化工学报 ›› 2020, Vol. 71 ›› Issue (S2): 314-320.DOI: 10.11949/0438-1157.20200355
• 材料化学工程与纳米技术 • 上一篇
收稿日期:2020-04-07
修回日期:2020-06-04
出版日期:2020-11-06
发布日期:2020-11-06
通讯作者:
刘亮
作者简介:刘亮(1982—),男,博士,工程师,基金资助:
Liang LIU1,2( ),Aizhi WU1,Yun HUANG2,Jian HUANG3
),Aizhi WU1,Yun HUANG2,Jian HUANG3
Received:2020-04-07
Revised:2020-06-04
Online:2020-11-06
Published:2020-11-06
Contact:
Liang LIU
摘要:
以适合工业储热的复合无机相变储热材料硝酸盐(KNO3、NaNO3)和碳酸盐(Li2CO3、K2CO3、Na2CO3和CaCO3)为相变组分,研究了4种不同配比硝酸盐相变组分和6种不同配比碳酸盐相变组分的热性能(熔点、潜热)差异,分别优选出一种熔融盐相变组分配比。利用多孔载体吸附原理,制备出两种最佳配比的复合熔融盐类储热材料,分析了储热材料在不同介质气氛中(Ar和air)分解难易程度,TG-DSC-MS联用测试的高温热分解产物表明,复合硝酸盐类储热材料在中温(300℃)储热时,物理化学性能稳定,安全性较好,但在高温(500℃以上)时易分解成NO和NO2,且air气氛中更易生成有毒气体;而复合碳酸盐类储热材料在air中比在Ar中更易生成CO而影响储热过程中的安全性。
中图分类号:
刘亮, 吴爱枝, 黄云, 黄剑. 两类复合无机相变储热材料高温热稳定性和安全性研究[J]. 化工学报, 2020, 71(S2): 314-320.
Liang LIU, Aizhi WU, Yun HUANG, Jian HUANG. Research on high temperature thermal stability and safety of two types of composite inorganic phase change thermal storage materials[J]. CIESC Journal, 2020, 71(S2): 314-320.
| 型号及名称 | 厂家 | 用途 |
|---|---|---|
| HC-07040玛瑙研钵 | 北京中镜科仪技术有限公司 | 原材料研磨 |
| JH-50700067标准过目筛 | 新乡金禾机械有限公司 | 原材料粒径筛选 |
| DZF-6050真空干燥箱 | 上海一恒科技有限公司 | 原材料烘干 |
| XS-204 电子天平 | METTLER TOLEDO | 原材料称量 |
| FYD-40-A电动手动台式压片机 | 北京鑫骉腾达仪器设备有限公司 | 压片 |
| SK-G08165真空/气氛管式电炉 | 天津中环电炉股份有限公司 | 烧结 |
表1 复合无机相变储热材料制备仪器
Table 1 Preparation instruments of composite inorganic phase change thermal storage materials
| 型号及名称 | 厂家 | 用途 |
|---|---|---|
| HC-07040玛瑙研钵 | 北京中镜科仪技术有限公司 | 原材料研磨 |
| JH-50700067标准过目筛 | 新乡金禾机械有限公司 | 原材料粒径筛选 |
| DZF-6050真空干燥箱 | 上海一恒科技有限公司 | 原材料烘干 |
| XS-204 电子天平 | METTLER TOLEDO | 原材料称量 |
| FYD-40-A电动手动台式压片机 | 北京鑫骉腾达仪器设备有限公司 | 压片 |
| SK-G08165真空/气氛管式电炉 | 天津中环电炉股份有限公司 | 烧结 |
| 配比编号 | KNO3∶NaNO3比例 |
|---|---|
| 1 | 6∶4 |
| 2 | 4∶6 |
| 3 | 1∶0 |
| 4 | 0∶1 |
表2 四种典型复合硝酸盐配比
Table 2 Proportion of four typical compound nitrates
| 配比编号 | KNO3∶NaNO3比例 |
|---|---|
| 1 | 6∶4 |
| 2 | 4∶6 |
| 3 | 1∶0 |
| 4 | 0∶1 |
| 配比编号 | Li2CO3∶K2CO3∶Na2CO3∶CaCO3比例 |
|---|---|
| 1 | 1∶0∶0∶0 |
| 2 | 1∶0∶1∶0 |
| 3 | 2∶6.5∶1.7∶1 |
| 4 | 2∶6.5∶2∶1 |
| 5 | 2∶6.5∶2.5∶1 |
| 6 | 1.5∶6.5∶2.5∶1 |
表3 六种典型复合碳酸盐配比
Table 3 Proportion of six typical composite carbonates
| 配比编号 | Li2CO3∶K2CO3∶Na2CO3∶CaCO3比例 |
|---|---|
| 1 | 1∶0∶0∶0 |
| 2 | 1∶0∶1∶0 |
| 3 | 2∶6.5∶1.7∶1 |
| 4 | 2∶6.5∶2∶1 |
| 5 | 2∶6.5∶2.5∶1 |
| 6 | 1.5∶6.5∶2.5∶1 |
| 储热材料类别 | 原材料 | 组分配比 |
|---|---|---|
| 硝酸盐类 | 硝酸钠∶二氧化硅∶石墨 | 7∶3∶1 |
| 碳酸盐类 | 碳酸钠∶碳酸锂∶氧化镁∶石墨 | 3∶3∶4∶1 |
表4 两类复合熔融盐类储热材料原材料最佳配比
Table 4 Optimum ratio of raw materials for two types of composite molten salt thermal storage materials
| 储热材料类别 | 原材料 | 组分配比 |
|---|---|---|
| 硝酸盐类 | 硝酸钠∶二氧化硅∶石墨 | 7∶3∶1 |
| 碳酸盐类 | 碳酸钠∶碳酸锂∶氧化镁∶石墨 | 3∶3∶4∶1 |
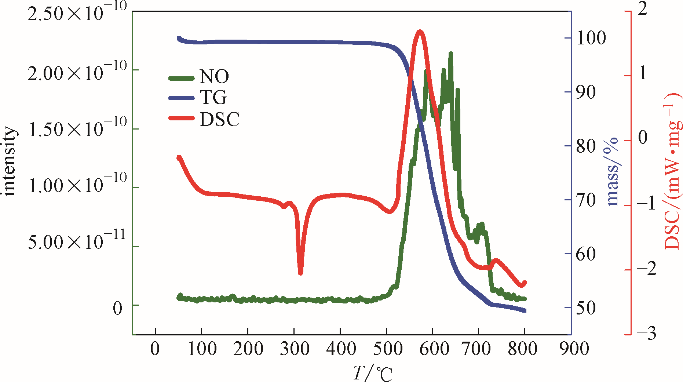
图3 复合硝酸盐类储热材料的热分析-质谱图(Ar气中,NO气体产物)
Fig.3 Thermal analysis-mass spectrometry diagram of composite nitrate thermal storage material (in Ar, NO gas product)
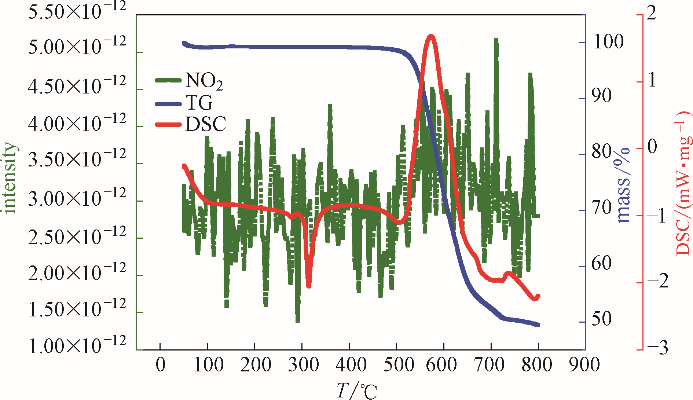
图4 复合硝酸盐类储热材料的热分析-质谱图(Ar气中, NO2气体产物)
Fig.4 Thermal analysis-mass spectrometry diagram of composite nitrate thermal storage material (in Ar,NO2 gas product)

图5 复合硝酸盐类储热材料的热分析-质谱图(air气中, NO气体产物)
Fig.5 Thermal analysis-mass spectrometry diagram of composite nitrate thermal storage material (in air, NO gas product)
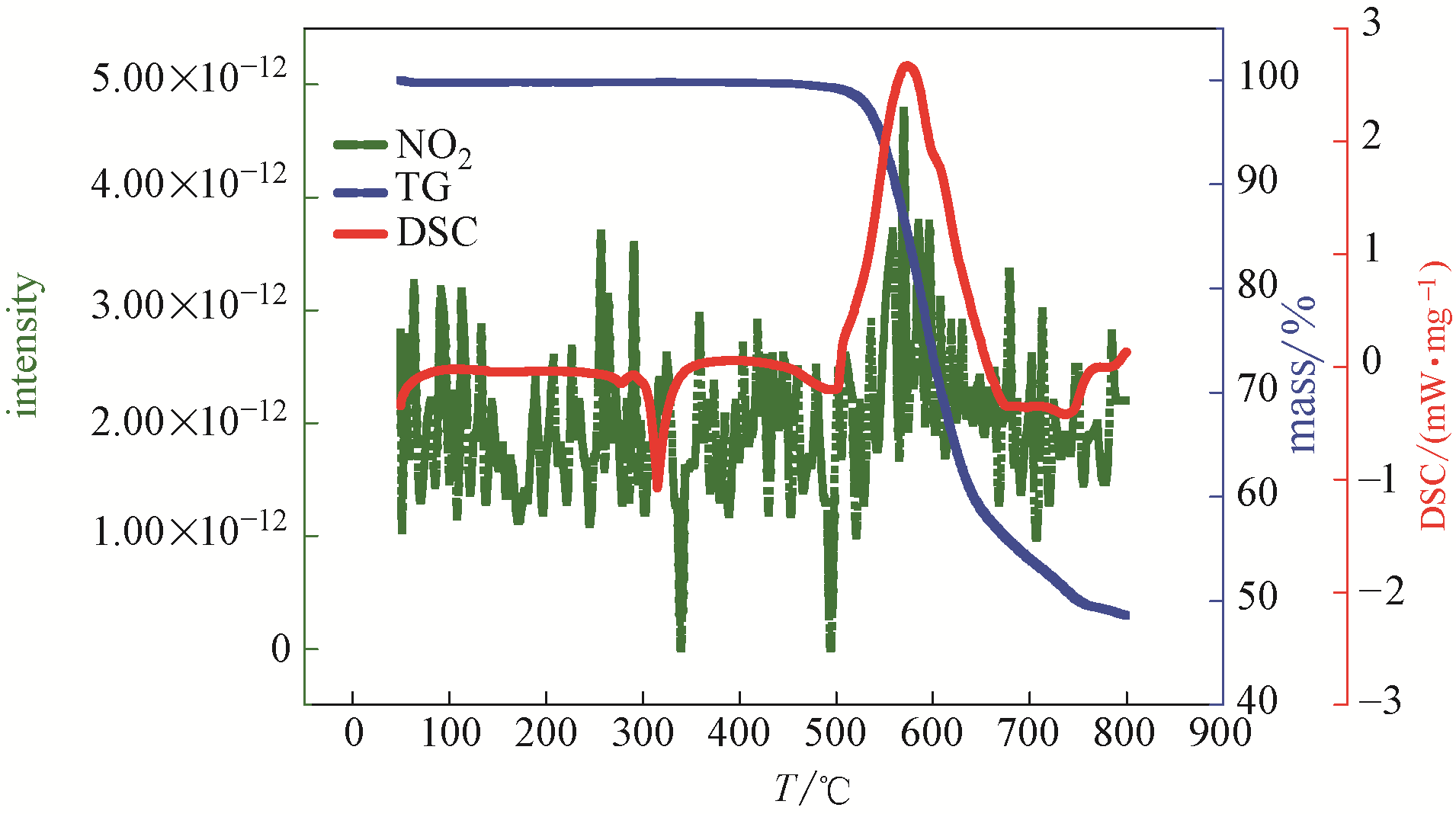
图6 复合硝酸盐类储热材料的热分析-质谱图(air气中, NO2气体产物)
Fig.6 Thermal analysis-mass spectrometry diagram of composite nitrate thermal storage material (in air, NO2 gas product)
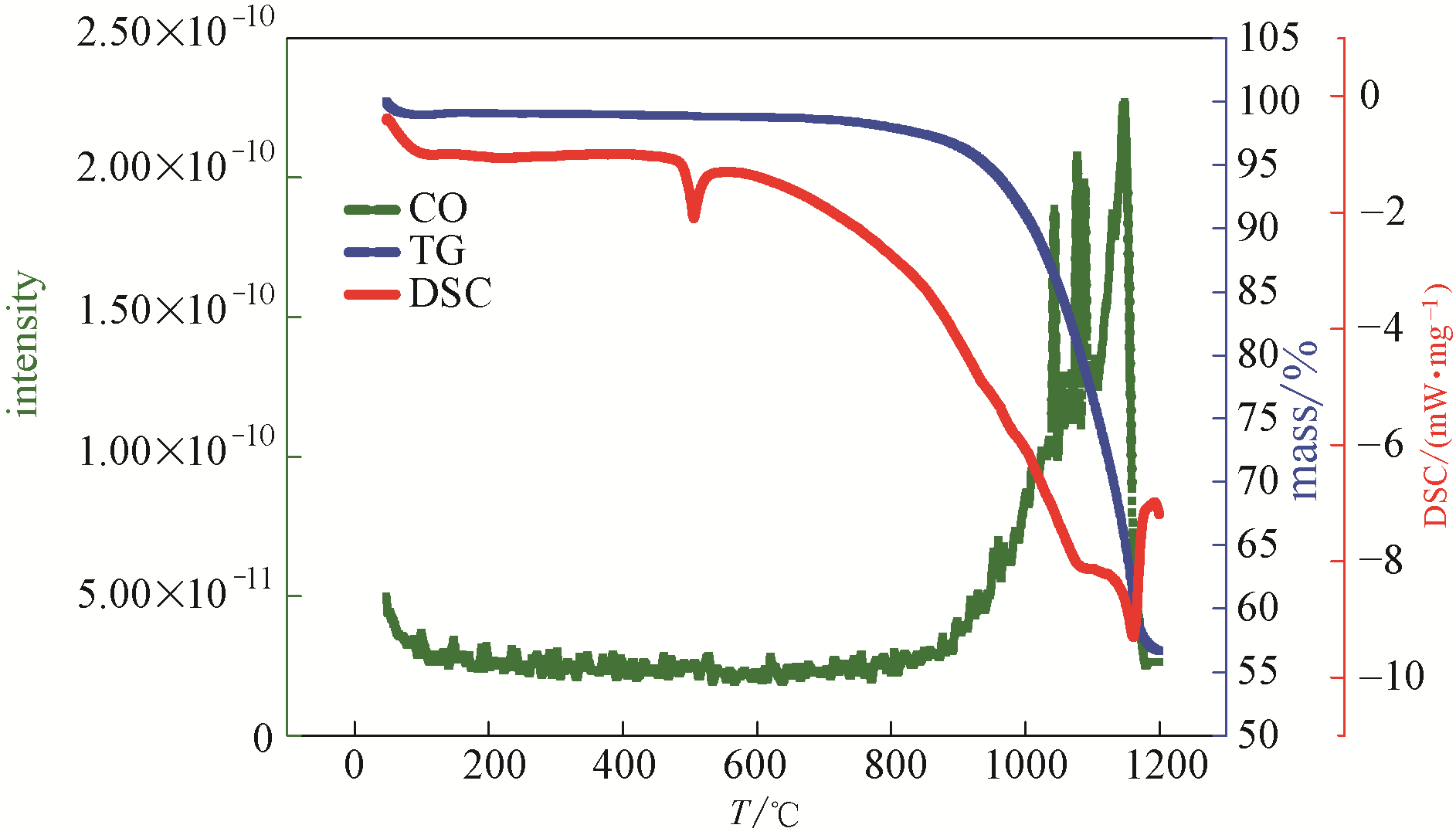
图7 复合碳酸盐类储热材料的热分析-质谱图(Ar气中, CO气体产物)
Fig.7 Thermal analysis-mass spectrometry diagram of composite carbonate thermal storage material (in Ar, CO gas product)

图8 复合碳酸盐类储热材料的热分析-质谱图(Ar气中, CO2气体产物)
Fig.8 Thermal analysis-mass spectrometry diagram of composite carbonate heat storage material (in Ar, product of CO2 gas)

图9 复合碳酸盐类储热材料的热分析-质谱图(air气中,CO气体产物)
Fig.9 Thermal analysis-mass spectrometry diagram of composite carbonate thermal storage material (in air, CO gas product)
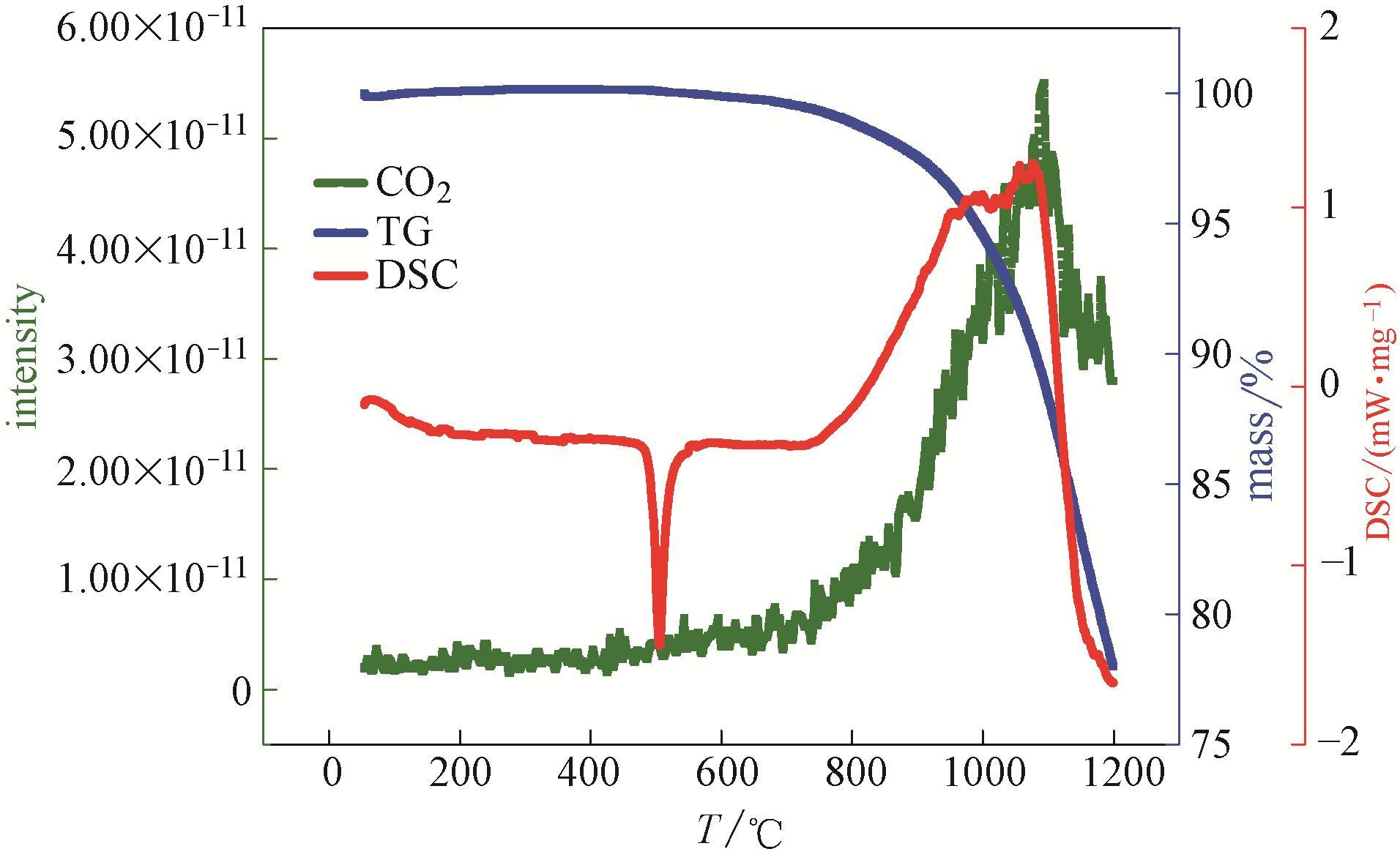
图10 复合碳酸盐类储热材料的热分析-质谱图(air气中, CO2气体产物)
Fig.10 Thermal analysis-mass spectrometry diagram of composite carbonate thermal storage material (in air, CO2 gas product)
| 1 | Hoshi A, Mills D R, Bittar A, et al. Screening of high melting point phase change materials (PCM) in solar thermal concentrating technology based on CLFR[J]. Solar Energy, 2005, 79(3): 332-339. |
| 2 | 张寅平, 胡汉平, 孔祥冬. 相变储热-理论与应用[M]. 合肥: 中国科技大学出版社, 1996. |
| Zhang Y P, Hu H P, Kong X D. Phase Change Thermal Storage-Theory and Application [M]. Hefei: University of Science and Technology of China Press, 1996. | |
| 3 | Pielichowska K, Pielichowski K. Phase change materials for thermal energy storage[J]. Progress in Materials Science, 2014, 65(10): 67-123. |
| 4 | 张贺磊, 方贤德, 赵颖杰. 相变储热材料及技术的研究进展[J]. 材料导报, 2014, 28(13): 26-32. |
| Zhang H L, Fang X D, Zhao Y J. Research progress of phase change thermal storage materials and technologies [J]. Materials Review, 2014, 28 (13): 26-32. | |
| 5 | Jamekhorshid A, Sadrameli S M, Barzin R, et al. Composite of wood-plastic and micro-encapsulated phase change material (MEPCM) used for thermal energy storage[J]. Applied Thermal Engineering, 2016, 112: 82-88. |
| 6 | Cheralathan M, Velraj R, Renganarayanan S. Heat transfer and parametric studies of an encapsulated phase change material based cool thermal energy storage system[J]. Journal of Zhejiang University-Science A(Applied Physics & Engineering), 2006, 7(11): 1886-1895. |
| 7 | Ho C J, Gao J Y. Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material[J]. International Communications in Heat & Mass Transfer, 2009, 36(5): 467-470. |
| 8 | 何天白, 胡汉杰. 功能高分子与新材料[M]. 北京: 化学工业出版社, 2001. |
| He T B, Hu H J. Functional Polymers and New Materials [M]. Beijing: Chemical Industry Press, 2001. | |
| 9 | 杨岳澔, 程晓敏, 李丹, 等. 硬脂酸/改性碳纳米管复合相变储热材料性能[J]. 储能科学与技术, 2019, 8(4): 759-763. |
| Yang Y D, Cheng X M, Li D, et al. Properties of stearic acid / modified carbon nanotube composite phase change thermal storage material [J]. Energy Storage Science and Technology, 2019, 8 (4): 759-763. | |
| 10 | 赵耀. 相变材料及梯级系统传热储热特性的理论与实验研究[D]. 上海: 上海交通大学, 2018. |
| Zhao Y. Theoretical and experimental study on heat transfer and storage characteristics of phase change materials and cascade systems[D]. Shanghai: Shanghai Jiao Tong University, 2018. | |
| 11 | Li M, Wu Z, Tan J. Properties of form-stable paraffin/silicon dioxide/expanded graphite phase change composites prepared by sol–gel method[J]. Applied Energy, 2012, 92(2): 456-461. |
| 12 | Zhang H, Wang X, Wu D. Silica encapsulation of n-octadecane via sol-gel process: a novel microencapsulated phase-change material with enhanced thermal conductivity and performance[J]. Journal of Colloid & Interface Science, 2010, 343(1): 246-255. |
| 13 | Yu S, Wang X, Wu D. Microencapsulation of n-octadecane phase change material with calcium carbonate shell for enhancement of thermal conductivity and serving durability: synthesis, microstructure, and performance evaluation[J]. Applied Energy, 2014, 114(2): 632-643. |
| 14 | Sarı A, Alkan C, Karaipekli A. Preparation, characterization and thermal properties of PMMA/ n-heptadecane microcapsules as novel solid-liquid microPCM for thermal energy storage[J]. Applied Energy, 2010, 87(5): 1529-1534. |
| 15 | Alkan C, Sarı A, Karaipekli A. Preparation, thermal properties and thermal reliability of microencapsulated n-eicosane as novel phase change material for thermal energy storage[J]. Energy Conversion & Management, 2011, 52(1): 687-692. |
| 16 | 吕社辉, 郭元强, 陈鸣才, 等. 复合高分子相变材料研究进展[J]. 高分子材料科学与工程, 2004, 20(3): 37-40. |
| Lyu S H, Guo Y Q, Chen M C, et al. Research progress of composite polymer phase change materials[J]. Polymer Materials Science and Engineering, 2004, 20(3): 37-40. | |
| 17 | 张东, 康韡, 李凯莉. 复合相变材料研究进展[J]. 功能材料, 2007, 38(12): 1936-1940. |
| Zhang D, Kang Y, Li K L. Research progress of composite phase change materials[J]. Functional Materials, 2007, 38(12): 1936-1940. | |
| 18 | Wang L B, Zhang K L, Pan H L, et al. 2D molybdenum nitride nanosheets as anode materials for improved lithium storage[J]. Nanoscale, 2018, 10(40): 18936-18941. |
| 19 | Ortega F, Nicolas C, Gil A, et al. Thermophysical characterization of a by-product from the steel industry to be used as a sustainable and low-cost thermal energy storage material[J]. Energy, 2015, 89: 601-609. |
| 20 | Nasef M M, Gürsel S A, Karabelli D, et al. Radiation-grafted materials for energy conversion and energy storage applications[J]. Progress in Polymer Science, 2016, 63: 1-41. |
| 21 | Rathod M K, Banerjee J. Experimental investigations on latent heat storage unit using paraffin wax as phase change material[J]. Experimental Heat Transfer, 2014, 27(1/2/3/4/5): 40-55. |
| 22 | Sick N, Blug M, Leker J. The influence of raw material prices on the development of hydrogen storage materials: the case of metal hydrides[J]. Journal of the Knowledge Economy, 2014, 5(4): 735-760. |
| 23 | Huang X, Alva G, Jia Y T, et al. Morphological characterization and applications of phase change materials in thermal energy storage: a review[J]. Renewable and Sustainable Energy Reviews, 2017, 72: 128-145. |
| 24 | Diarce G, Corro-Martínez, E, Quant L, et al. The sodium nitrate–urea binary mixture as a phase change material for medium temperature thermal energy storage(Ⅰ): Determination of the phase diagram and main thermal properties[J]. Solar Energy Materials and Solar Cells, 2016, 157: 1065-1075. |
| 25 | Zhang X, Ma J, Chen K. Impact of morphology of conductive agent and anode material on lithium storage properties[J]. Nano-Micro Letters, 2015, 7(4): 360-367. |
| 26 | Banu D, Feldman D, Haghighat F, et al. Energy-storing wallbnant: flammability test[J]. Joumal of Materials in Civil Engineerin, 1998, 10(2): 98-105. |
| 27 | Zalba B, Marín J M, Cabeza L F, et al. Review on thermal energy storage with phase change: materials, heat transfer analysis andapplications[J]. Applied Thermal Engineering, 2003, 23(3): 251-283. |
| 28 | 贺万玉, 闫全英. 熔融盐相变储热材料[J]. 材料导报: 纳米与新材料专辑, 2015, (S1): 128-130. |
| He W Y, Yan Q Y. Molten salt phase change heat storage material [J]. Materials Review: Nano and New Materials Special, 2015, (S1): 128-130. | |
| 29 | Jamekhorshid A, Sadrameli S M, Barzin R, et al. Composite of wood-plastic and micro-encapsulated phase change material (MEPCM) used for thermal energy storage[J]. Applied thermal Engineering, 2017, 112: 82-88. |
| 30 | Ruiz M L, Lick I D, Ponzi M I, et al. Thermal decomposition of supported lithium nitrate catalysts[J]. Thermochimica Acta, 2010, 499(1): 21-26. |
| [1] | 徐银龙, 郑文杰, 王琳, 薛中飞, 谢毅鑫. 壳聚糖联合酶诱导碳酸盐沉淀处理铜废水的劣化现象和强化机理研究[J]. 化工学报, 2022, 73(5): 2222-2232. |
| [2] | 王琴, 徐会金, 韩兴超, 赵长颖. MgO/Mg(OH)2热化学储热反应的第一性原理研究[J]. 化工学报, 2021, 72(3): 1242-1252. |
| [3] | 胡南,陈雪,张辉,李艾书,李广悦,王永东,丁德馨. Sporosarcina pasteurii诱导碳酸盐-铀共沉淀修复低浓度铀废水的试验研究[J]. 化工学报, 2021, 72(10): 5354-5361. |
| [4] | 王雪莹,黄雪莉,黄河,罗清龙,邹雪净. -15℃下Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O体系相平衡研究[J]. 化工学报, 2020, 71(11): 5059-5066. |
| [5] | 于强, 鹿院卫, 张晓盼, 吴玉庭. 纳米粒子对熔盐复合蓄热材料热物性的影响[J]. 化工学报, 2019, 70(S1): 217-225. |
| [6] | 李德生, 张超, 邓时海, 胡智丰, 李金龙, 刘元辉. 基于铁基质高效催化还原污水中硝酸盐氮的实验研究[J]. 化工学报, 2019, 70(3): 1065-1074. |
| [7] | 朱益, 王浩, 陈利平, 郭子超, 何中其, 陈网桦. 基于数值计算方法计算最大反应速率到达时间[J]. 化工学报, 2019, 70(1): 379-387. |
| [8] | 桑丽霞, 李锋. 碳酸盐复合蓄热材料的制备及热物性研究[J]. 化工学报, 2018, 69(S1): 129-135. |
| [9] | 赵海谦, 董明, 汪怀远, 刘立君, 李栋, 刘晓燕. 不同尺寸反应器内H2O2热分解氧化NO特性与氧化产物分析[J]. 化工学报, 2018, 69(9): 4037-4043. |
| [10] | 吴东灵, 李廷贤, 何峰, 王如竹. 三水醋酸钠相变储能复合材料改性制备及储/放热特性[J]. 化工学报, 2018, 69(7): 2860-2868. |
| [11] | 李洪伟, 徐飞扬, 夏曼曼, 程扬帆, 龚悦. 用C80量热仪研究重铵油炸药的热分解特性[J]. 化工学报, 2018, 69(4): 1799-1805. |
| [12] | 程爱华, 钱大鹏. 棉花模板Zn/Ti/Fe-LDO吸附水中硝酸盐机制[J]. 化工学报, 2018, 69(12): 5283-5291. |
| [13] | 董泽, 陈利平, 陈网桦, 马莹莹. 40%DCP溶液的热分解模型[J]. 化工学报, 2017, 68(5): 1773-1779. |
| [14] | 修瑞瑞, 何世颖, 宋海亮, 杨林章, 张婉. 改性硅藻土负载纳米零价铁去除水中硝酸盐氮[J]. 化工学报, 2016, 67(9): 3888-3894. |
| [15] | 谢丽, 殷紫, 尹志轩, 王悦超, 周琪. 一段式厌氧氨氧化工艺亚硝酸盐氧化菌抑制方法研究进展[J]. 化工学报, 2016, 67(7): 2647-2655. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号