化工学报 ›› 2020, Vol. 71 ›› Issue (11): 5059-5066.DOI: 10.11949/0438-1157.20200059
收稿日期:2020-01-15
修回日期:2020-06-30
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
黄雪莉
作者简介:王雪莹(1988—),女,博士研究生,基金资助:
Xueying WANG( ),Xueli HUANG(
),Xueli HUANG( ),He HUANG,Qinglong LUO,Xuejing ZOU
),He HUANG,Qinglong LUO,Xuejing ZOU
Received:2020-01-15
Revised:2020-06-30
Online:2020-11-05
Published:2020-11-05
Contact:
Xueli HUANG
摘要:
新疆卤水硝酸盐矿主要含有Na+、K+、Mg2+、Cl-、NO3-、SO42-六种离子,属于高元复杂体系,其合理利用和开发需要不同温度下的相平衡研究作为理论支撑。采用等温溶解平衡法,对Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O体系在-15℃、NaCl·2H2O饱和条件下的相平衡进行了研究,并构建了相图。相图中有六个零变量点和八个两盐结晶区,只存在一种复盐KCl·MgCl2·6H2O。八个两盐结晶区,分别对应于NaCl·2H2O+Na2SO4·10H2O、NaCl·2H2O+NaNO3、NaCl·2H2O+KCl、NaCl·2H2O+KNO3、NaCl·2H2O+MgSO4·7H2O、NaCl·2H2O+MgCl2·8H2O、NaCl·2H2O+Mg(NO3)2·6H2O和NaCl·2H2O+KCl·MgCl2·6H2O,其中NaCl·2H2O+Na2SO4·10H2O共晶区最大,在低温时,硫酸钠的溶解度最小,降温过程中较易结晶析出。与该体系在25℃下的相图相比,复盐种类减少5种,零变量点减少19个,相关系得以极大简化。
中图分类号:
王雪莹,黄雪莉,黄河,罗清龙,邹雪净. -15℃下Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O体系相平衡研究[J]. 化工学报, 2020, 71(11): 5059-5066.
Xueying WANG,Xueli HUANG,He HUANG,Qinglong LUO,Xuejing ZOU. Study on phase equilibrium of system Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O at -15℃[J]. CIESC Journal, 2020, 71(11): 5059-5066.
| No. | Composition of solution, wB/% | Solid phase | |||||
|---|---|---|---|---|---|---|---|
| K+ | Mg2+ | Cl- | NO3- | SO42- | Na+ | ||
| 1,A1 | 0.79 | 6.35 | 20.24 | 0.00 | 0.00 | 0.65 | Hy+Sy+Car |
| 2,A2 | 0.04 | 8.26 | 24.29 | 0.00 | 0.00 | 0.11 | Hy+Car+M8 |
| 3,A3 | 0.00 | 8.08 | 18.66 | 9.44 | 0.00 | 0.32 | Hy+NN+Nit |
| 4,A4 | 0.00 | 8.42 | 20.01 | 8.66 | 0.00 | 0.26 | Hy+M8+Nit |
| 5,A5① | 2.06 | 0.00 | 9.88 | 17.41 | 0.00 | 11.66 | Hy+KN+NN |
| 6,A6① | 4.21 | 0.00 | 14.49 | 4.91 | 0.00 | 8.75 | Hy+KN+Sy |
| 7,A7① | 0.00 | 0.00 | 9.75 | 14.42 | 0.23 | 11.78 | Hy+NN+S10 |
| 8,A8① | 0.00 | 4.45 | 15.45 | 0.00 | 2.52 | 2.81 | Hy+Eps+S10 |
| 9,A9① | 0.00 | 7.94 | 23.12 | 0.00 | 0.48 | 0.19 | Hy+Eps+M8 |
| 10,A10① | 3.30 | 0.00 | 15.60 | 0.00 | 0.34 | 8.34 | Hy+S10+Sy |
| 11,B1 | 0.71 | 6.63 | 16.64 | 9.36 | 0.00 | 1.30 | Hy+NN+KN+Nit |
| 12,B2 | 1.37 | 5.91 | 18.78 | 2.82 | 0.00 | 1.24 | Hy+KN+Sy+Car |
| 13,B3 | 0.42 | 7.72 | 18.03 | 9.67 | 0.00 | 0.43 | Hy+KN+Car+Nit |
| 14,B4 | 0.15 | 8.36 | 20.34 | 8.52 | 0.00 | 0.45 | Hy+Car+Nit+M8 |
| 15,B5 | 0.00 | 4.79 | 14.02 | 8.39 | 1.15 | 3.69 | Hy+Eps+S10+NN |
| 16,B6 | 0.00 | 8.24 | 18.76 | 9.86 | 0.54 | 0.50 | Hy+Eps+Nit+NN |
| 17,B7 | 0.00 | 8.50 | 19.97 | 8.82 | 0.77 | 0.51 | Hy+Eps+M8+Nit |
| 18,B8① | 2.03 | 0.00 | 9.81 | 17.34 | 0.24 | 11.71 | Hy+NN+S10+KN |
| 19,B9① | 4.13 | 0.00 | 14.34 | 4.97 | 0.29 | 8.86 | Hy+S10+Sy+KN |
| 20,B10① | 1.72 | 4.12 | 16.07 | 0.00 | 2.37 | 2.76 | Hy+Eps+S10+Sy |
| 21,B11① | 0.84 | 6.45 | 19.45 | 0.00 | 0.93 | 0.37 | Hy+Eps+Car+Sy |
| 22,B12① | 0.04 | 8.57 | 24.92 | 0.00 | 0.57 | 0.21 | Hy+Eps+Car+M8 |
| 23,C1 | 0.37 | 7.70 | 17.45 | 11.04 | 0.79 | 1.00 | Hy+Eps+Nit+NN+KN |
| 24,C2 | 0.29 | 7.40 | 19.28 | 4.99 | 0.60 | 0.46 | Hy+Eps+Sy+Car+KN |
| 25,C3 | 0.09 | 8.36 | 19.98 | 8.78 | 0.76 | 0.70 | Hy+Eps+Nit+Car+KN |
| 26,C4 | 0.09 | 8.56 | 19.62 | 9.00 | 0.55 | 0.08 | Hy+Eps+M8+Car+Nit |
| 27,C5 | 0.84 | 5.83 | 15.14 | 8.84 | 0.81 | 1.97 | Hy+Eps+NN+S10+KN |
| 28,C6 | 1.28 | 6.22 | 18.62 | 2.81 | 0.89 | 1.03 | Hy+Eps+Sy+S10+KN |
| 29 | 0.67 | 7.14 | 17.09 | 9.90 | 0.61 | 1.14 | Hy+NN+KN+Nit |
| 30 | 1.07 | 6.37 | 18.75 | 3.27 | 0.93 | 1.15 | Hy+KN+Sy+Car |
| 31 | 0.62 | 6.96 | 19.59 | 4.22 | 0.78 | 1.12 | Hy+KN+Sy+Car |
| 32 | 0.10 | 8.48 | 19.74 | 9.01 | 0.58 | 0.33 | Hy+KN+Car+Nit |
| 33 | 0.10 | 8.39 | 19.66 | 8.68 | 1.21 | 0.61 | Hy+Car+Nit+M8 |
| 34 | 0.09 | 8.35 | 20.12 | 8.83 | 0.69 | 0.80 | Hy+Car+Nit+M8 |
| 35 | 2.09 | 1.63 | 10.38 | 16.52 | 0.33 | 8.71 | Hy+NN+S10+KN |
| 36 | 1.01 | 4.71 | 13.87 | 9.37 | 1.20 | 3.54 | Hy+NN+S10+KN |
| 37 | 0.91 | 5.03 | 14.07 | 8.96 | 1.37 | 3.06 | Hy+NN+Eps+S10 |
| 38 | 0.84 | 5.65 | 15.05 | 8.72 | 0.83 | 2.20 | Hy+NN+Eps+S10 |
| 39 | 1.52 | 5.40 | 17.06 | 2.92 | 1.28 | 1.65 | Hy+Eps+S10+Sy |
| 40 | 0.10 | 8.35 | 19.86 | 8.66 | 0.62 | 0.52 | Hy+NN+Eps+Nit |
| 41 | 0.27 | 7.59 | 20.61 | 3.26 | 0.74 | 0.41 | Hy+Eps+Car+Sy |
| 42 | 0.10 | 7.98 | 21.92 | 3.75 | 0.60 | 0.73 | Hy+Eps+Car+Sy |
| 43 | 0.05 | 8.38 | 21.66 | 4.24 | 0.60 | 0.01 | Hy+Eps+Car+Sy |
| 44 | 0.07 | 8.31 | 20.46 | 6.32 | 0.56 | 0.13 | Hy+Eps+Car+Sy |
| 45 | 0.05 | 8.26 | 21.84 | 3.29 | 0.68 | 0.05 | Hy+Eps+Car+M8 |
| 46 | 0.03 | 8.71 | 23.37 | 3.70 | 0.71 | 0.37 | Hy+Eps+Car+M8 |
| 47 | 0.05 | 8.45 | 21.03 | 6.66 | 0.73 | 0.44 | Hy+Eps+Car+M8 |
| 48 | 0.09 | 8.39 | 19.29 | 8.62 | 0.56 | 0.05 | Hy+Eps+Nit+M8 |
| 49 | 0.09 | 8.49 | 19.51 | 8.60 | 0.65 | 0.03 | Hy+Eps+Nit+M8 |
| 50 | 3.61 | 1.32 | 15.13 | 4.15 | 0.54 | 7.00 | Hy+Sy+KN+S10 |
| 51 | 3.12 | 2.27 | 14.84 | 3.76 | 0.84 | 5.29 | Hy+Sy+KN+S10 |
表1 -15 ℃,在NaCl·2H2O饱和下Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O的溶解度
Table 1 The solubilities of Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O saturated with NaCl·2H2O at -15℃
| No. | Composition of solution, wB/% | Solid phase | |||||
|---|---|---|---|---|---|---|---|
| K+ | Mg2+ | Cl- | NO3- | SO42- | Na+ | ||
| 1,A1 | 0.79 | 6.35 | 20.24 | 0.00 | 0.00 | 0.65 | Hy+Sy+Car |
| 2,A2 | 0.04 | 8.26 | 24.29 | 0.00 | 0.00 | 0.11 | Hy+Car+M8 |
| 3,A3 | 0.00 | 8.08 | 18.66 | 9.44 | 0.00 | 0.32 | Hy+NN+Nit |
| 4,A4 | 0.00 | 8.42 | 20.01 | 8.66 | 0.00 | 0.26 | Hy+M8+Nit |
| 5,A5① | 2.06 | 0.00 | 9.88 | 17.41 | 0.00 | 11.66 | Hy+KN+NN |
| 6,A6① | 4.21 | 0.00 | 14.49 | 4.91 | 0.00 | 8.75 | Hy+KN+Sy |
| 7,A7① | 0.00 | 0.00 | 9.75 | 14.42 | 0.23 | 11.78 | Hy+NN+S10 |
| 8,A8① | 0.00 | 4.45 | 15.45 | 0.00 | 2.52 | 2.81 | Hy+Eps+S10 |
| 9,A9① | 0.00 | 7.94 | 23.12 | 0.00 | 0.48 | 0.19 | Hy+Eps+M8 |
| 10,A10① | 3.30 | 0.00 | 15.60 | 0.00 | 0.34 | 8.34 | Hy+S10+Sy |
| 11,B1 | 0.71 | 6.63 | 16.64 | 9.36 | 0.00 | 1.30 | Hy+NN+KN+Nit |
| 12,B2 | 1.37 | 5.91 | 18.78 | 2.82 | 0.00 | 1.24 | Hy+KN+Sy+Car |
| 13,B3 | 0.42 | 7.72 | 18.03 | 9.67 | 0.00 | 0.43 | Hy+KN+Car+Nit |
| 14,B4 | 0.15 | 8.36 | 20.34 | 8.52 | 0.00 | 0.45 | Hy+Car+Nit+M8 |
| 15,B5 | 0.00 | 4.79 | 14.02 | 8.39 | 1.15 | 3.69 | Hy+Eps+S10+NN |
| 16,B6 | 0.00 | 8.24 | 18.76 | 9.86 | 0.54 | 0.50 | Hy+Eps+Nit+NN |
| 17,B7 | 0.00 | 8.50 | 19.97 | 8.82 | 0.77 | 0.51 | Hy+Eps+M8+Nit |
| 18,B8① | 2.03 | 0.00 | 9.81 | 17.34 | 0.24 | 11.71 | Hy+NN+S10+KN |
| 19,B9① | 4.13 | 0.00 | 14.34 | 4.97 | 0.29 | 8.86 | Hy+S10+Sy+KN |
| 20,B10① | 1.72 | 4.12 | 16.07 | 0.00 | 2.37 | 2.76 | Hy+Eps+S10+Sy |
| 21,B11① | 0.84 | 6.45 | 19.45 | 0.00 | 0.93 | 0.37 | Hy+Eps+Car+Sy |
| 22,B12① | 0.04 | 8.57 | 24.92 | 0.00 | 0.57 | 0.21 | Hy+Eps+Car+M8 |
| 23,C1 | 0.37 | 7.70 | 17.45 | 11.04 | 0.79 | 1.00 | Hy+Eps+Nit+NN+KN |
| 24,C2 | 0.29 | 7.40 | 19.28 | 4.99 | 0.60 | 0.46 | Hy+Eps+Sy+Car+KN |
| 25,C3 | 0.09 | 8.36 | 19.98 | 8.78 | 0.76 | 0.70 | Hy+Eps+Nit+Car+KN |
| 26,C4 | 0.09 | 8.56 | 19.62 | 9.00 | 0.55 | 0.08 | Hy+Eps+M8+Car+Nit |
| 27,C5 | 0.84 | 5.83 | 15.14 | 8.84 | 0.81 | 1.97 | Hy+Eps+NN+S10+KN |
| 28,C6 | 1.28 | 6.22 | 18.62 | 2.81 | 0.89 | 1.03 | Hy+Eps+Sy+S10+KN |
| 29 | 0.67 | 7.14 | 17.09 | 9.90 | 0.61 | 1.14 | Hy+NN+KN+Nit |
| 30 | 1.07 | 6.37 | 18.75 | 3.27 | 0.93 | 1.15 | Hy+KN+Sy+Car |
| 31 | 0.62 | 6.96 | 19.59 | 4.22 | 0.78 | 1.12 | Hy+KN+Sy+Car |
| 32 | 0.10 | 8.48 | 19.74 | 9.01 | 0.58 | 0.33 | Hy+KN+Car+Nit |
| 33 | 0.10 | 8.39 | 19.66 | 8.68 | 1.21 | 0.61 | Hy+Car+Nit+M8 |
| 34 | 0.09 | 8.35 | 20.12 | 8.83 | 0.69 | 0.80 | Hy+Car+Nit+M8 |
| 35 | 2.09 | 1.63 | 10.38 | 16.52 | 0.33 | 8.71 | Hy+NN+S10+KN |
| 36 | 1.01 | 4.71 | 13.87 | 9.37 | 1.20 | 3.54 | Hy+NN+S10+KN |
| 37 | 0.91 | 5.03 | 14.07 | 8.96 | 1.37 | 3.06 | Hy+NN+Eps+S10 |
| 38 | 0.84 | 5.65 | 15.05 | 8.72 | 0.83 | 2.20 | Hy+NN+Eps+S10 |
| 39 | 1.52 | 5.40 | 17.06 | 2.92 | 1.28 | 1.65 | Hy+Eps+S10+Sy |
| 40 | 0.10 | 8.35 | 19.86 | 8.66 | 0.62 | 0.52 | Hy+NN+Eps+Nit |
| 41 | 0.27 | 7.59 | 20.61 | 3.26 | 0.74 | 0.41 | Hy+Eps+Car+Sy |
| 42 | 0.10 | 7.98 | 21.92 | 3.75 | 0.60 | 0.73 | Hy+Eps+Car+Sy |
| 43 | 0.05 | 8.38 | 21.66 | 4.24 | 0.60 | 0.01 | Hy+Eps+Car+Sy |
| 44 | 0.07 | 8.31 | 20.46 | 6.32 | 0.56 | 0.13 | Hy+Eps+Car+Sy |
| 45 | 0.05 | 8.26 | 21.84 | 3.29 | 0.68 | 0.05 | Hy+Eps+Car+M8 |
| 46 | 0.03 | 8.71 | 23.37 | 3.70 | 0.71 | 0.37 | Hy+Eps+Car+M8 |
| 47 | 0.05 | 8.45 | 21.03 | 6.66 | 0.73 | 0.44 | Hy+Eps+Car+M8 |
| 48 | 0.09 | 8.39 | 19.29 | 8.62 | 0.56 | 0.05 | Hy+Eps+Nit+M8 |
| 49 | 0.09 | 8.49 | 19.51 | 8.60 | 0.65 | 0.03 | Hy+Eps+Nit+M8 |
| 50 | 3.61 | 1.32 | 15.13 | 4.15 | 0.54 | 7.00 | Hy+Sy+KN+S10 |
| 51 | 3.12 | 2.27 | 14.84 | 3.76 | 0.84 | 5.29 | Hy+Sy+KN+S10 |
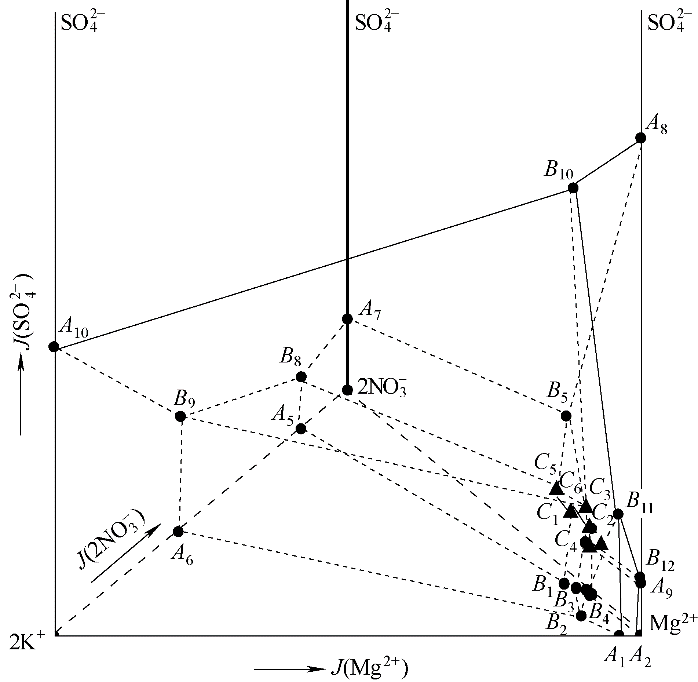
图1 -15℃,NaCl·2H2O饱和下六元体系Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O的相图(仅绘出共饱点)
Fig.1 Phase diagram of the system Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O saturated with NaCl·2H2O at -15℃(only invariant points)
| 卤水样品 | 组分/%(质量) | ||||||
|---|---|---|---|---|---|---|---|
| K+ | Mg2+ | Cl- | NO3- | SO42- | Na+ | H2O | |
| 1# | 0.270 | 0.194 | 14.90 | 0.824 | 1.831 | 10.32 | 71.66 |
| 2# | 0.291 | 0.115 | 14.81 | 1.275 | 1.505 | 10.41 | 71.60 |
表2 卤水组成
Table 2 Brine compositions
| 卤水样品 | 组分/%(质量) | ||||||
|---|---|---|---|---|---|---|---|
| K+ | Mg2+ | Cl- | NO3- | SO42- | Na+ | H2O | |
| 1# | 0.270 | 0.194 | 14.90 | 0.824 | 1.831 | 10.32 | 71.66 |
| 2# | 0.291 | 0.115 | 14.81 | 1.275 | 1.505 | 10.41 | 71.60 |
| 1 | 张明刚. 新疆盐湖卤水水化学特征研究[J]. 盐湖研究, 1993, 1(1): 17-32. |
| Zhang M G. Study on the chemical characteristics of brine in Xinjiang salt lake[J]. Journal of Salt Lake Research, 1993, 1(1): 17-32. | |
| 2 | 郑喜玉. 新疆盐湖的形成演化环境[J]. 盐湖研究, 1993, 1(1): 1-10. |
| Zheng X Y. Formation and evolution environment of salt lake in Xinjiang[J]. Journal of Salt Lake Research, 1993, 1(1): 1-10. | |
| 3 | 黄雪莉. 298.16 K下,Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O体系液固相平衡及应用基础研究[D]. 大连: 大连理工大学, 2007. |
| Huang X L. Study on liquid-solid equilibrium and application of the Na+, K+, Mg2+//Cl-, NO3-, SO42--H2O system at298.16 K[D]. Dalian: Dalian University of Technology, 2007. | |
| 4 | 刘福云, 黄雪莉, 黄文婷, 等. 三元水盐体系冰点和共晶点的测定、相图表达及计算[J]. 化工学报, 2017, 68(9): 3336-3342. |
| Liu F Y, Huang X L, Huang W L, et al. Determination, graphics expression and calculation of freezing point and eutectic point in ternary salt-water system[J]. CIESC Journal, 2017, 68(9): 3336-3342. | |
| 5 | 廖玲, 黄雪莉, 宋欢. 258.15 K下五元体系Na+, K+//Cl-, NO3-, SO42--H2O相平衡研究[J]. 高校化学工程学报,2016, 30(1): 7-12. |
| Liao L, Huang X L, Song H. Phase equilibria of quinary system Na+, K+//Cl-, NO3-, SO42--H2O at 258.15 K[J]. Journal of Chemical Engineering of Chinese Universities, 2016, 30(1): 7-12. | |
| 6 | 朱巧丽, 黄雪莉. -15℃下Na+, K+, Mg2+//Cl-, SO42--H2O体系相平衡[J]. 化工学报, 2015, 66(4): 1252-1257. |
| Zhu Q L, Huang X L. Liquid-solid phase equilibrium of Na+, K+, Mg2+//Cl-, SO42--H2O system at -15℃[J]. CIESC Journal, 2015, 66(4): 1252-1257. | |
| 7 | Shestov A S, Marchenko A V. The consolidation of saline ice blocks in water of varying freezing points: laboratory experiments and computer simulations[J]. Cold Regions Science & Technology, 2015, 122: 71-79. |
| 8 | Karamoddin M, Varaminian F. Water purification by freezing and gas hydrate processes and removal of dissolved minerals (Na+, K+, Mg2+, Ca2+)[J]. Journal of Molecular Liquids, 2016, 223: 1021-1031. |
| 9 | Hasan M, Rotich N, John M, et al. Salt recovery from wastewater by air-cooled eutectic freeze crystallization[J]. Chemical Engineering Journal, 2017, 326: 192-200. |
| 10 | John M, Häkkinen A, Louhi-Kultanen M. Purification efficiency of natural freeze crystallization for urban wastewaters[J]. Cold Regions Science and Technology, 2020, 170: 102953. |
| 11 | Bian S J, Li D D, Gao D D, et al. Hydrometallurgical processing of lithium, potassium, and boron for the comprehensive utilization of da Qaidam lake brine via natural evaporation and freezing[J]. Hydrometallurgy, 2017, 173: 80-83. |
| 12 | 于旭东, 黄琴, 王林, 等. KCl-PEG4000-H2O三元体系288、298、308 K相平衡测定及计算[J]. 化工学报, 2019, 70(3): 830-839. |
| Yu X D, Huang Q, Wang L, et al. Measurements and simulation for ternary system KCl-PEG4000-H2O at 288, 298 and 308 K[J]. CIESC Journal, 2019, 70(3): 830-839. | |
| 13 | Spenser R, Moller N, Weare J. The prediction of mineral solubilities in natural waters: a chemical equilibrium model for the Na-K-Ca-Mg-Cl-SO4-H2O system at temperatures below 25℃[J]. Geochim. Cosmochim. Acta, 1990, 54: 575–590. |
| 14 | Marion G M, Farren R E.Mineral solubilities in the Na-K-Mg-Ca-Cl-SO4-H2O system: a re-evaluation of the sulfate chemistry in the Spencer-Moller-Weare model[J]. Geochimica Et Cosmochimica Acta, 1999, 63(9): 1305-1318. |
| 15 | Fu C, Sang S H, Zhou M F, et al. Phase equilibria in the ternary systems Li2B4O7-MgB4O7-H2O and K2B4O7-MgB4O7-H2O at 273 K[J]. Journal of Chemical & Engineering Data, 2016, 61(3): 1071-1077. |
| 16 | 桑世华, 张婷婷, 傅超, 等. 四元体系Li+, K+, Mg2+//B4O72--H2O 273 K相平衡[J]. 化工学报, 2017, 68(9): 3343-3349. |
| Sang S H, Zhang T T, Fu C, et al. Phase equilibrium of the quaternary system Li+, K+, Mg2+//B4O72--H2O at 273 K[J]. CIESC Journal, 2017, 68(9): 3343-3349. | |
| 17 | Zhao X P, Zhang X P, Yang Y Y, et al. Phase equilibria in the quaternary systems KCl-K2B4O7-K2SO4-H2O and MgCl2-MgB4O7-MgSO4-H2O at 273 K[J]. Journal of Chemical & Engineering Data, 2017, 62(4): 1377-1383. |
| 18 | 曾英, 林晓峰, 郑志远. Li+, K+//SO42-, B4O72--H2O交互四元体系273 K介稳相平衡研究[J]. 高校化学工程学报, 2009, 23(1): 7-11. |
| Zeng Y, Lin X F, Zheng Z Y. Study on metastable phase equilibrium of the reciprocal quaternary system Li+, K+//SO42-, B4O72--H2O at 273 K[J]. J. Chem. Eng. Chin. Univ., 2009, 23(1): 7-11. | |
| 19 | Li D, Meng L Z, Deng T L, et al. Thermodynamic study of solid–liquid equilibrium in NaCl-NaBr-H2O system at 288.15 K[J]. Russian Journal of Physical Chemistry A, 2018, 92(6): 1213-1218. |
| 20 | Dou S Y, Zhao B, Li L, et al. Phase diagram of Mg2+, NH4+//Cl-, SO42--H2O system at 0℃ and their application[J]. Fluid Phase Equilibria, 2016, 409: 264-270. |
| 21 | Zhang J, Guo H F, Cao J L, et al. Phase equilibrium of the quaternary system Na2SO4-MgSO4-(NH4)2SO4-H2O at 0℃[J]. Journal of Chemical & Engineering Data, 2013, 58(9): 2622-2628. |
| 22 | 牛自得, 程芳琴. 水盐体系相图及其应用[M]. 天津: 天津大学出版社, 2002. |
| Niu Z D, Cheng F Q. Phase Diagram of Salt-water System and Its Applications[M]. Tianjin: Tianjin University Press, 2002. | |
| 23 | 中国科学院青海盐湖所. 卤水和盐的分析方法[M]. 北京: 科学与技术出版社, 1984. |
| Institute of Qinghai Salt-Lake of Chinese Academy of Sciences. Analytical Methods of Brines and Salts[M]. Beijing: Chinese Science Press, 1984. | |
| 24 | 王明. 分析化学手册[M]. 3版. 北京: 化学工业出版社, 2016. |
| Wang M. Handbook of Analytical Chemistry[M]. 3rd ed. Beijing: Chemical Industry Press, 2016. | |
| 25 | Howards S, Slicok H L. Solubililies of Inorganic and Organic CompoundsM]. 3rd ed. New York: Pergamon Press, 1979. |
| 26 | Drebushchak V A, Ogienko A G, Yunoshev A S. Metastable eutectic melting in the NaCl-H2O system[J]. Thermochim. Acta, 2017, 647: 94-100. |
| 27 | Archer D G. Thermodynamic properties of the NaCl+H2O system(Ⅱ): Thermodynamic properties of NaCl(aq), NaCl·2H2O(cr) and phase equilibria[J]. Journal of Physical and Chemical Reference Data, 1992, 21(4): 793-829. |
| 28 | Wang X Y, Wang X F, Luo Q L, et al. Phase equilibria in the aqueous quinary system Na+, Mg2+//Cl-, NO3-, SO42--H2O and its subsystem at 258 K[J]. Journal of Chemical & Engineering Data, 2020, 65(1): 264-273. |
| 29 | Christov C. Chemical equilibrium model of solution behavior and bishofite (MgCl2·6H2O(cr)) and hydrogen-carnallite (HCl·MgCl2·7H2O(cr)) solubility in the MgCl2+H2O and HCl·MgCl2+H2O systems to high acid concentration at (0 to 100)℃[J]. Journal of Chemical & Engineering Data, 2009, 54(9): 2599-2608. |
| 30 | Pillay V, Gärtner R S, Himawan C, et al. MgSO4+H2O system at eutectic conditions and thermodynamic solubility products of MgSO4·12H2O(s) and MgSO4·7H2O(s)[J]. Journal of Chemical & Engineering Data, 2009, 50(2): 551-555. |
| 31 | Steiger M, Linnow K, Ehrhardt D, et al. Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl--SO42--H2O systems with implications for Mars[J]. Geochimica Et Cosmochimica Acta, 2011, 75(12): 3600-3626. |
| 32 | Li D D, Zeng D W, Yin X, et al. Phase diagrams and thermochemical modeling of salt lake brine systems(Ⅲ): Li2SO4+H2O, Na2SO4+H2O, K2SO4+H2O, MgSO4+H2O and CaSO4+H2O systems[J]. Calphad, 2018, 60: 163-176. |
| [1] | 常明慧, 王林, 苑佳佳, 曹艺飞. 盐溶液蓄能型热泵循环特性研究[J]. 化工学报, 2023, 74(S1): 329-337. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 程小松, 殷勇高, 车春文. 不同工质在溶液除湿真空再生系统中的性能对比[J]. 化工学报, 2023, 74(8): 3494-3501. |
| [5] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [6] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [7] | 周璇, 李孟亚, 孙杰, 岑振凯, 吕强三, 周立山, 王海涛, 韩丹丹, 龚俊波. 添加剂对氨基酸晶体生长的影响[J]. 化工学报, 2023, 74(2): 500-510. |
| [8] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [9] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [10] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [11] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [12] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [13] | 方辉煌, 程金星, 罗宇, 陈崇启, 周晨, 江莉龙. 氨电氧化催化剂及其低温直接氨碱性膜燃料电池性能的研究进展[J]. 化工学报, 2022, 73(9): 3802-3814. |
| [14] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [15] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号