化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4861-4871.DOI: 10.11949/0438-1157.20210283
王立晖1( ),刘焕1,李赫宇2,郑晓冰1,3(
),刘焕1,李赫宇2,郑晓冰1,3( ),姜艳军1,3,高静1
),姜艳军1,3,高静1
收稿日期:2021-02-23
修回日期:2021-05-05
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
郑晓冰
作者简介:王立晖(1981—),男,博士研究生,基金资助:
Lihui WANG1( ),Huan LIU1,Heyu LI2,Xiaobing ZHENG1,3(
),Huan LIU1,Heyu LI2,Xiaobing ZHENG1,3( ),Yanjun JIANG1,3,Jing GAO1
),Yanjun JIANG1,3,Jing GAO1
Received:2021-02-23
Revised:2021-05-05
Online:2021-09-05
Published:2021-09-05
Contact:
Xiaobing ZHENG
摘要:
为了提升脂肪酶的稳定性并构建新型固定化酶催化体系,利用改进的Winsor Ⅲ微乳液双连续相体系合成了超顺磁性Fe3O4内核和树枝状纤维形氧化硅外壳的核壳结构磁性有机硅纳米粒子(MMOSNs),用于固定化南极假丝酵母脂肪酶B(CALB)。优化条件后CALB负载量为177.49 mg/g,比水解活性为27390 U/g。磁性有机硅通过与CLAB分子之间疏水相互作用及表面孔道结构,可有效激活CALB的界面活性并保护活性构象免受破坏,比游离酶和磁性无机硅固定化酶表现出更好的活性和稳定性。除此之外,将CALB@MMOSNs用于催化乙酰丙酸与十二醇的酯化反应最高转化率为85.05%,重复使用9次后仍保留68.94%转化率,而商业化N435只保留29.83%。证明疏水性磁性核壳结构有机硅是固定化CALB的良好载体,可有效扩展脂肪酶的工业应用。
中图分类号:
王立晖, 刘焕, 李赫宇, 郑晓冰, 姜艳军, 高静. 核壳结构磁性树枝状纤维形有机硅固定化脂肪酶制备及其应用[J]. 化工学报, 2021, 72(9): 4861-4871.
Lihui WANG, Huan LIU, Heyu LI, Xiaobing ZHENG, Yanjun JIANG, Jing GAO. Preparation and application of core-shell hydrophobic magnetic dendritic fibrous organosilica immobilized lipase[J]. CIESC Journal, 2021, 72(9): 4861-4871.
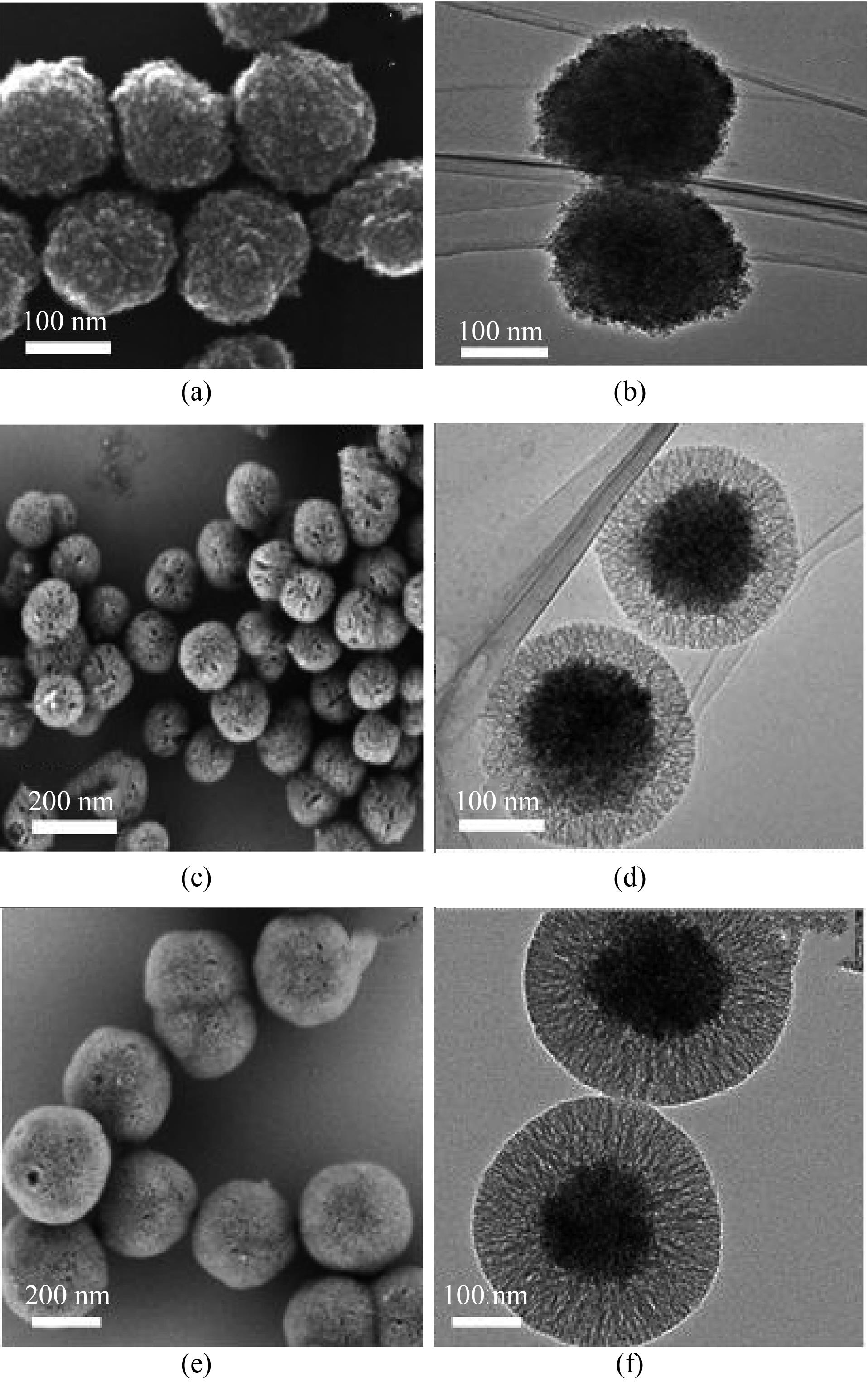
图2 Fe3O4 (a)、MMOSNs (c)和MMSNs (e)的SEM图片;Fe3O4(b)、MMOSNs (d)和MMSNs (f)的TEM图片
Fig.2 SEM images of Fe3O4 (a), MMOSNs (c) and MMSNs (e); TEM images of Fe3O4 (b), MMOSNs (d) and MMSNs (f)

图4 MMOSNs、CALB@MMOSNs的N2吸附-脱附曲线(a)和孔径分布(b)
Fig.4 Nitrogen adsorption-desorption isotherms (a) and pore size distribution profile (b) of the MMOSNs and CALB@MMOSNs

图7 MMOSNs在不同酶浓度下的吸附进程曲线(a);初始酶浓度对载体的蛋白负载量及CALB@MMOSNs酶活的影响(b)
Fig.7 Adsorption process of CALB at different concentrations on the MMOSNs (a) and effect of initial lipase concentration on CALB loading and specific activity of CALB@MMOSNs(b)
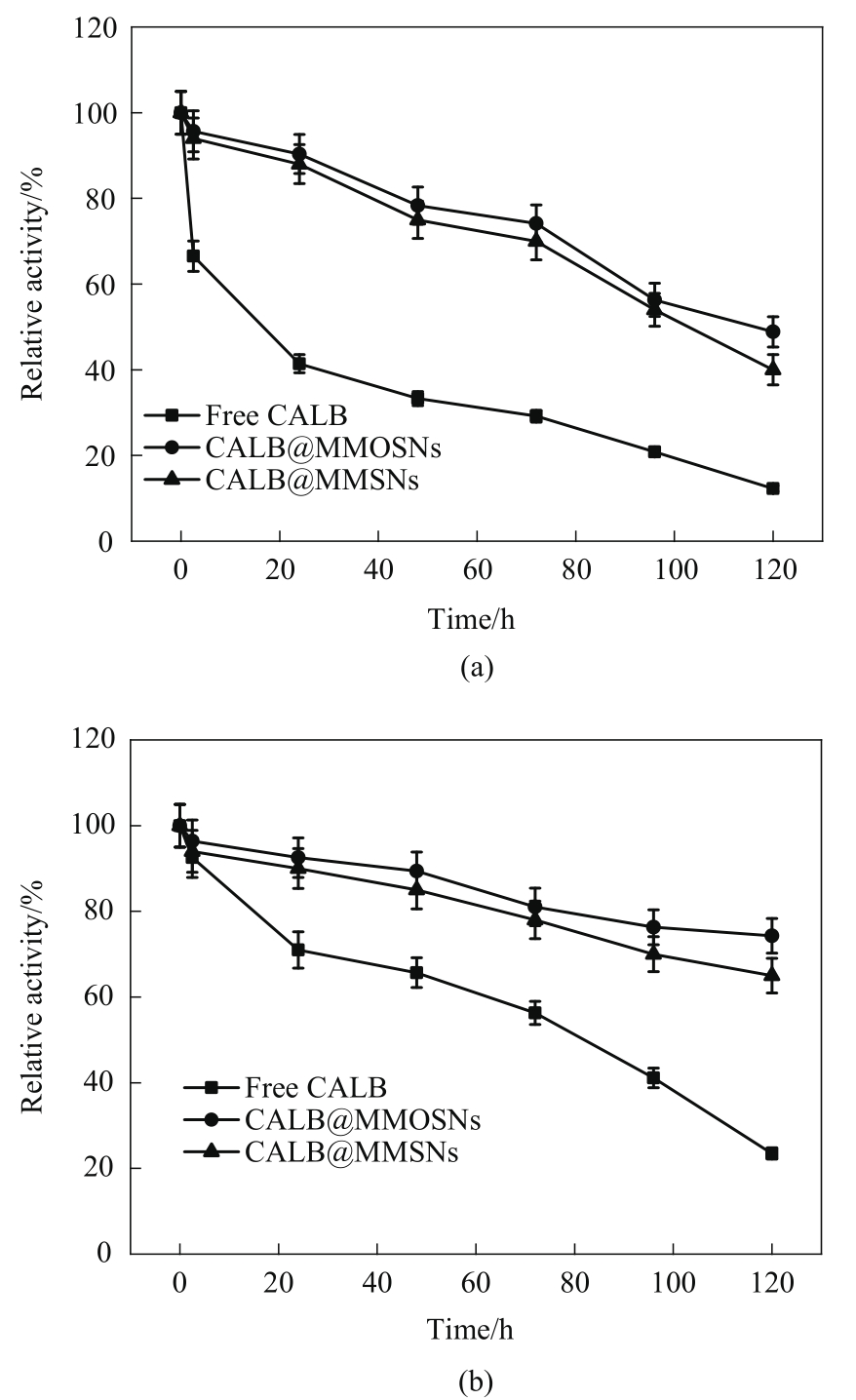
图8 游离酶、CALB@MMSNs和CALB@MMOSNs在pH=4.0 (a)和pH=10.0 (b)的缓冲溶液中的稳定性
Fig.8 The stabilities of free lipase, CALB@MMSNs and CALB@MMOSNs at pH=4.0 (a) and pH=10.0 (b)

图12 温度(a)、LA/十二醇摩尔比(b)和时间(c)对LA和十二醇酯化反应的影响
Fig.12 Effect of temperature (a), LA/n-lauryl alcohol molar ratio (b) and time (c) on esterification reaction
| 1 | Shi J, Wu Y, Zhang S, et al. Bioinspired construction of multi-enzyme catalytic systems[J]. Chemical Society Reviews, 2018, 47(12): 4295-4313. |
| 2 | Tamura T, Hamachi I. Chemistry for covalent modification of endogenous/native proteins: from test tubes to complex biological systems[J]. Journal of the American Chemical Society, 2019, 141(7): 2782-2799. |
| 3 | Liu Z H, Wang K, Chen Y, et al. Third-generation biorefineries as the means to produce fuels and chemicals from CO2[J]. Nature Catalysis, 2020, 3(3): 274-288. |
| 4 | Benjamin S, Pandey A. Candida rugosa lipases: molecular biology and versatility in biotechnology[J]. Yeast, 1998, 14(12): 1069-1087. |
| 5 | Jakovetić Tanasković S, Jokić B, Grbavčić S, et al. Immobilization of Candida antarctica lipase B on Kaolin and its application in synthesis of lipophilic antioxidants[J]. Applied Clay Science, 2017, 135: 103-111. |
| 6 | Adlercreutz P. Immobilisation and application of lipases in organic media[J]. Chemical Society Reviews, 2013, 42(15): 6406-6436. |
| 7 | Ansorge-Schumacher M B, Thum O. Immobilised lipases in the cosmetics industry[J]. Chemical Society Reviews, 2013, 42(15): 6475-6490. |
| 8 | Kuwahara Y, Yamanishi T, Kamegawa T, et al. Activity, recyclability, and stability of lipases immobilized on oil-filled spherical silica nanoparticles with different silica shell structures[J]. ChemCatChem, 2013, 5(8): 2527-2536. |
| 9 | Rodrigues R C, Ortiz C, Berenguer-Murcia Á, et al. Modifying enzyme activity and selectivity by immobilization[J]. Chemical Society Reviews, 2013, 42(15): 6290-6307. |
| 10 | Rueda N, dos Santos J C S, Ortiz C, et al. Chemical modification in the design of immobilized enzyme biocatalysts: drawbacks and opportunities[J]. The Chemical Record, 2016, 16(3): 1436-1455. |
| 11 | Kalantari M, Kazemeini M, Tabandeh F, et al. Lipase immobilisation on magnetic silica nanocomposite particles: effects of the silica structure on properties of the immobilised enzyme[J]. Journal of Materials Chemistry, 2012, 22(17): 8385-8393. |
| 12 | Yue Q, Sun J G, Kang Y J, et al. Advances in the interfacial assembly of mesoporous silica on magnetite particles[J]. Angewandte Chemie, 2020, 132(37): 15936-15949. |
| 13 | Lei Z L, Ren N, Li Y L, et al. Fe3O4/SiO2-g-PSStNa polymer nanocomposite microspheres (PNCMs) from a surface-initiated atom transfer radical polymerization (SI-ATRP) approach for pectinase immobilization[J]. Journal of Agricultural and Food Chemistry, 2009, 57(4): 1544-1549. |
| 14 | Hou C, Wang Y, Zhu H, et al. Formulation of robust organic-inorganic hybrid magnetic microcapsules through hard-template mediated method for efficient enzyme immobilization[J]. Journal of Materials Chemistry B, 2015, 3(14): 2883-2891. |
| 15 | Chen Z, Xu W, Jin L, et al. Synthesis of amine functionalized Fe3O4@C nanoparticles for lipase immobilization[J]. Journal of Materials Chemistry A, 2014, 2: 18339-18344. |
| 16 | Zhao M, Zhang X, Deng C. Rational synthesis of novel recyclable Fe₃O₄@MOF nanocomposites for enzymatic digestion[J]. Chemical Communications (Cambridge, England), 2015, 51(38): 8116-8119. |
| 17 | Polshettiwar V, Cha D, Zhang X X, et al. High-surface-area silica nanospheres (KCC-1) with a fibrous morphology[J]. Angewandte Chemie, 2010, 122(50): 9846-9850. |
| 18 | Moon D S, Lee J K. Tunable synthesis of hierarchical mesoporous silica nanoparticles with radial wrinkle structure[J]. Langmuir, 2012, 28(33): 12341-12347. |
| 19 | Moon D S, Lee J K. Formation of wrinkled silica mesostructures based on the phase behavior of pseudoternary systems[J]. Langmuir, 2014, 30(51): 15574-15580. |
| 20 | Schmid R D, Verger R. Lipases: interfacial enzymes with attractive applications[J]. Angewandte Chemie International Edition, 1998, 37(12): 1608-1633. |
| 21 | Verger R. 'Interfacial activation' of lipases: facts and artifacts[J]. Trends in Biotechnology, 1997, 15(1): 32-38. |
| 22 | Brzozowski A M, Derewenda U, Derewenda Z S, et al. A model for interfacial activation in lipases from the structure of a fungal lipase-inhibitor complex[J]. Nature, 1991, 351(6326): 491-494. |
| 23 | Rodrigues R C, Virgen-Ortíz J J, dos Santos J C S, et al. Immobilization of lipases on hydrophobic supports: immobilization mechanism, advantages, problems, and solutions[J]. Biotechnology Advances, 2019, 37(5): 746-770. |
| 24 | Gao J, Kong W X, Zhou L Y, et al. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization[J]. Chemical Engineering Journal, 2017, 309: 70-79. |
| 25 | Kalantari M, Yu M, Yang Y, et al. Tailoring mesoporous-silica nanoparticles for robust immobilization of lipase and biocatalysis[J]. Nano Research, 2017, 10(2): 605-617. |
| 26 | Bilal M, Zhao Y P, Rasheed T, et al. Magnetic nanoparticles as versatile carriers for enzymes immobilization: a review[J]. International Journal of Biological Macromolecules, 2018, 120: 2530-2544. |
| 27 | Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J]. Analytical Biochemistry, 1976, 72(1/2): 248-254. |
| 28 | Bouhrara M, Ranga C, Fihri A, et al. Nitridated fibrous silica (KCC-1) as a sustainable solid base nanocatalyst[J]. ACS Sustainable Chemistry & Engineering, 2013, 1(9): 1192-1199. |
| 29 | Zhou L Y, He Y, Ma L, et al. Conversion of levulinic acid into alkyl levulinates: using lipase immobilized on meso-molding three-dimensional macroporous organosilica as catalyst[J]. Bioresource Technology, 2018, 247: 568-575. |
| 30 | Jiang Y J, Liu H, Wang L H, et al. Virus-like organosilica nanoparticles for lipase immobilization: characterization and biocatalytic applications[J]. Biochemical Engineering Journal, 2019, 144: 125-134. |
| 31 | Linsha V, Aboo Shuhailath K, MA·hesh K V, et al. Biocatalytic conversion efficiency of steapsin lipase immobilized on hierarchically porous biomorphic aerogel supports[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(9): 4692-4703. |
| 32 | Ali Z, Tian L, Zhang B, et al. Synthesis of paramagnetic dendritic silica nanomaterials with fibrous pore structure (Fe3O4@ KCC-1) and their application in immobilization of lipase from Candida rugosa with enhanced catalytic activity and stability[J]. New Journal of Chemistry, 2017, 41(16): 8222-8231. |
| 33 | Shao B B, Liu Z F, Zeng G M, et al. Immobilization of laccase on hollow mesoporous carbon nanospheres: noteworthy immobilization, excellent stability and efficacious for antibiotic contaminants removal[J]. Journal of Hazardous Materials, 2019, 362: 318-326. |
| 34 | Tong S M, Zhu L L, Wang X N, et al. Optimization of cephalosporin C acylase immobilization[J]. E3S Web of Conferences, 2019, 78: 02003. |
| 35 | Al-Lolage F, Bartlett P N, Gounel S, et al. Site-directed immobilization of bilirubin oxidase for electrocatalytic oxygen reduction[J]. ACS Catalysis, 2019, 9(3): 2068-2078. |
| 36 | Liu J Y, Liu Y, Jin D X, et al. Immobilization of trypsin onto large-pore mesoporous silica and optimization enzyme activity via response surface methodology[J]. Solid State Sciences, 2019, 89: 15-24. |
| 37 | Shikha S, Thakur K G, Bhattacharyya M S. Facile fabrication of lipase to amine functionalized gold nanoparticles to enhance stability and activity[J]. RSC Advances, 2017, 7(68): 42845-42855. |
| 38 | Sharma S, Kanwar S S. Organic solvent tolerant lipases and applications[J]. The Scientific World Journal, 2014, 2014: 625258. |
| 39 | Jiang Y J, Sun W Y, Zhou L Y, et al. Improved performance of lipase immobilized on tannic acid-templated mesoporous silica nanoparticles[J]. Applied Biochemistry and Biotechnology, 2016, 179(7): 1155-1169. |
| 40 | Gao J, Wang Y, Du Y J, et al. Construction of biocatalytic colloidosome using lipase-containing dendritic mesoporous silica nanospheres for enhanced enzyme catalysis[J]. Chemical Engineering Journal, 2017, 317: 175-186. |
| 41 | Jeong H, Jang S K, Hong C Y, et al. Levulinic acid production by two-step acid-catalyzed treatment of Quercus mongolica using dilute sulfuric acid[J]. Bioresource Technology, 2017, 225: 183-190. |
| 42 | Xie W L, Zang X Z. Covalent immobilization of lipase onto aminopropyl-functionalized hydroxyapatite-encapsulated-γ-Fe2O3 nanoparticles: a magnetic biocatalyst for interesterification of soybean oil[J]. Food Chemistry, 2017, 227: 397-403. |
| 43 | Poppe J K, Garcia-Galan C, Matte C R, et al. Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized on hydrophobic supports[J]. Journal of Molecular Catalysis B: Enzymatic, 2013, 94: 51-56. |
| 44 | Lee A, Chaibakhsh N, Rahman M B A, et al. Optimized enzymatic synthesis of levulinate ester in solvent-free system[J]. Industrial Crops and Products, 2010, 32(3): 246-251. |
| [1] | 何瑞宁, 邹昀, 石萌, 李洋, 徐晶, 童张法. 固载离子液体[HSO3-BMIM][HSO4]/SiO2的制备及其催化乙酸乙醇酯化反应的研究[J]. 化工学报, 2022, 73(9): 3880-3894. |
| [2] | 王吴玉, 史玉竹, 严龙, 张兴华, 马隆龙, 张琦. 负载型Co基双功能催化剂上戊酸酯生物燃料的制备[J]. 化工学报, 2022, 73(2): 689-698. |
| [3] | 张因, 郭健健, 任欢杰, 程娟, 李海涛, 武建兵, 赵永祥. 插层阴离子对以类水滑石为前体Ni-Al2O3催化剂催化乙酰丙酸加氢性能的影响[J]. 化工学报, 2020, 71(8): 3614-3624. |
| [4] | 徐浩,李洋,夏成康,何瑞宁,邹昀,童张法. 吡啶硫酸氢盐离子液体催化甘油与乙酸酯化反应动力学[J]. 化工学报, 2020, 71(11): 5178-5187. |
| [5] | 王杰, 张因, 郭健健, 赵丽丽, 赵永祥. Ni/ZrO2-SiO2催化剂催化乙酰丙酸加氢合成γ-戊内酯[J]. 化工学报, 2018, 69(8): 3452-3459. |
| [6] | 吕喜蕾, 阮厚航, 陈皓, 吕秀阳. 近临界乙醇中Zr-SBA-15催化糠醛一步法制备乙酰丙酸乙酯[J]. 化工学报, 2018, 69(6): 2488-2495. |
| [7] | 岳东敏, 张欠之, 孙德, 李冰冰, 毛钦烨, 彭从康. PVA/SO42--AAO催化-渗透汽化双功能复合膜合成乙酸乙酯[J]. 化工学报, 2018, 69(6): 2775-2781. |
| [8] | 常春, 白净, 安冉, 邓琳, 戚小各, 徐艳丽. 硫酸铁催化生物基糠醇制取乙酰丙酸丁酯[J]. 化工学报, 2017, 68(6): 2368-2375. |
| [9] | 胡晶晶, 赵地顺, 胡甜甜, 李静静, 翟建华. SBA-15固载酸性离子液体催化酯化反应性能[J]. 化工学报, 2016, 67(5): 1907-1914. |
| [10] | 安冉, 孔鹏飞, 徐桂转, 常春, 白净, 方书起. 脱铝超稳Y沸石负载Cu催化纤维素醇解合成乙酰丙酸乙酯[J]. 化工学报, 2016, 67(11): 4643-4651. |
| [11] | 常翠荣, 王华, 韩金玉. 固体酸表面B酸和L酸与果糖转化制乳酸甲酯产物分布[J]. 化工学报, 2015, 66(9): 3428-3436. |
| [12] | 张阳阳, 罗璇, 庄绪丽, 仝新利. 混合酸催化葡萄糖选择性转化合成乙酰丙酸甲酯[J]. 化工学报, 2015, 66(9): 3490-3495. |
| [13] | 沈忠权, 余锡孟, 陈纪忠. 新型磺化竹炭材料催化酯化反应[J]. 化工学报, 2015, 66(8): 3072-3077. |
| [14] | 姜楠1,谢楠1,齐崴1,2,3,苏荣欣1,2,3,何志敏1,2,3. 硫酸催化葡萄糖制备乙酰丙酸的过程强化[J]. 化工进展, 2014, 33(11): 2888-2893. |
| [15] | 高学艺,武彦伟,王克冰. 沙柳酸催化水解制备乙酰丙酸及分离提纯[J]. 化工进展, 2014, 33(01): 242-246. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号