70.7%/1000次循环/1.0C | 98.6/10C | [ |
| Na(Fe0.2Co0.15Cu0.05Ni0.2Mn0.2Ti0.2)B0.02O2 | 120.5 | 2.0~4.1 | 95%/100次循环/1.0C | 103.3/2.0C | [ |
| Na0.95Li0.06Ni0.25Cu0.05Fe0.15Mn0.49O2 | 141.2 | 2.0~4.2 | 83.2/500次循环/8.0C | 83.5/20C | [ |
| Na0.89Li0.05Cu0.11Ni0.11Fe0.3Mn0.43O1.97F0.03 | 145 | 1.5~4.0 | 80%/300次循环/1.0C | 109/10C | [ |
| Na0.85Li0.05Ni0.25Cu0.025Mg0.025Fe0.05Al0.05Mn0.5Ti0.05O2 | 122 | 2.0~4.3 | 89%/1000次循环/10C | 81.8/10C | [ |
| Na0.85Li0.05Ni0.3Fe0.1Mn0.5Ti0.05O2 | 182.2 | 1.5~4.3 | 94.3/10次循环/10C | 68.4/10C | [ |
Table 2 Summary of layered cathodes with high-entropy configurations in SIBs
| 正极材料 | 放电比容量/(mAh·g-1) | 电压范围/V | 循环稳定性 | 倍率性能/(mAh·g-1) | 文献 |
|---|---|---|---|---|---|
| Na0.83Li0.1Ni0.25Co0.2Mn0.15Ti0.15Sn0.15O2-δ | 109.4 | 2.0~4.2 | 87.2%/200次循环/2.0C | 83.3 /10C | [ |
| NaCu0.1Ni0.25Co0.15Mn0.35Li0.05Ti0.05Sn0.05O2 | 144.5 | 2.2~4.4 | 90.1%/100次循环/1.0C | 76.7/5.0C | [ |
| NaNi0.3Cu0.1Fe0.2Mn0.3Ti0.1O2 | 141.5 | 2.0~4.0 | 85%/500次循环/1.0C | 120/5.0C | [ |
| NaNi0.2Fe0.2Mn0.35Cu0.05Zn0.1Sn0.1O2 | 128 | 2.0~4.0 | 87%/500次循环/3.0C | 64.3/2.0C | [ |
| Na2/3Li1/6Fe1/6Co1/6Ni1/6Mn1/3O2 | 171.2 | 2.0~4.5 | 90%/30次/0.3C | 78.2/10C | [ |
| [Na0.67Zn0.05]Ni0.22Cu0.06Mn0.66Ti0.01O2 | 146.1 | 2.0~4.3 | 92.7%/100次循环/1.0C | 91.54/10C | [ |
| Na0.67Mn0.6Cu0.08Ni0.09Fe0.18Ti0.05O2 | 150.3 | 2.0~4.5 | 100%/500次循环/10C | 62.5/10C | [ |
| Na[FeCoNiTi]1/6Mn1/4Zn1/12O2 | 127.3 | 2.0~4.1 | 88%/1000次循环/1.0C | 30.7/10C | [ |
| Na0.9Ni0.2Fe0.2Co0.2Mn0.2Ti0.15Cu0.05O2 | 117.8 | 2.2~4.1 | 70.7%/1000次循环/1.0C | 98.6/10C | [ |
| Na(Fe0.2Co0.15Cu0.05Ni0.2Mn0.2Ti0.2)B0.02O2 | 120.5 | 2.0~4.1 | 95%/100次循环/1.0C | 103.3/2.0C | [ |
| Na0.95Li0.06Ni0.25Cu0.05Fe0.15Mn0.49O2 | 141.2 | 2.0~4.2 | 83.2/500次循环/8.0C | 83.5/20C | [ |
| Na0.89Li0.05Cu0.11Ni0.11Fe0.3Mn0.43O1.97F0.03 | 145 | 1.5~4.0 | 80%/300次循环/1.0C | 109/10C | [ |
| Na0.85Li0.05Ni0.25Cu0.025Mg0.025Fe0.05Al0.05Mn0.5Ti0.05O2 | 122 | 2.0~4.3 | 89%/1000次循环/10C | 81.8/10C | [ |
| Na0.85Li0.05Ni0.3Fe0.1Mn0.5Ti0.05O2 | 182.2 | 1.5~4.3 | 94.3/10次循环/10C | 68.4/10C | [ |
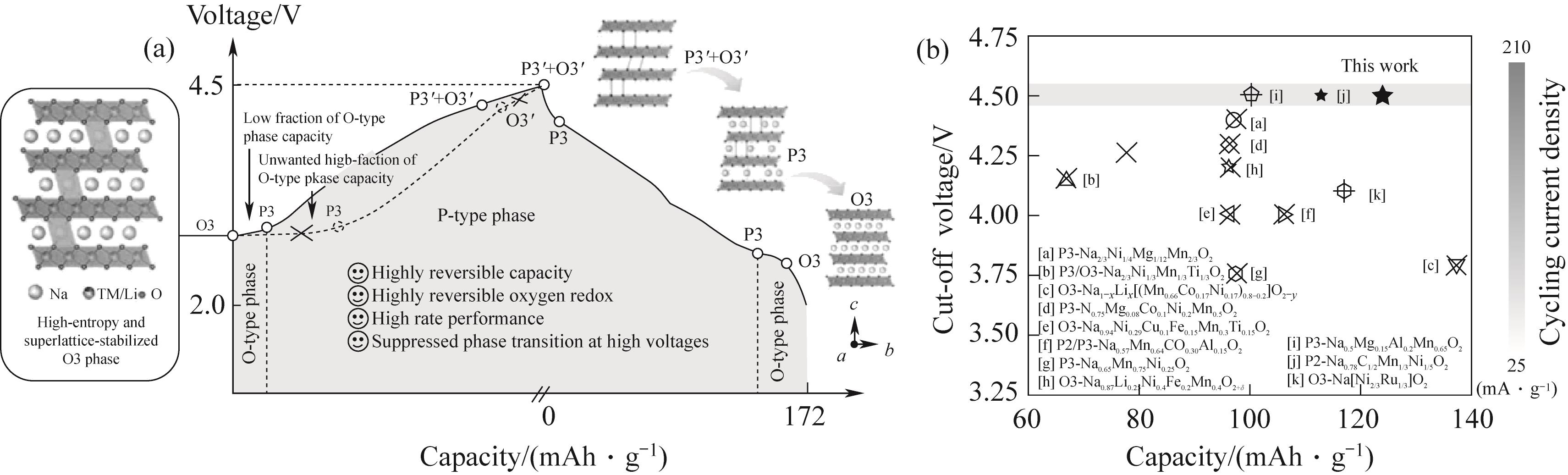
Fig.4 (a) Schematic illustration of charge/discharge behaviors for high-entropy and superlattice-stabilized O3-type cathodes proposed in this work,where the O3-P3 phase transition at the low-voltage region is facilitated and the P3-O3 phase transition at the high-voltage region is suppressed; (b) Electrochemical performance comparison between NaLFCNM and previously reported O3/P3-type cathodes, in which all cathodes havebeen cycled for more than 50 cycles[39]
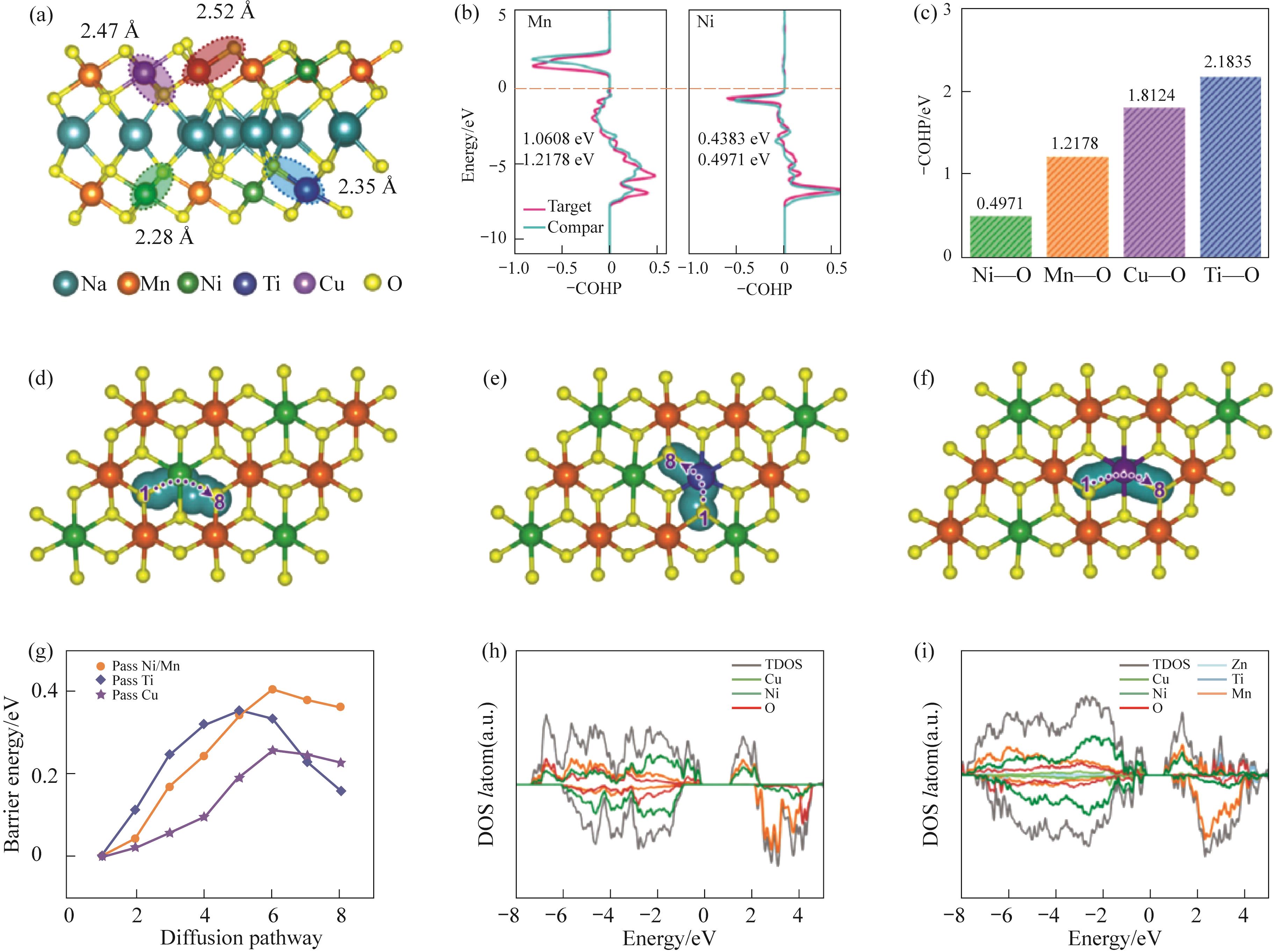
Fig.5 (a) The optimized structures of entropy-tuned NZNCMTO; (b) Comparison of COHP values of Ni—O and Mn—O bonds in NZNCMTO and NNMO; (c) COHP results of entropy-tuned NZNCMTO; (d)—(g) Schematic diagram of the migration paths and migration energy barrier of sodium ions through Ni/Mn, Ti, and Cu in NNMO and entropy-tuned NZNCMTO; (h) Total density of states (DOS) of NNMO and (i) entropy-tuned NZNCMTO of the initial state[40]
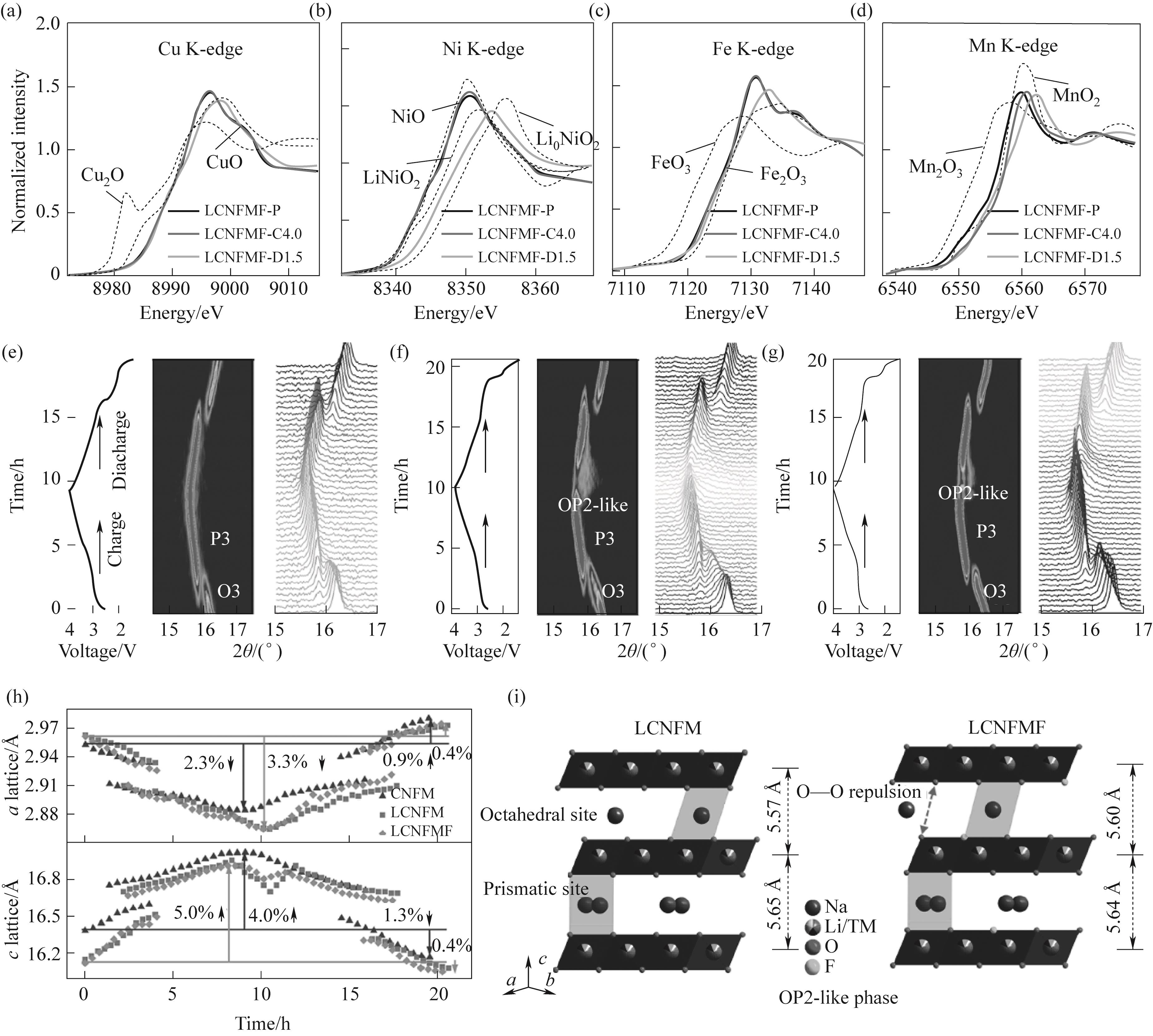
Fig.10 Redox mechanism and crystal structural evolution: (a)—(d) Normalized Cu, Ni, Fe, and Mn K-edge XANES spectra at different charge- discharge states; (e)—(g) Voltage profile and corresponding in situ XRD evolution of CNFM, LCNFM, and LCNFMF; (h) The a/c-lattice parameters change in the three samples obtained by fitting the in situ XRD data; (i) Schematic illustration of the crystal structural evolution at the end of charging of LCNFM and LCNFMF[46]
| [1] | Guo S H, Yi J, Sun Y, et al. Recent advances in titanium-based electrode materials for stationary sodium-ion batteries[J]. Energy & Environmental Science, 2016, 9(10): 2978-3006. |
| [2] | Delmas C. Sodium and sodium-ion batteries: 50 years of research[J]. Advanced Energy Materials, 2018, 8(17): 1703137. |
| [3] | Yabuuchi N, Kubota K, Dahbi M, et al. Research development on sodium-ion batteries[J]. Chemical Reviews, 2014, 114(23): 11636-11682. |
| [4] | Liu Y C, Liu X B, Wang T S, et al. Research and application progress on key materials for sodium-ion batteries[J]. Sustainable Energy & Fuels, 2017, 1(5): 986-1006. |
| [5] | Deng J Q, Luo W B, Chou S L, et al. Sodium-ion batteries: from academic research to practical commercialization[J]. Advanced Energy Materials, 2018, 8(4): 1701428. |
| [6] | Bommier C, Ji X L. Electrolytes, SEI formation, and binders: a review of nonelectrode factors for sodium-ion battery anodes[J]. Small, 2018, 14(16): e1703576. |
| [7] | Chen M Z, Liu Q N, Wang S W, et al. High-abundance and low-cost metal-based cathode materials for sodium-ion batteries: problems, progress, and key technologies[J]. Advanced Energy Materials, 2019, 9(14): 1803609. |
| [8] | Xiang X D, Zhang K, Chen J. Recent advances and prospects of cathode materials for sodium-ion batteries[J]. Advanced Materials, 2015, 27(36): 5343-5364. |
| [9] | Hao Z Q, Shi X Y, Yang Z, et al. The distance between phosphate-based polyanionic compounds and their practical application for sodium-ion batteries[J]. Advanced Materials, 2024, 36(7): 2305135. |
| [10] | Zhao L N, Bi S Y, Li J Y, et al. Prussian blue analogues for advanced non-aqueous sodium ion batteries: redox mechanisms, key challenges and modification strategies[J]. Energy Storage Materials, 2025, 78: 104256. |
| [11] | Gao Y, Zhang H, Liu X H, et al. Low-cost polyanion-type sulfate cathode for sodium-ion battery[J]. Advanced Energy Materials, 2021, 11(42): 2101751. |
| [12] | Li J J, Li H J, Huang Q, et al. Study on the mechanism of the influence of doping on the properties of cathode materials of sodium ion batteries[J]. Prog Chem, 2022, 34(4): 857-869. |
| [13] | Delmas C, Fouassier C, Hagenmuller P. Structural classification and properties of the layered oxides[J]. Physica B+C, 1980, 99(1/2/3/4): 81-85. |
| [14] | Wei F L, Zhang Q P, Zhang P, et al. Review: research progress on layered transition metal oxide cathode materials for sodium ion batteries[J]. Journal of the Electrochemical Society, 2021, 168(5): 050524. |
| [15] | Zuo W H, Qiu J M, Liu X S, et al. The stability of P2-layered sodium transition metal oxides in ambient atmospheres[J]. Nature Communications, 2020, 11(1): 3544. |
| [16] | Li X, Wang Y, Wu D, et al. Jahn-Teller assisted Na diffusion for high performance Na ion batteries[J]. Chemistry of Materials, 2016, 28(18): 6575-6583. |
| [17] | Zhang L, Wang C C, Liu Y C, et al. Suppressing interlayer-gliding and Jahn-Teller effect in P2-type layered manganese oxide cathode via Mo doping for sodium-ion batteries[J]. Chemical Engineering Journal, 2021, 426: 130813. |
| [18] | Zhang R W, Liang J N, Zeng C, et al. Air degradation and rehealing of high-voltage Na0.7Ni0.35Sn0.65O2 cathode for sodium ion batteries[J]. Science China Materials, 2023, 66(1): 88-96. |
| [19] | Gao X, Liu H Q, Chen H Y, et al. Cationic-potential tuned biphasic layered cathodes for stable desodiation/sodiation[J]. Science Bulletin, 2022, 67(15): 1589-1602. |
| [20] | Brugnetti G, Triolo C, Massaro A, et al. Structural evolution of air-exposed layered oxide cathodes for sodium-ion batteries: an example of Ni-doped Na x MnO2 [J]. Chemistry of Materials, 2023, 35(20): 8440-8454. |
| [21] | Wang Y Y, Cao Z T, Du Z Y, et al. Research progress of iron-based polyanionic cathode materials for sodium-ion batteries[J]. Acta Physico-Chimica Sinica, 2025, 41(4): 100035. |
| [22] | Yao H, Gao Y, Lin X H, et al. Prussian blue analogues for aqueous sodium-ion batteries: progress and commercialization assessment[J]. Advanced Energy Materials, 2024, 14(32): 2401984. |
| [23] | Zhang J L, Yu D Y W. Stabilizing Na0.7MnO2 cathode for Na-ion battery via a single-step surface coating and doping process[J]. Journal of Power Sources, 2018, 391: 106-112. |
| [24] | Li S Y, Fan X Q, Wang S M, et al. Probing the account of phase transition upon electrochemical cycling of the P2-Na0.67Ni0.15Fe0.2Mn0.65O2 layered oxide cathodes for sodium-ion batteries[J]. Materials Research Express, 2024, 11(3): 035504. |
| [25] | Kong W J, Wang H B, Sun L M, et al. Understanding the synergic roles of MgO coating on the cycling and rate performance of Na0.67Mn0.5Fe0.5O2 cathode[J]. Applied Surface Science, 2019, 497: 143814. |
| [26] | Fan Z W, Song W D, Yang N, et al. Insights into the phase purity and storage mechanism of nonstoichiometric Na3.4Fe2.4(PO4)1.4P2O7 cathode for high-mass-loading and high-power-density sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(8): e202316957. |
| [27] | Chen C, Huang W Y, Li Y W, et al. P2/O3 biphasic Fe/Mn-based layered oxide cathode with ultrahigh capacity and great cyclability for sodium ion batteries[J]. Nano Energy, 2021, 90: 106504. |
| [28] | Cai C C, Li X Y, Li J T, et al. Transition metal vacancy and position engineering enables reversible anionic redox reaction for sodium storage[J]. Nature Communications, 2025, 16(1): 100. |
| [29] | Wu L R, Zhang Y H, Wu Z, et al. Stabilized O3-type layered sodium oxides with enhanced rate performance and cycling stability by dual-site Ti4+/K+ substitution[J]. Advanced Science, 2023, 10(32): 2304067. |
| [30] | Feng S, Zheng C J, Song Z Y, et al. Boosting fast ionic transport and stability of O3-NaNi1/3Fe1/3Mn1/3O2 cathode via Al/Cu synergistically modulating microstructure for high-rate sodium-ion batteries[J]. Chemical Engineering Journal, 2023, 475: 146090. |
| [31] | Ahmad N, Yu L, Muzaffar M U, et al. Dual-pillar effect in P2-type Na0.67Ni0.33Mn0.67O2 through Na site substitution achieve superior electrochemical and air/water dual-stability as cathode for sodium-ion batteries[J]. Advanced Energy Materials, 2025, 15(20): 2404093. |
| [32] | Zhou B, Wong D, Fu Z H, et al. K-doping suppresses oxygen redox in P2-Na0.67Ni0.11Cu0.22Mn0.67O2 cathode materials for sodium-ion batteries[J]. Small, 2024, 20(43): 2402991. |
| [33] | Xiao B, Wu G, Wang T D, et al. High-entropy oxides as advanced anode materials for long-life lithium-ion batteries[J]. Nano Energy, 2022, 95: 106962. |
| [34] | Huang L P, Zhu J T, Liu J X, et al. Emerging high-entropy strategy: a booster to the development of cathode materials for power batteries[J]. Journal of Advanced Ceramics, 2024, 13(8): 1093-1118. |
| [35] | Wang H J, Gao X, Zhang S, et al. High-entropy Na-deficient layered oxides for sodium-ion batteries[J]. ACS Nano, 2023, 17(13): 12530-12543. |
| [36] | Wang H J, Gao J Q, Mei Y, et al. Halting oxygen evolution to achieve long cycle life in sodium layered cathodes[J]. Angewandte Chemie International Edition, 2025, 64(6): e202418605. |
| [37] | Ding F X, Ji P X, Han Z, et al. Tailoring planar strain for robust structural stability in high-entropy layered sodium oxide cathode materials[J]. Nature Energy, 2024, 9: 1529-1539. |
| [38] | Wang B, Ma J, Wang K J, et al. High-entropy phase stabilization engineering enables high-performance layered cathode for sodium-ion batteries[J]. Advanced Energy Materials, 2024, 14(23): 2401090. |
| [39] | Yao L B, Zou P C, Wang C Y, et al. High-entropy and superstructure-stabilized layered oxide cathodes for sodium-ion batteries[J]. Advanced Energy Materials, 2022, 12(41): 2201989. |
| [40] | Liu J, Huang W Y, Liu R B, et al. Entropy tuning stabilizing P2-type layered cathodes for sodium-ion batteries[J]. Advanced Functional Materials, 2024, 34(24): 2315437. |
| [41] | Liu Z G, Liu R X, Xu D S, et al. Achieving a deeply desodiated stabilized cathode material by the high entropy strategy for sodium-ion batteries[J]. Angewandte Chemie International Edition, 2024, 63(29): e202405620. |
| [42] | Zeng Z Y, Abulikemu A, Zhang J K, et al. High-entropy O3-type cathode enabling low-temperature performance for sodium-ion batteries[J]. Nano Energy, 2024, 128: 109813. |
| [43] | Wang X Z, Zuo Y T, Qin Y B, et al. Fast Na+ kinetics and suppressed voltage hysteresis enabled by a high-entropy strategy for sodium oxide cathodes[J]. Advanced Materials, 2024, 36(24): 2312300. |
| [44] | Dang Y Z, Xu Z, Wu Y R, et al. Boron-doped high-entropy oxide toward high-rate and long-cycle layered cathodes for wide-temperature sodium-ion batteries[J]. Journal of Energy Chemistry, 2024, 95: 577-587. |
| [45] | Cai T X, Cai M Z, Mu J X, et al. High-entropy layered oxide cathode enabling high-rate for solid-state sodium-ion batteries[J]. Nano-Micro Letters, 2023, 16(1): 10. |
| [46] | Ding F X, Wang H B, Zhang Q H, et al. Tailoring electronic structure to achieve maximum utilization of transition metal redox for high-entropy Na layered oxide cathodes[J]. Journal of the American Chemical Society, 2023, 145(25): 13592-13602. |
| [47] | Mu J X, Cai T X, Dong W J, et al. Biphasic high-entropy layered oxide as a stable and high-rate cathode for sodium-ion batteries[J]. Chemical Engineering Journal, 2023, 471: 144403. |
| [48] | Hao D B, Zhang G Y, Ning D, et al. Design of high-entropy P2/O3 hybrid layered oxide cathode material for high-capacity and high-rate sodium-ion batteries[J]. Nano Energy, 2024, 125: 109562. |
| [49] | Hu J, Guo T Q, Zhong X Y, et al. In situ reconstruction of high-entropy heterostructure catalysts for stable oxygen evolution electrocatalysis under industrial conditions[J]. Advanced Materials, 2024, 36(14): 2310918. |
| [50] | Gao X D, Zhang X Y, Liu X Y, et al. Recent advances for high-entropy based layered cathodes for sodium ion batteries[J]. Small Methods, 2023, 7(9): 2300152. |
| [51] | Yeh J W, Chen S K, Lin S J, et al. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes[J]. Advanced Engineering Materials, 2004, 6(5): 299-303. |
| [52] | Rost C M, Sachet E, Borman T, et al. Entropy-stabilized oxides[J]. Nature Communications, 2015, 6: 8485. |
| [53] | Aamlid S S, Oudah M, Rottler J, et al. Understanding the role of entropy in high entropy oxides[J]. Journal of the American Chemical Society, 2023, 145(11): 5991-6006. |
| [54] | George E P, Raabe D, Ritchie R O. High-entropy alloys[J]. Nature Reviews Materials, 2019, 4(8): 515-534. |
| [55] | Zhang W T, Wang X Q, Zhang F Q, et al. Frontiers in high entropy alloys and high entropy functional materials[J]. Rare Metals, 2024, 43(10): 4639-4776. |
| [56] | Zhang R Z, Reece M J. Review of high entropy ceramics: design, synthesis, structure and properties[J]. Journal of Materials Chemistry A, 2019, 7(39): 22148-22162. |
| [57] | Xu T Y, Feng H W, Liu W, et al. Opportunities and challenges of high-entropy materials in lithium-ion batteries[J]. Rare Metals, 2024, 43(10): 4884-4902. |
| [58] | Wang L, Sunariwal N, He Y F, et al. Elemental stability rules for high entropy disordered rocksalt type Li-ion battery positive electrodes[J]. Advanced Energy Materials, 2025, 15(22): 2404982. |
| [59] | Fracchia M, Ghigna P, Pozzi T, et al. Stabilization by configurational entropy of the Cu(Ⅱ) active site during CO oxidation on Mg0.2Co0.2Ni0.2Cu0.2Zn0.2O[J]. The Journal of Physical Chemistry Letters, 2020, 11(9): 3589-3593. |
| [60] | Zheng W, Liang G M, Liu Q, et al. The promise of high-entropy materials for high-performance rechargeable Li-ion and Na-ion batteries[J]. Joule, 2023, 7(12): 2732-2748. |
| [61] | Kong X K, Gu R, Jin Z Z, et al. Maximizing interface stability in all-solid-state lithium batteries through entropy stabilization and fast kinetics[J]. Nature Communications, 2024, 15(1): 7247. |
| [62] | Li M, Zhuo H X, Lei J W, et al. Unravelling the structure-stability interplay of O3-type layered sodium cathode materials via precision spacing engineering[J]. Nature Communications, 2025, 16(1): 2010. |
| [63] | Wang J H, Xu F T, Fan X M, et al. Study on the impact of cutoff voltage on structural and electrochemical stability of sodium-ion layered cathodes[J]. Chemical Engineering Journal, 2024, 500: 157032. |
| [64] | Huang Q, Wang M Y, Zhang L, et al. Shear-resistant interface of layered oxide cathodes for sodium ion batteries[J]. Energy Storage Materials, 2022, 45: 389-398. |
| [65] | Liu S Q, Liu F Z, Zhao S, et al. A high-entropy engineering on sustainable anionic redox Mn-based cathode with retardant stress for high-rate sodium-ion batteries[J]. Angewandte Chemie International Edition, 2025, 64(10): e202421089. |
| [66] | Tian K H, Dang Y Z, Xu Z, et al. A three-in-one strategy of high-entropy, single-crystal, and biphasic approaches to design O3- type layered cathodes for sodium-ion batteries[J]. Energy Storage Materials, 2024, 73: 103841. |
| [67] | Sekine S, Hosaka T, Maejima H, et al. Na[Mn0.36Ni0.44Ti0.15Fe0.05]O2 predicted via machine learning for high energy Na-ion batteries[J]. Journal of Materials Chemistry A, 2024, 12(45): 31103-31107. |
| [68] | Guo R N, Yang Y, Zhao C C, et al. The role of high-entropy materials in lithium-based rechargeable batteries[J]. Advanced Functional Materials, 2024, 34(18): 2313168. |
| [69] | Quinn A, Moutinho H, Usseglio-Viretta F, et al. Electron backscatter diffraction for investigating lithium-ion electrode particle architectures[J]. Cell Reports Physical Science, 2020, 1(8): 100137. |
| [70] | Chen D, Indris S, Schulz M, et al. In situ scanning electron microscopy on lithium-ion battery electrodes using an ionic liquid[J]. Journal of Power Sources, 2011, 196(15): 6382-6387. |
| [71] | He K, Zhang S, Li J, et al. Visualizing non-equilibrium lithiation of spinel oxide via in situ transmission electron microscopy[J]. Nature Communications, 2016, 7: 11441. |
| [72] | Wu K, Ran P L, Yin W, et al. Dynamic evolution of antisite defect and coupling anionic redox in high-voltage ultrahigh-Ni cathode[J]. Angewandte Chemie International Edition, 2024, 63(42): e202410326. |
| [1] | Shengmei ZHANG, Ming LI, Ying ZHANG, Xi YI, Yiting YANG, Yali LIU. Effects of emulsifier and reacting temperature on characteristics of phase change microcapsules [J]. CIESC Journal, 2025, 76(S1): 444-452. |
| [2] | Songyuan GUO, Xiaoqing ZHOU, Wubing MIAO, Bin WANG, Rui ZHUAN, Qingtai CAO, Chengcheng CHEN, Guang YANG, Jingyi WU. Numerical study on characteristics of pressurized discharge in liquid oxygen tank equipped with porous plate in the ascent period of rocket [J]. CIESC Journal, 2025, 76(S1): 62-74. |
| [3] | Lanhao LOU, Lipeng YANG, Xiaoguang YANG. Review of parameter identification for physics-based lithium-ion battery models [J]. CIESC Journal, 2025, 76(9): 4369-4382. |
| [4] | Yufeng WANG, Xiaoxue LUO, Hongliang FAN, Baijing WU, Cunpu LI, Zidong WEI. Green organic electrosynthesis coupled with water electrolysis to produce hydrogen—overview of electrode interface regulation strategies [J]. CIESC Journal, 2025, 76(8): 3753-3771. |
| [5] | Linkai WU, Zhimin LIN, Liangbi WANG. Improvement and numerical validation of quasi-steady-state frosting model based on thermal and mass transfer effect [J]. CIESC Journal, 2025, 76(8): 4004-4016. |
| [6] | Ning YANG, Haonan LI, Xiao LIN, Stella GEORGIADOU, Wen-Feng LIN. Application of plastic-derived carbon@CoMoO4 composites as an efficient electrocatalyst for hydrogen evolution reaction in water electrolysis [J]. CIESC Journal, 2025, 76(8): 4081-4094. |
| [7] | Jiaxin LUO, Yan YUAN. Research progress of piezoelectric materials in solid-state metal secondary batteries [J]. CIESC Journal, 2025, 76(8): 3822-3833. |
| [8] | Wenjia LIU, Ruxue DU, Siqi WANG, Tingxian LI. Research status and application of functional phase change materials for electro-thermal conversion in thermal energy storage [J]. CIESC Journal, 2025, 76(7): 3185-3196. |
| [9] | Ziheng WANG, Wenhuai LI, Wei ZHOU. Application of patterned electrodes in solid oxide fuel cell [J]. CIESC Journal, 2025, 76(7): 3153-3171. |
| [10] | Xinran LI, Longjiao CHANG, Shaohua LUO, Yongbing LI, Ruifen YANG, Zenglei HOU, Jie ZOU. Modification mechanism of Ho doped NCM622 induced local electron remodeling to inhibit cationic mixing [J]. CIESC Journal, 2025, 76(7): 3733-3741. |
| [11] | Lixiao WU, Xixi YAN, Suna ZHANG, Yiming XU, Jiaying QIAN, Yongmin QIAO, Lijun WANG. The preparation of phosphorus-doped microcrystalline graphite and its electrochemical performance as an anode material for lithium-ion batteries [J]. CIESC Journal, 2025, 76(7): 3615-3625. |
| [12] | Tianwei XIA, Anci WANG, Zihan JU, Xiaoxia SUN, Dinghua HU. Study on thermal storage and release characteristics of TPMS-based high density thermal storage device [J]. CIESC Journal, 2025, 76(7): 3605-3614. |
| [13] | Guoqing SUN, Haibo LI, Zhiyang DING, Wenhui GUO, Hao XU, Yanxia ZHAO. Research progress of silicon based anode materials [J]. CIESC Journal, 2025, 76(7): 3197-3211. |
| [14] | Peiqiang CHEN, Qun ZHENG, Yuting JIANG, Chunhua XIONG, Jinmao CHEN, Xudong WANG, Long HUANG, Man RUAN, Wanli XU. Effects of electrolyte flow rate and current density on the output performance of seawater-activated batteries [J]. CIESC Journal, 2025, 76(7): 3235-3245. |
| [15] | Jia KANG, Huan LIU, Haiyan LI, Maoliang LUO, Hong YAO. Corrosion behavior and coating performance of carbon steel in HCl/NaOH thermal medium in wide temperature zone [J]. CIESC Journal, 2025, 76(6): 2872-2885. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||