• •
赵明月1( ), 孟凡宇1, 闫昊1(
), 孟凡宇1, 闫昊1( ), 冯翔1(
), 冯翔1( ), 刘熠斌1, 陈小博1, 杨朝合1, 陈德2
), 刘熠斌1, 陈小博1, 杨朝合1, 陈德2
收稿日期:2025-09-09
修回日期:2025-10-29
出版日期:2025-10-29
通讯作者:
闫昊,冯翔
作者简介:赵明月(1996—),女,博士研究生,2965155534@qq.com
基金资助:
Mingyue ZHAO1( ), Fanyu MENG1, Hao YAN1(
), Fanyu MENG1, Hao YAN1( ), Xiang FENG1(
), Xiang FENG1( ), Yibin LIU1, Xiaobo CHEN1, Chaohe YANG1, DE Chen2
), Yibin LIU1, Xiaobo CHEN1, Chaohe YANG1, DE Chen2
Received:2025-09-09
Revised:2025-10-29
Online:2025-10-29
Contact:
Hao YAN, Xiang FENG
摘要:
低碳多元醇(来源于石油、煤或生物质)的选择性氧化是制备乙醇酸、甘油酸等高附加值化学品的关键途径。开发以分子氧为氧化剂的高效绿色催化工艺,对实现多元醇高值化转化及促进高端化工产业链绿色升级具有重要意义,其核心在于开发高性能负载型催化剂。本文系统综述了以电子效应精准调控为核心的纳米金催化剂在伯羟基氧化制羧酸、仲羟基氧化制酮及C-C键断裂制短链酸三大路径的研究进展,重点分析载体工程与双金属协同策略如何通过调变金的电子结构解决羟基选择性活化、过度氧化抑制等关键问题,探讨了电子结构与催化性能之间构效关系及反应机理,并对未来催化剂设计方向与潜在应用前景进行了展望,为绿色催化工艺的开发提供理论依据与技术参考。
中图分类号:
赵明月, 孟凡宇, 闫昊, 冯翔, 刘熠斌, 陈小博, 杨朝合, 陈德. 电子效应精准调控的纳米金催化剂在低碳多元醇选择性氧化研究进展[J]. 化工学报, DOI: 10.11949/0438-1157.20251015.
Mingyue ZHAO, Fanyu MENG, Hao YAN, Xiang FENG, Yibin LIU, Xiaobo CHEN, Chaohe YANG, DE Chen. Progress in Research of Selective Oxidation of Low Carbon Polyols over Nano-Au Catalysts with Precise Electronic Effect Regulation[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251015.
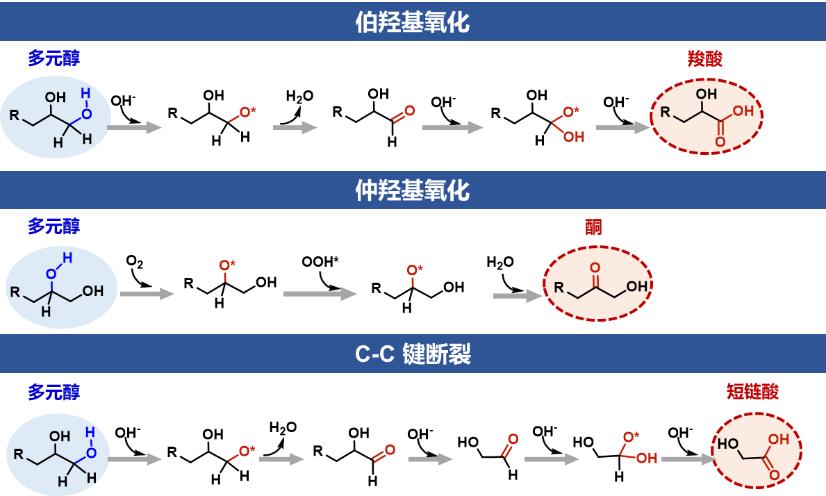
图2 多元醇伯羟基氧化、仲羟基氧化和C-C键断裂氧化的反应机理示意图[47].
Fig. 2 Proposed reaction mechanism of primary, secondary hydroxyl and C-C bond cleavage oxidation of polyols[47].
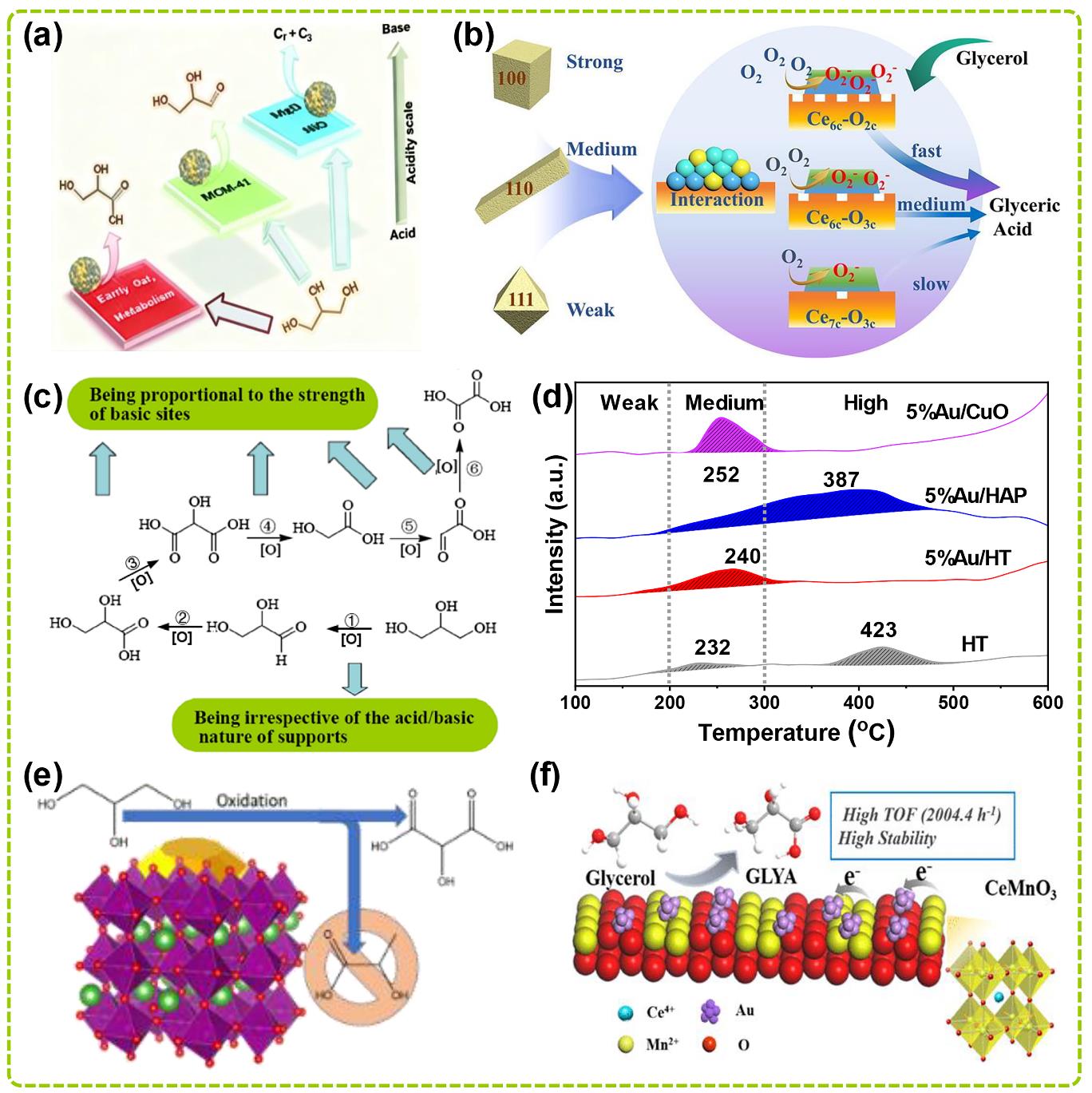
图3 多元醇伯羟基选择性氧化制羧酸相关催化剂载体工程研究:(a) 不同酸碱性质载体负载的AuPt催化剂调控甘油氧化产物[55];(b) Au1Pt3/CeO2催化剂上甘油氧化的可能反应原理图[56];(c) 载体的酸/碱性质与反应速率的关系[57];(d) 5Au/HT、5Au/HAP、5Au/CuO和水滑石(HT)催化剂的CO2程序升温脱附(CO2-TPD)谱图[58];(e) LaMnO3钙钛矿负载的Au催化剂在甘油氧化制丙醇二酸中的特殊选择性[59];(f) CeMnO3钙钛矿及Au/CeMnO3催化剂制备过程示意图及催化甘油氧化为甘油酸性能[60].
Fig. 3 Researches on catalyst support engineering related to selective oxidation of primary hydroxyl groups of polyols to carboxylic acids: (a) Regulation of glycerol oxidation Products by AuPt catalysts loaded on supports with different acid-base properties[55]; (b) Possible reaction mechanism of glycerol oxidation on Au1Pt3/CeO2 catalysts[56]; (c) Relationship between acid/base properties of supports and reaction rates[57]; (d) CO2 temperature programmed desorption (CO2-TPD) spectra of 5Au/HT, 5Au/HAP, 5Au/CuO, and Hydrotalcite (HT) catalysts[58]; (e) Unique selectivity of Au catalysts supported on LaMnO3 perovskite for glycerol oxidation to tartronic acid[59]; (f) Schematic diagram of CeMnO3 perovskite and Au/CeMnO3 catalyst preparation process and performance in catalytic oxidation of glycerol to glyceric acid[60].
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF/ h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 5Au/HT | 60 | 4 | 10 | 0.1/甘油 | 0.2 | 89.5 | 61.2/甘油酸 | - | [ |
| AuPt/H-SiO2 | 80 | 4 | 3 | 0.3/甘油 | 0 | 30.0 | 61.0/甘油酸 | - | [ |
| Au-Pt/HT | 60 | 4 | 2 | 0.3/甘油 | 0 | 64.0 | 69.0/甘油酸 | - | [ |
| Au-Pt/MgO | 60 | 4 | 2 | 0.3/甘油 | 0 | 52.0 | 48.0/甘油酸 | - | [ |
| Au1Pt3/NC | 80 | 2 | 1 | 0.3/甘油 | 0 | 83.7 | 60.5/甘油酸 | 0.26 | [ |
| Au-Pt/Hβ | 100 | 3 | 3 | 0.3/甘油 | 0 | 68.0 | 68.0/甘油酸 | - | [ |
| Au-Pt/N-TiO2 | 100 | 6 | 3 | 0.3/甘油 | 0 | 92.1 | 79.9/甘油酸 | - | [ |
| Au/LaMnO3 | 100 | 24 | 3 | 0.3/甘油 | 1.2 | 92.0 | 87.0/丙醇二酸 | 390 | [ |
| Au/CeMnO3 | 90 | 10 | 10 | 0.4/甘油 | 1.0 | 71.0 | 77.0/甘油酸 | 2004 | [ |
| Au/Mg(OH)2 | 60 | 6 | 3 | 0.68/1,2-丙二醇 | 1.36 | 94.4 | 89.4/乳酸 | - | [ |
表1 多元醇伯羟基选择性氧化中不同载体类型的催化性能总结
Table 1 Summary of catalytic performance of different support types for the selective oxidation of primary hydroxyl groups in polyols
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF/ h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 5Au/HT | 60 | 4 | 10 | 0.1/甘油 | 0.2 | 89.5 | 61.2/甘油酸 | - | [ |
| AuPt/H-SiO2 | 80 | 4 | 3 | 0.3/甘油 | 0 | 30.0 | 61.0/甘油酸 | - | [ |
| Au-Pt/HT | 60 | 4 | 2 | 0.3/甘油 | 0 | 64.0 | 69.0/甘油酸 | - | [ |
| Au-Pt/MgO | 60 | 4 | 2 | 0.3/甘油 | 0 | 52.0 | 48.0/甘油酸 | - | [ |
| Au1Pt3/NC | 80 | 2 | 1 | 0.3/甘油 | 0 | 83.7 | 60.5/甘油酸 | 0.26 | [ |
| Au-Pt/Hβ | 100 | 3 | 3 | 0.3/甘油 | 0 | 68.0 | 68.0/甘油酸 | - | [ |
| Au-Pt/N-TiO2 | 100 | 6 | 3 | 0.3/甘油 | 0 | 92.1 | 79.9/甘油酸 | - | [ |
| Au/LaMnO3 | 100 | 24 | 3 | 0.3/甘油 | 1.2 | 92.0 | 87.0/丙醇二酸 | 390 | [ |
| Au/CeMnO3 | 90 | 10 | 10 | 0.4/甘油 | 1.0 | 71.0 | 77.0/甘油酸 | 2004 | [ |
| Au/Mg(OH)2 | 60 | 6 | 3 | 0.68/1,2-丙二醇 | 1.36 | 94.4 | 89.4/乳酸 | - | [ |
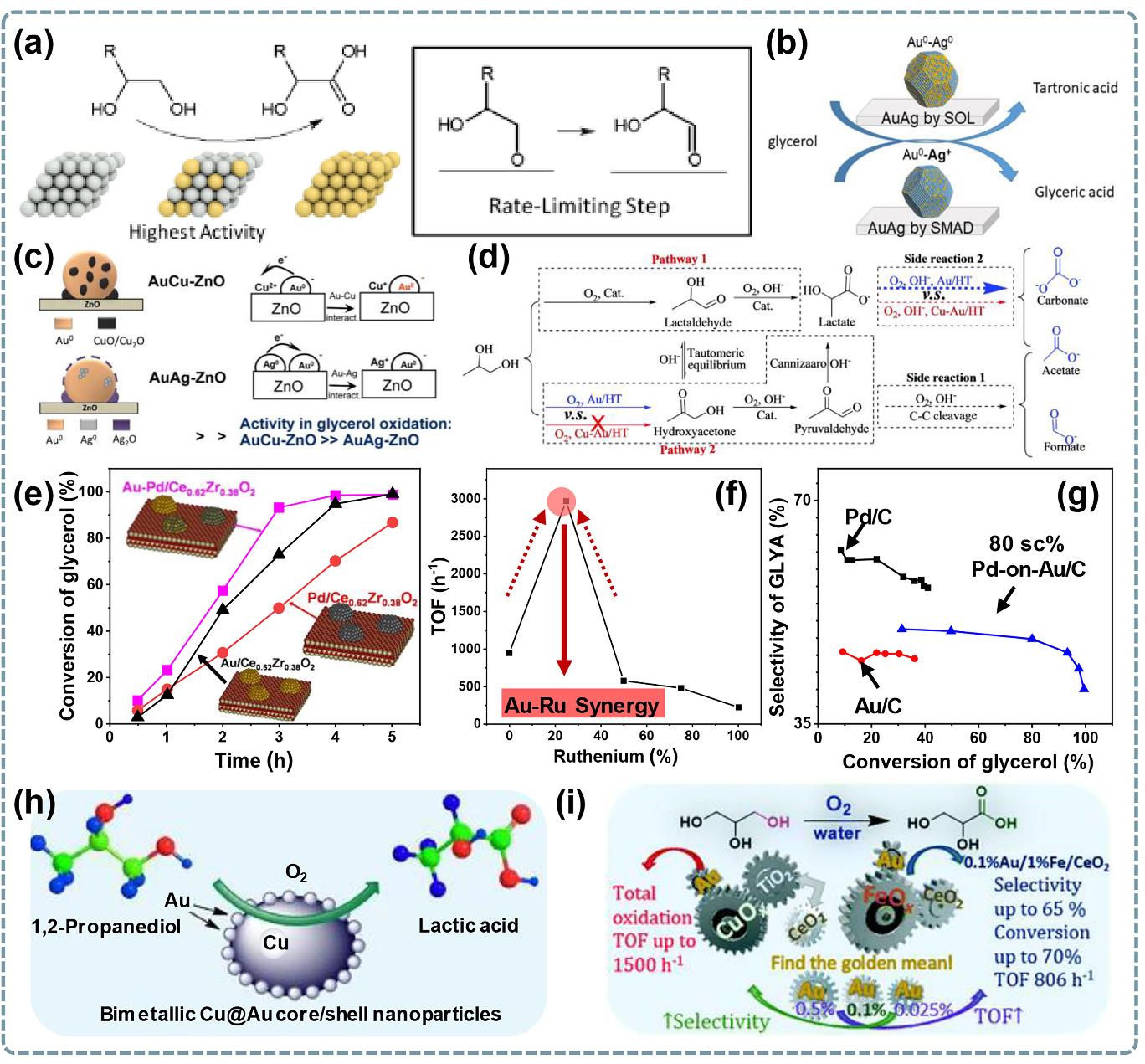
图4 多元醇伯羟基选择性氧化相关催化剂双金属协同研究:(a) Au-Pd/C催化剂上乙二醇氧化反应原理图及速控步骤[64];(b)溶胶固定(Sol)和溶剂化金属原子沉积(SMAD)制备AuAg/Al2O3催化剂产物分布图[29];(c) AuAg-ZnO和AuCu-ZnO催化剂性能对比[66];(d) Au-Cu/HT和Au/HT催化1,2-丙二醇氧化路径[70];(e) 2.2AuPd/CeZrOx与单金属性能对比图[65];(f) Au1Ru0.5/CeZrOx催化剂的Au/Ru比例与活性关系图[67];(g) Pd-on-Au/C与单金属性能对比图[69];(h) Cu@Au核/壳纳米颗粒上1,2-丙二醇转化为乳酸[68];(i) Au/Fe-CeO2与Au/Cu-CeO2性能对比图[28].
Fig. 4 Researches on bimetallic synergy in catalysts related to selective oxidation of primary hydroxyl groups of polyols: (a) Schematic diagram of glycol oxidation reaction mechanism and rate determination step on Au-Pd/C catalyst[64]; (b) Oxidation product distribution over AuAg/Al2O3 catalysts prepared by sol immobilization (Sol) and solvated metal atom deposition (SMAD) methods[29]; (c) Performance comparison between AuAg-ZnO and AuCu-ZnO catalysts[66]; (d) Proposed reaction pathways of 1,2-propanediol over Au-Cu/HT and Au/HT catalysts[70]; (e) Performance comparison between 2.2AuPd/CeZrOx catalyst and its monometallic counterparts[65]; (f) Relationship between Au/Ru ratio of Au1Ru0.5/CeZrOx catalyst and activity[67]; (g) Performance comparison between Pd-on-Au/C and monometallic catalysts[69]; (h) Conversion of 1,2-propanediol to lactic acid on Cu@Au core/shell nanoparticles[68]; (i) Reaction performance comparison between Au/Fe-CeO2 and Au/Cu-CeO2 catalysts[28].
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF/h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Au-Pd/C | 60 | 5 | 10 | 0.1/乙二醇 | 1 | 90.0 | 100/乙醇酸 | 600 | [ |
| 0.1Au/Fe-CeO2 | 90 | 4 | 5 | 0.3/甘油 | 0.5 | 68.0 | 63.0/甘油酸 | 0.22 | [ |
| AuAg/Al2O3-SMAD | 50 | 6 | 3 | 0.3/甘油 | 1.2 | 66.7 | 52.0/甘油酸 | 0.28 | [ |
| AuAg/Al2O3-Sol | 50 | 2 | 3 | 0.3/甘油 | 1.2 | 83.3 | 44.0/甘油酸 | 0.83 | [ |
| 2.2AuPd/CeZrOx | 60 | 3 | 3 | 0.3/甘油 | 0.6 | 93.6 | 59.8/甘油酸 | 0.09 | [ |
| Pd-on-Au/C | 60 | 3 | - | 0.1/甘油 | 0.4 | 99.4 | 42.3/甘油酸 | 1.7 | [ |
| AuCu-ZnO | 60 | 5 | 6 | 1.0/甘油 | 2.0 | 10.0 | 77.0/甘油酸 | - | [ |
| AuAg-ZnO | 60 | 5 | 6 | 1.0/甘油 | 2.0 | 95.0 | 59.0/甘油酸 | - | [ |
| AuPd/C | 60 | 5 | 10 | 0.3/甘油 | 0.6 | 50.0 | 84.0/甘油酸 | 4.6 | [ |
| Au1Ru0.5/CeZrOx-500 | 50 | 2 | 3 | 0.3/甘油 | 0.3 | 98.0 | 78.0/甘油酸 | 0.66 | [ |
| Cu0.985Au0.015 | 100 | 4 | 10 | 0.28/1,2-丙二醇 | 0.56 | 89.3 | 76.1/乳酸 | 917 | [ |
| 0.5Cu-Au/HT | 80 | 6 | 10 | 1.3/1,2-丙二醇 | 2.6 | 98.5 | 88.5/乳酸 | - | [ |
| AuPt/H-丝光沸石 | 100 | 2 | 3 | 0.3/甘油 | 0 | 70.0 | 83.0/甘油酸 | - | |
| Au1-Pt3/MgO | 23 | 24 | 3 | 0.3/甘油 | 0 | 43.0 | 85.0/甘油酸 | - | |
| AuPt/H-SiO2 | 80 | 4 | 3 | 0.3/甘油 | 0 | 30.0 | 61.0/甘油酸 | - | [ |
| Au-Pt/N-TiO2 | 100 | 6 | 3 | 0.3/甘油 | 0 | 92.1 | 79.9/甘油酸 | - | [ |
| Au1Pt3/NC | 80 | 2 | 1 | 0.3/甘油 | 0 | 83.7 | 60.5/甘油酸 | - | [ |
| Au-Pt/HT | 60 | 4 | 2 | 0.3/甘油 | 0 | 64.0 | 69.0/甘油酸 | - | [ |
| Au-Pt/Hβ | 100 | 3 | 3 | 0.3/甘油 | 0 | 68.0 | 68.0/甘油酸 | - | [ |
表2 多元醇伯羟基选择性氧化中不同双金属组合的催化性能协同效应总结
Table 2 Summary of catalytic performance and synergistic effects of different bimetallic combinations for selective oxidation of primary hydroxyl groups in polyols
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF/h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Au-Pd/C | 60 | 5 | 10 | 0.1/乙二醇 | 1 | 90.0 | 100/乙醇酸 | 600 | [ |
| 0.1Au/Fe-CeO2 | 90 | 4 | 5 | 0.3/甘油 | 0.5 | 68.0 | 63.0/甘油酸 | 0.22 | [ |
| AuAg/Al2O3-SMAD | 50 | 6 | 3 | 0.3/甘油 | 1.2 | 66.7 | 52.0/甘油酸 | 0.28 | [ |
| AuAg/Al2O3-Sol | 50 | 2 | 3 | 0.3/甘油 | 1.2 | 83.3 | 44.0/甘油酸 | 0.83 | [ |
| 2.2AuPd/CeZrOx | 60 | 3 | 3 | 0.3/甘油 | 0.6 | 93.6 | 59.8/甘油酸 | 0.09 | [ |
| Pd-on-Au/C | 60 | 3 | - | 0.1/甘油 | 0.4 | 99.4 | 42.3/甘油酸 | 1.7 | [ |
| AuCu-ZnO | 60 | 5 | 6 | 1.0/甘油 | 2.0 | 10.0 | 77.0/甘油酸 | - | [ |
| AuAg-ZnO | 60 | 5 | 6 | 1.0/甘油 | 2.0 | 95.0 | 59.0/甘油酸 | - | [ |
| AuPd/C | 60 | 5 | 10 | 0.3/甘油 | 0.6 | 50.0 | 84.0/甘油酸 | 4.6 | [ |
| Au1Ru0.5/CeZrOx-500 | 50 | 2 | 3 | 0.3/甘油 | 0.3 | 98.0 | 78.0/甘油酸 | 0.66 | [ |
| Cu0.985Au0.015 | 100 | 4 | 10 | 0.28/1,2-丙二醇 | 0.56 | 89.3 | 76.1/乳酸 | 917 | [ |
| 0.5Cu-Au/HT | 80 | 6 | 10 | 1.3/1,2-丙二醇 | 2.6 | 98.5 | 88.5/乳酸 | - | [ |
| AuPt/H-丝光沸石 | 100 | 2 | 3 | 0.3/甘油 | 0 | 70.0 | 83.0/甘油酸 | - | |
| Au1-Pt3/MgO | 23 | 24 | 3 | 0.3/甘油 | 0 | 43.0 | 85.0/甘油酸 | - | |
| AuPt/H-SiO2 | 80 | 4 | 3 | 0.3/甘油 | 0 | 30.0 | 61.0/甘油酸 | - | [ |
| Au-Pt/N-TiO2 | 100 | 6 | 3 | 0.3/甘油 | 0 | 92.1 | 79.9/甘油酸 | - | [ |
| Au1Pt3/NC | 80 | 2 | 1 | 0.3/甘油 | 0 | 83.7 | 60.5/甘油酸 | - | [ |
| Au-Pt/HT | 60 | 4 | 2 | 0.3/甘油 | 0 | 64.0 | 69.0/甘油酸 | - | [ |
| Au-Pt/Hβ | 100 | 3 | 3 | 0.3/甘油 | 0 | 68.0 | 68.0/甘油酸 | - | [ |
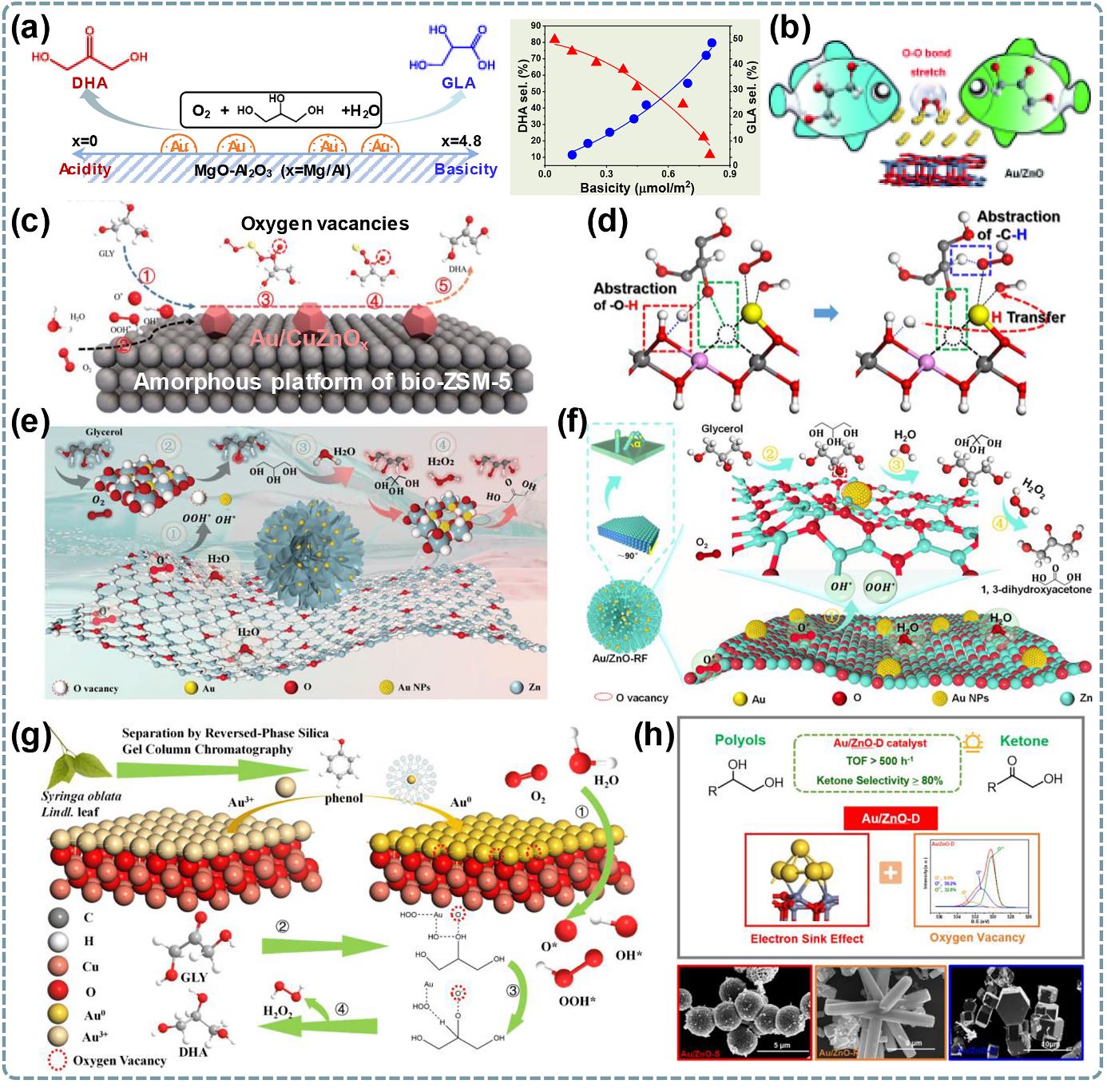
图5 多元醇仲羟基选择性氧化催化剂载体工程研究:(a) Au/MgO-Al2O3载体酸度提高和碱度降低来提高二羟基丙酮选择性[77];(b)O2在Au/ZnO(001)上吸附模型[26];(c) Au/CuZnOx@bio-ZSM-5催化剂制备图及甘油催化机理[79];(d)在甘油选择性氧化生成二羟基丙酮过程中,羟基空位与ZnII-O-Au3+位点的额外协同作用[81];(e) Au/ZnO-花形催化剂合成图和甘油选择性氧化机理[78];(f)不同形貌Au/ZnO-Z催化剂制备图和仲羟基氧化机理[80];(g)由丁香叶提取物S3制备的Au/CuO用于甘油氧化的机理图[76];(h) Au/ZnO-球、棒、盘上甘油氧化制二羟基丙酮性能[82].
Fig. 5 Researches on catalyst support engineering related to selective oxidation of secondary hydroxyl groups of polyols: (a) Enhanced acidity and reduced basicity of Au/MgO-Al2O3 support to improve dihydroxyacetone selectivity[77]; (b) Adsorption model of O2 on Au/ZnO(001)[26]; (c) Preparation of Au/CuZnOx@bio-ZSM-5 catalyst and oxidation mechanism[79]; (d) Additional synergistic effect between hydroxyl vacancies and ZnII-O-Au3+sites during oxidation of glycerol to dihydroxyacetone[81]; (e) Synthesis of Au/ZnO-flower catalyst and oxidation mechanism of glycerol[78]; (f) Preparation for Au/ZnO-Z nanocatalysts with different morphologies and mechanism of secondary alcohol oxidation[80]; (g) Reaction mechanism diagram of Au/CuO prepared from S3 clove leaf extract for oxidation of glycerol[76]; (h) Performance of glycerol selective oxidation to dihydroxyacetone over Au/ZnO-sphere, rod, and disk catalysts [82].
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF /h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Au/Al2O3 | 80 | 3 | 10 | 0.1/甘油 | 0 | 11.9 | 81.7/DHA | 43.0 | [ |
| Au/MgO-Al2O3(0.1) | 80 | 0.5 | 10 | 0.1/甘油 | 0 | 16.4 | 74.1/DHA | 358.0 | [ |
| Au/CuMgAl-HTs | 60 | 4 | 3 | 0.3/甘油 | 0 | 42.0 | 64.0/DHA | 292.0 | [ |
| Au/CuO | 60 | 4 | 5 | 0.1/甘油 | 0 | 36.6 | 81.6/DHA | 92.0 | [ |
| Au/Cu-NPC-15 %-H | 60 | 4 | 5 | 0.1/甘油 | 0 | 65.6 | 92.1/DHA | 177.7 | [ |
| Au/Cu0.95Zr0.05O1.05 | 50 | 4 | 2 | 0.1/甘油 | 0 | 72.8 | 96.2/DHA | 32.1 | [ |
| Au/CuO-SnO2-3:1 | 80 | 2 | 10 | 0.1/甘油 | 0 | 100.0 | 94.7/DHA | 874.8 | [ |
| 0.98Au/Zn2.15Ga1.0-LDHs | 60 | 4 | 5 | 0.1/甘油 | 0 | 73.1 | 63.6/DHA | 267.3 | [ |
| 0.98Au/Mg2.10Al1.0-LDHs | 60 | 4 | 5 | 0.1/甘油 | 0 | 71.2 | 57.0/DHA | 245.3 | [ |
| S3-Au/CuO | 100 | 2 | 10 | 0.1/甘油 | 0 | 86.6 | 82.0/DHA | 430.3 | [ |
| Au/ZnO-NF | 100 | 1 | 10 | 0.1/甘油 | 0 | 92.9 | 69.5/DHA | - | [ |
| Au/CuZnOx@bio-ZSM-5 | 80 | 2 | 10 | 0.1/甘油 | 0 | 93.0 | 86.3/DHA | - | [ |
| Au/CuZnOx | 80 | 2 | 10 | 0.1/甘油 | 0 | 88.0 | 83.0/DHA | - | [ |
| Au/ZnO-RF | 100 | 1 | 1 | 0.1/甘油 | 0 | 91.1 | 76.2/DHA | - | [ |
| Au/ZnO-D | 60 | 4 | 10 | 0.1/甘油 | 0 | 58.0 | 68.5/DHA | 521 | [ |
| Au/CuAlO-3 | 80 | 2 | 10 | 0.1/甘油 | 0 | 76.7 | 97.3/DHA | 80.4 | [ |
表3 多元醇仲羟基选择性氧化中不同载体类型的催化性能总结
Table 3 Summary of catalytic performance of different support types for selective oxidation of secondary hydroxyl groups in polyols
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF /h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Au/Al2O3 | 80 | 3 | 10 | 0.1/甘油 | 0 | 11.9 | 81.7/DHA | 43.0 | [ |
| Au/MgO-Al2O3(0.1) | 80 | 0.5 | 10 | 0.1/甘油 | 0 | 16.4 | 74.1/DHA | 358.0 | [ |
| Au/CuMgAl-HTs | 60 | 4 | 3 | 0.3/甘油 | 0 | 42.0 | 64.0/DHA | 292.0 | [ |
| Au/CuO | 60 | 4 | 5 | 0.1/甘油 | 0 | 36.6 | 81.6/DHA | 92.0 | [ |
| Au/Cu-NPC-15 %-H | 60 | 4 | 5 | 0.1/甘油 | 0 | 65.6 | 92.1/DHA | 177.7 | [ |
| Au/Cu0.95Zr0.05O1.05 | 50 | 4 | 2 | 0.1/甘油 | 0 | 72.8 | 96.2/DHA | 32.1 | [ |
| Au/CuO-SnO2-3:1 | 80 | 2 | 10 | 0.1/甘油 | 0 | 100.0 | 94.7/DHA | 874.8 | [ |
| 0.98Au/Zn2.15Ga1.0-LDHs | 60 | 4 | 5 | 0.1/甘油 | 0 | 73.1 | 63.6/DHA | 267.3 | [ |
| 0.98Au/Mg2.10Al1.0-LDHs | 60 | 4 | 5 | 0.1/甘油 | 0 | 71.2 | 57.0/DHA | 245.3 | [ |
| S3-Au/CuO | 100 | 2 | 10 | 0.1/甘油 | 0 | 86.6 | 82.0/DHA | 430.3 | [ |
| Au/ZnO-NF | 100 | 1 | 10 | 0.1/甘油 | 0 | 92.9 | 69.5/DHA | - | [ |
| Au/CuZnOx@bio-ZSM-5 | 80 | 2 | 10 | 0.1/甘油 | 0 | 93.0 | 86.3/DHA | - | [ |
| Au/CuZnOx | 80 | 2 | 10 | 0.1/甘油 | 0 | 88.0 | 83.0/DHA | - | [ |
| Au/ZnO-RF | 100 | 1 | 1 | 0.1/甘油 | 0 | 91.1 | 76.2/DHA | - | [ |
| Au/ZnO-D | 60 | 4 | 10 | 0.1/甘油 | 0 | 58.0 | 68.5/DHA | 521 | [ |
| Au/CuAlO-3 | 80 | 2 | 10 | 0.1/甘油 | 0 | 76.7 | 97.3/DHA | 80.4 | [ |
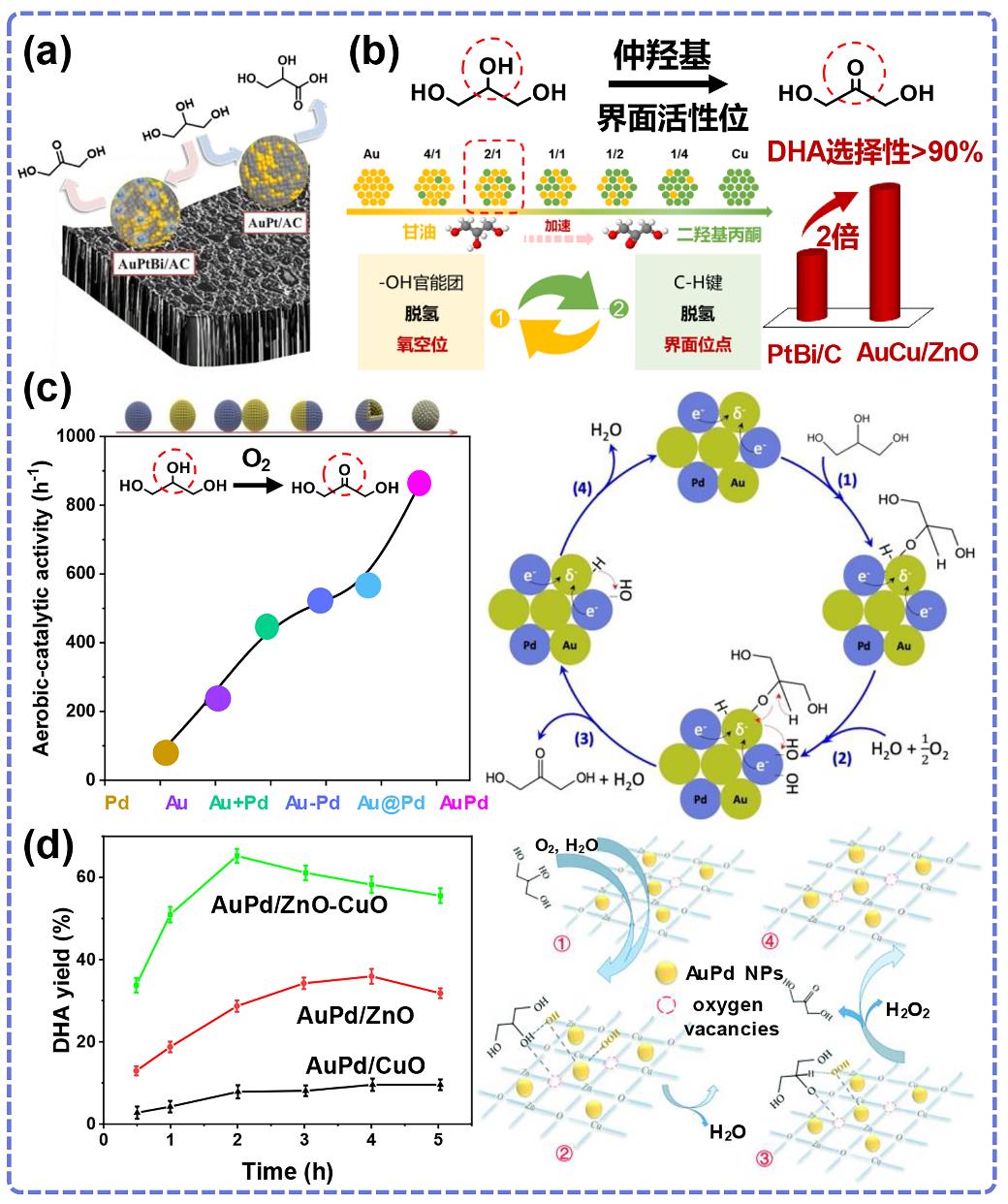
图6 多元醇仲羟基选择性氧化相关催化剂双金属协同研究:(a)铋改性AuPt/AC催化甘油氧化生成二羟基丙酮[88];(b)不同比例AuCu/ZnO催化剂甘油氧化性能[18];(c) AuPd合金、Au@Pd核壳、Au-Pd Janus颗粒和不同构型Au+Pd混合催化剂选择性氧化甘油制DHA性能与机理[89];(d) AuPd/ZnO-CuO界面上甘油仲羟基氧化性能与机理[90].
Fig. 6 Researches on bimetallic synergy in catalysts related to selective oxidation of secondary hydroxyl groups of polyols: (a) Bismuth-modified AuPt/AC catalyst for glycerol oxidation to dihydroxyacetone[88]; (b) Performance of glycerol oxidation over AuCu/ZnO with different ratios[18]; (c) Catalytic performance and mechanism for glycerol oxidation to DHA over supported AuPd alloy, Au@Pd core-shell, Au-Pd Janus particles, and differently configured Au+Pd mixed catalysts[89]; (d) Performance and mechanism of glycerol secondary hydroxyl oxidation at AuPd/ZnO-CuO interface[90].
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF /h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| AuCu/ZnO | 60 | 5 | 10 | 0.105/甘油 | 0 | 90.6 | 83.4/DHA | 402.5 | [ |
| 0.1Bi-AuPt/AC | 80 | 2 | 3 | 0.3/甘油 | 0 | 80.0 | 63.0/DHA | 585.0 | [ |
| AuPd/ZnO | 110 | 12 | 10 | 0.1/甘油 | 0 | 87.0 | 70.1/DHA | 754.8 | [ |
| AuPd/ZnO-CuO | 80 | 2 | 1 | 0.05/甘油 | 0 | 75.0 | 86.0/DHA | 687.1 | [ |
| Au0.5-Pt0.5/MgO | 40 | 4 | 3 | 0.6/1,2-丙二醇 | 0 | 40.0 | 65.0/α-羟基丙酮 | 400.0 | [ |
| Au0.5-Pt0.5/C | 100 | 24 | 10 | 0.6/1,2-丙二醇 | 0 | 67.0 | 52.0/α-羟基丙酮 | 112.0 | [ |
| Au0.5-Pt0.5/C | 100 | 24 | 3 | 0.6/1,2-丁二醇 | 0 | 75.0 | 42.0/1-羟基-2-丁酮 | - | [ |
| Au0.5-Pt0.5/C | 100 | 24 | 3 | 0.6/1,3-丁二醇 | 0 | 67.0 | 39.0/4-羟基-2-丁酮 | - | [ |
| Au0.5-Pt0.5/C | 100 | 24 | 3 | 0.6/2,3-丁二醇 | 0 | 62.0 | 84.0/3-羟基-2-丁酮 | - | [ |
表4 多元醇仲羟基选择性氧化中不同双金属组合的催化性能协同效应总结
Table 4 Summary of catalytic performance and synergistic effects of different bimetallic combinations for the selective oxidation of secondary hydroxyl groups in polyols
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF /h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| AuCu/ZnO | 60 | 5 | 10 | 0.105/甘油 | 0 | 90.6 | 83.4/DHA | 402.5 | [ |
| 0.1Bi-AuPt/AC | 80 | 2 | 3 | 0.3/甘油 | 0 | 80.0 | 63.0/DHA | 585.0 | [ |
| AuPd/ZnO | 110 | 12 | 10 | 0.1/甘油 | 0 | 87.0 | 70.1/DHA | 754.8 | [ |
| AuPd/ZnO-CuO | 80 | 2 | 1 | 0.05/甘油 | 0 | 75.0 | 86.0/DHA | 687.1 | [ |
| Au0.5-Pt0.5/MgO | 40 | 4 | 3 | 0.6/1,2-丙二醇 | 0 | 40.0 | 65.0/α-羟基丙酮 | 400.0 | [ |
| Au0.5-Pt0.5/C | 100 | 24 | 10 | 0.6/1,2-丙二醇 | 0 | 67.0 | 52.0/α-羟基丙酮 | 112.0 | [ |
| Au0.5-Pt0.5/C | 100 | 24 | 3 | 0.6/1,2-丁二醇 | 0 | 75.0 | 42.0/1-羟基-2-丁酮 | - | [ |
| Au0.5-Pt0.5/C | 100 | 24 | 3 | 0.6/1,3-丁二醇 | 0 | 67.0 | 39.0/4-羟基-2-丁酮 | - | [ |
| Au0.5-Pt0.5/C | 100 | 24 | 3 | 0.6/2,3-丁二醇 | 0 | 62.0 | 84.0/3-羟基-2-丁酮 | - | [ |
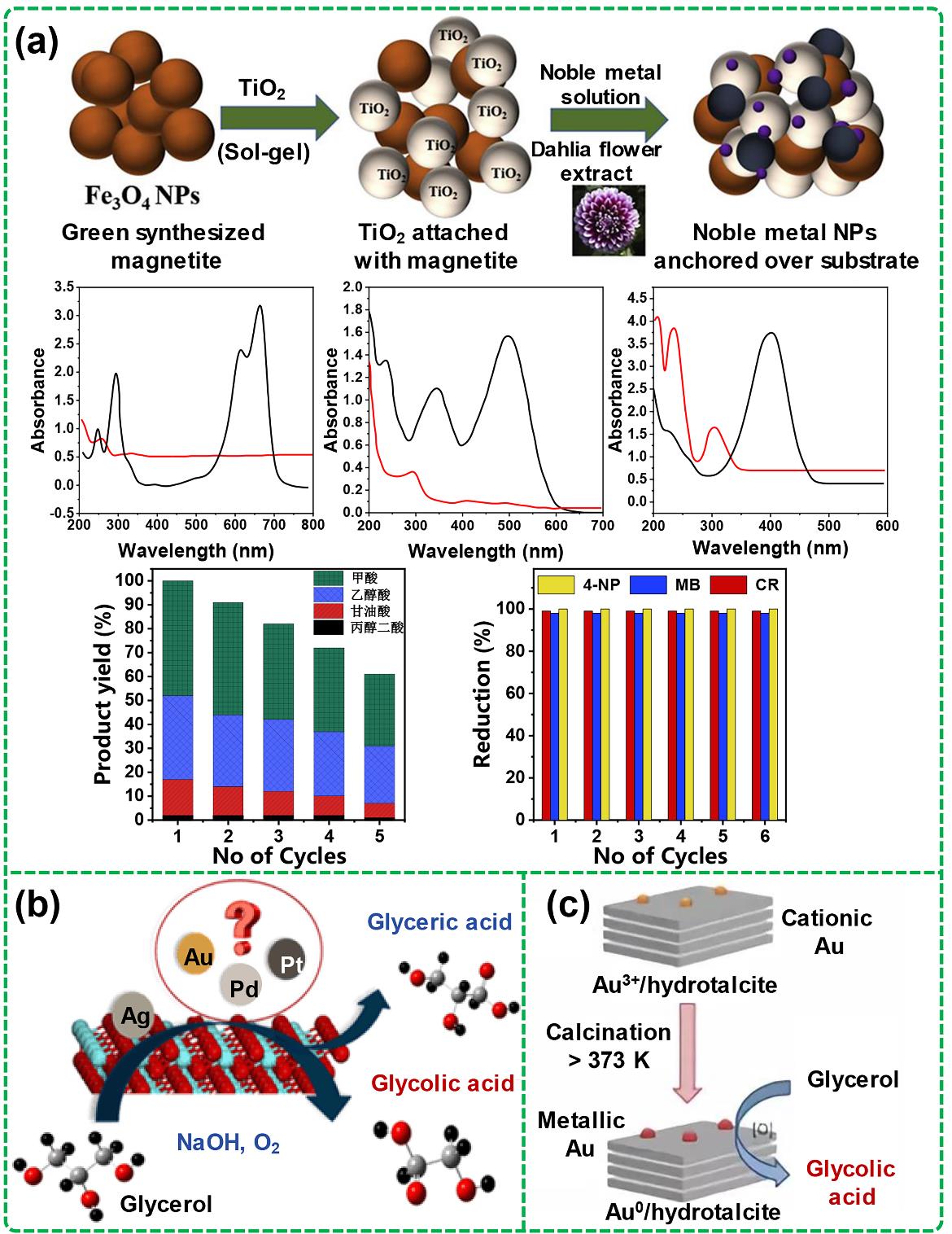
图7 多元醇C-C键断裂相关催化剂研究:(a) 可磁分离Au-Pt@TiO2纳米复合材料的绿色合成及用于甘油选择性氧化和回收测试[93];(b) 贵金属M (M = Au, Pd或Pt)促进银基甘油液相氧化催化剂[94];(c) 经高温焙烧处理后的水滑石负载催化活性金纳米颗粒无碱选择性氧化甘油成乙醇酸[98].
Fig. 7 Researches on catalysts for C-C bond cleavage of polyols: (a) Green synthesis of magnetically separable Au-Pt@TiO2 nanocomposites for selective oxidation of glycerol and recycling performance[93]; (b) Noble metal M (M = Au, Pd or Pt) promoted silver-based catalysts for liquid-phase oxidation of glycerol[94]; (c) Selective oxidation of glycerol to glycolic acid over hydrotalcite-supported gold nanoparticles activated by high-temperature calcination[98].
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF /h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Au-Pt/C | 50 | 0.5 | 3 | 0.3/甘油 | 1.2 | 90.0 | 54.2/乙醇酸 | 1258 | [ |
| Au-Pt/C-NaBH4 | 50 | 0.5 | 3 | 0.3/甘油 | 1.2 | 75.8 | 59.9/乙醇酸 | 758 | [ |
| Au-Pt/C-H2 | 50 | 0.5 | 3 | 0.3/甘油 | 1.2 | 98.3 | 46.9/乙醇酸 | 878 | [ |
| Fe3O4@TiO2-Au | 60 | 4 | 4 | 0.3/甘油 | 1.2 | 16.5 | 68.5/乙醇酸 | - | [ |
| Ag-Au/CeO2 | 60 | 5 | 4 | 0.3/甘油 | 1.2 | 43.8 | 46.2/乙醇酸 | 115 | [ |
| Au/HT | 20 | 72 | 1 | 0.1/甘油 | 0 | 97.3 | 73.8/乙醇酸 | - | [ |
| Au-Pt/C | 50 | 0.5 | 3 | 0.3/甘油 | 1.2 | 90.0 | 54.2/乙醇酸 | 1258 | [ |
表5 多元醇C-C键断裂相关催化剂性能总结
Table 5 Summary of catalytic performance of catalysts for C-C bond cleavage of polyols
| 催化剂 | 温度/℃ | 时间/h | 压力/bar | 底物/M | 碱/M | 转化率/% | 产物选择性/% | TOF /h-1 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| Au-Pt/C | 50 | 0.5 | 3 | 0.3/甘油 | 1.2 | 90.0 | 54.2/乙醇酸 | 1258 | [ |
| Au-Pt/C-NaBH4 | 50 | 0.5 | 3 | 0.3/甘油 | 1.2 | 75.8 | 59.9/乙醇酸 | 758 | [ |
| Au-Pt/C-H2 | 50 | 0.5 | 3 | 0.3/甘油 | 1.2 | 98.3 | 46.9/乙醇酸 | 878 | [ |
| Fe3O4@TiO2-Au | 60 | 4 | 4 | 0.3/甘油 | 1.2 | 16.5 | 68.5/乙醇酸 | - | [ |
| Ag-Au/CeO2 | 60 | 5 | 4 | 0.3/甘油 | 1.2 | 43.8 | 46.2/乙醇酸 | 115 | [ |
| Au/HT | 20 | 72 | 1 | 0.1/甘油 | 0 | 97.3 | 73.8/乙醇酸 | - | [ |
| Au-Pt/C | 50 | 0.5 | 3 | 0.3/甘油 | 1.2 | 90.0 | 54.2/乙醇酸 | 1258 | [ |
| [1] | 王雪鹏. 草酸二甲酯加氢制备乙二醇催化剂稳定性研究[D]. 金华: 浙江师范大学, 2020. |
| Wang X P. Study on stability of catalyst for hydrogenation of dimethyl oxalate to ethylene glycol[D]. Jinhua: Zhejiang Normal University, 2020. | |
| [2] | Taniguchi I, Yoshida S, Hiraga K, et al. Biodegradation of PET: current status and application aspects[J]. ACS Catalysis, 2019, 9(5): 4089-4105. |
| [3] | Yue H R, Zhao Y J, Ma X B, et al. Ethylene glycol: properties, synthesis, and applications[J]. Chemical Society Reviews, 2012, 41(11): 4218-4244. |
| [4] | Xia R, Chen Y Q, Chang Y X, et al. Electrosynthesis of ethylene glycol from ethylene coupled with CO2 capture[J]. Nature Catalysis, 2025, 8(8): 833-842. |
| [5] | Sun Y L, Chai G L. Direct synthesis of ethylene glycol from syngas[J]. ChemistrySelect, 2022, 7(6): e202103642. |
| [6] | Fan L, Zhao Y L, Chen L, et al. Selective production of ethylene glycol at high rate via cascade catalysis[J]. Nature Catalysis, 2023, 6(7): 585-595. |
| [7] | Zhu Z Y, Li W W, Wang D, et al. Constructing a new pathway for ethylene glycol biosynthesis and its coenzyme reuse mechanism[J]. Fermentation, 2024, 10(11): 558. |
| [8] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| Zhu F H, Cen X C, Chen Z. Research progress of diols production by microbes[J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. | |
| [9] | 季旭. 磷酸转酮酶定向进化及3-羟基丙酸生产应用[D]. 北京: 北京化工大学, 2021. |
| Ji X. Directed evolution of phosphoketolase and applications of 3-hydroxypropionic acid production[D]. Beijing: Beijing University of Chemical Technology, 2021. | |
| [10] | 吴云. 不同预处理方式对生物基多元醇制备的影响[D]. 马鞍山: 安徽工业大学, 2019. |
| Wu Y. Effect of different pretreatment methods on preparation of bio-based polyols[D]. Maanshan: Anhui University of Technology, 2019. | |
| [11] | Yan H, Yao S, Liang W, et al. Ni–Co oxide catalysts with lattice distortions for enhanced oxidation of glycerol to glyceric acid[J]. Journal of Catalysis, 2020, 381: 248-260. |
| [12] | Yan H, Yao S, Yin B, et al. Synergistic effects of bimetallic PtRu/MCM-41 nanocatalysts for glycerol oxidation in base-free medium: Structure and electronic coupling dependent activity[J]. Applied Catalysis B: Environmental, 2019, 259: 118070. |
| [13] | Yan H, Qin H, Feng X, et al. Synergistic Pt/MgO/SBA-15 nanocatalysts for glycerol oxidation in base-free medium: Catalyst design and mechanistic study[J]. Journal of Catalysis, 2019, 370: 434-446. |
| [14] | Yan H, Yao S, Zhao S M, et al. Insight into the basic strength-dependent catalytic performance in aqueous phase oxidation of glycerol to glyceric acid[J]. Chemical Engineering Science, 2021, 230: 116191. |
| [15] | Yan H, Yao S, Wang J Y, et al. Engineering Pt-Mn2O3 interface to boost selective oxidation of ethylene glycol to glycolic acid[J]. Applied Catalysis B: Environmental, 2021, 284: 119803. |
| [16] | Yan H, Shen Q, Sun Y H, et al. Tailoring facets of α-Mn2O3 microcrystalline catalysts for enhanced selective oxidation of glycerol to glycolic acid[J]. ACS Catalysis, 2021, 11(11): 6371-6383. |
| [17] | Yan H, Zhao M Y, Feng X, et al. PO4 3- coordinated robust single-atom platinum catalyst for selective polyol oxidation[J]. Angewandte Chemie International Edition, 2022, 61(21): e202116059. |
| [18] | Zhao M Y, Yan H, Lu R L, et al. Insight into the selective oxidation mechanism of glycerol to 1, 3-dihydroxyacetone over AuCu–ZnO interface[J]. AIChE Journal, 2022, 68(11): e17833. |
| [19] | Yan H, Li J, Rao Z Q, et al. Fully exposed Ptn cluster catalysts enable cascade oxidation of polyol to dicarboxylic acid[J]. Nature Communications, 2025, 16: 7030. |
| [20] | Song Z N, Yan H, Yuan J C, et al. Engineering a coordinatively unsaturated Au–O–Ti3+ structure toward unprecedented H2 efficiency for low-temperature propene epoxidation with H2 and O2 [J]. Engineering, 2023, 9(6): 144-156. |
| [21] | Zhang Y S, Liu J X, Qian K, et al. Structure sensitivity of Au-TiO2 strong metal–support interactions[J]. Angewandte Chemie International Edition, 2021, 60(21): 12074-12081. |
| [22] | Guo Z L, Jing G H, Tolba S A, et al. Design and construction of an O-Au-O coordination environment in Au single atom-doped Ti4+ defected TiO2 for an enhanced oxidative ability of lattice oxygen for Hg0 oxidation[J]. Chemical Engineering Journal, 2023, 451: 138895.. |
| [23] | Wang L, Xu W D, Yuan Z R, et al. Tannic acid assisted construction of Au-Ti integrated catalyst regulating the local environment of framework Ti toward propylene epoxidation under H2/O2 [J]. Molecular Catalysis, 2023, 545: 113206. |
| [24] | Li Y C, Li X S, Zhu B, et al. Boosting low-temperature water gas shift reaction over Au/TiO2 nanocatalyst activated by oxygen plasma[J]. Chemical Engineering Journal, 2022, 430: 133013. |
| [25] | Yao S Y, Zhang X, Zhou W, et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction[J]. Science, 2017, 357(6349): 389-393. |
| [26] | Meng Y, Zou S H, Zhou Y H, et al. Activating molecular oxygen by Au/ZnO to selectively oxidize glycerol to dihydroxyacetone[J]. Catalysis Science & Technology, 2018, 8(10): 2524-2528. |
| [27] | 陶伟. Au/HAP、CuPd、CuPdAu纳米金属催化剂催化氧化1, 2-丙二醇制备乳酸研究[D]. 镇江: 江苏大学, 2021. |
| Tao W. Study on the catalytic oxidation of 1, 2-propan diol tolactic acid over Au/HAP, CuPd and CuPdAu nano-metal catalysts[D]. Zhenjiang: Jiangsu University, 2021. | |
| [28] | Redina E A, Kapustin G I, Tkachenko O P, et al. Effect of ultra-low amount of gold in oxide-supported bimetallic Au–Fe and Au–Cu catalysts on liquid-phase aerobic glycerol oxidation in water[J]. Catalysis Science & Technology, 2021, 11(17): 5881-5897. |
| [29] | Jouve A, Nagy G, Somodi F, et al. Gold-silver catalysts: Effect of catalyst structure on the selectivity of glycerol oxidation[J]. Journal of Catalysis, 2018, 368: 324-335. |
| [30] | Pan Y N, Wu G D, He Y F, et al. Identification of the Au/ZnO interface as the specific active site for the selective oxidation of the secondary alcohol group in glycerol[J]. Journal of Catalysis, 2019, 369: 222-232. |
| [31] | Yan H, Qin H S, Liang W, et al. Enhanced performance of bimetallic PtCo/MCM-41 catalysts for glycerol oxidation in base-free medium[J]. Catalysis Science & Technology, 2019, 9(18): 4909-4919. |
| [32] | Xiong L Q, Yu Z N, Cao H C, et al. Converting glycerol into valuable trioses by Cuδ+-single-atom-decorated WO3 under visible light[J]. Angewandte Chemie International Edition, 2024, 63(12): e202318461. |
| [33] | 于志奕, 方俊彦, 陈文尧, 等. Pt-Bi界面结构调控及其催化甘油选择性氧化反应性能[J]. 化工学报, 2024, 75(10): 3568-3578. |
| Yu Z Y, Fang J Y, Chen W Y, et al. Regulation of Pt-Bi interfaces for selective catalytic oxidation of glycerol[J]. CIESC Journal, 2024, 75(10): 3568-3578. | |
| [34] | de Nazaré de Oliveira A, Melchiorre M, Farias da Costa A A, et al. Glycerol: a green solvent for synthetic chemistry[J]. Sustainable Chemistry and Pharmacy, 2024, 41: 101656. |
| [35] | Ke J W, Chi M F, Zhao J K, et al. Dynamically reversible interconversion of molecular catalysts for efficient electrooxidation of propylene into propylene glycol[J]. Journal of the American Chemical Society, 2023, 145(16): 9104-9111. |
| [36] | Liu X C, Wang T, Zhang Z M, et al. Reaction mechanism and selectivity tuning of propene oxidation at the electrochemical interface[J]. Journal of the American Chemical Society, 2022, 144(45): 20895-20902. |
| [37] | 李庆远, 王超, 许世佩, 等. PBS前体1, 4-丁二醇合成的反应工艺和催化剂研究进展[J]. 化工进展, 2022, 41(11): 5771-5782. |
| Li Q Y, Wang C, Xu S P, et al. Research progress on reaction process and catalysts for PBS precursor of 1, 4-butanediol synthesis[J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5771-5782. | |
| [38] | Zhu Y, Yang J M, Mei F, et al. Bio-based 1, 4-butanediol and tetrahydrofuran synthesis: perspective[J]. Green Chemistry, 2022, 24(17): 6450-6466. |
| [39] | Liu Y F, Wang W, Zeng A P. Biosynthesizing structurally diverse diols via a general route combining oxidative and reductive formations of OH-groups[J]. Nature Communications, 2022, 13: 1595. |
| [40] | Torbina V V, Nedoseykina N S, Ivanchikova I D, et al. Propylene glycol oxidation with hydrogen peroxide over Zr-containing metal-organic framework UiO-66[J]. Catalysis Today, 2019, 333: 47-53. |
| [41] | Lin H, Xu J Y, Sun W L, et al. Efficient 1-hydroxy-2-butanone production from 1, 2-butanediol by whole cells of engineered E. coli[J]. Catalysts, 2021, 11(10): 1184. |
| [42] | Wang H Y, Zhu C H, Liu Q Y, et al. Selective conversion of cellulose to hydroxyacetone and 1-hydroxy-2-butanone with Sn–Ni bimetallic catalysts[J]. ChemSusChem, 2019, 12(10): 2154-2160. |
| [43] | Cai T, Sun H B, Qiao J, et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide[J]. Science, 2021, 373(6562): 1523-1527. |
| [44] | Zhan T, Liu W B, Teng J J, et al. Selective oxidation of glycerol to tartronic acid over Pt/N-doped mesoporous carbon with extra framework magnesium catalysts under base-free conditions[J]. Chemical Communications, 2019, 55(18): 2620-2623. |
| [45] | Zhang H, Zhang R Z, Zhang W D, et al. Base-free selective oxidation of 5-hydroxymethylfurfural over Pt nanoparticles on surface Nb-enriched Co-Nb oxide[J]. Applied Catalysis B: Environmental, 2023, 330: 122670. |
| [46] | Dou J, Zhang B W, Liu H, et al. Carbon supported Pt9Sn1 nanoparticles as an efficient nanocatalyst for glycerol oxidation[J]. Applied Catalysis B: Environmental, 2016, 180: 78-85. |
| [47] | Kaminski P, Ziolek M, van Bokhoven J A. Mesoporous cerium–zirconium oxides modified with gold and copper–synthesis, characterization and performance in selective oxidation of glycerol[J]. RSC Advances, 2017, 7(13): 7801-7819. |
| [48] | Davis S E, Ide M S, Davis R J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles[J]. Green Chemistry, 2013, 15(1): 17-45. |
| [49] | Han Z Y, Xie R F, Song Y H, et al. Efficient and stable platinum nanocatalysts supported over Ca-doped ZnAl2O4 spinels for base-free selective oxidation of glycerol to glyceric acid[J]. Molecular Catalysis, 2019, 477: 110559. |
| [50] | Xu S G, Xiao Y, Zhang W Y, et al. Relay catalysis of copper-magnesium catalyst on efficient valorization of glycerol to glycolic acid[J]. Chemical Engineering Journal, 2022, 428: 132555. |
| [51] | Xiao Y H, Wang M R, Liu D, et al. Selective photoelectrochemical oxidation of glycerol to glyceric acid on (002) facets exposed WO3 nanosheets[J]. Angewandte Chemie International Edition, 2024, 63(11): e202319685. |
| [52] | Pakrieva E, Kolobova E, German D, et al. Glycerol oxidation over supported gold catalysts: the combined effect of Au particle size and basicity of support[J]. Processes, 2020, 8(9): 1016. |
| [53] | Martínez Q H, Neira J A, Amaya Á A, et al. Selective oxidation of glycerol mediated by surface plasmon of gold nanoparticles deposited on titanium dioxide nanowires[J]. Chemosphere, 2024, 364: 142995. |
| [54] | Villa A, Dimitratos N, Chan-Thaw C E, et al. Glycerol oxidation using gold-containing catalysts[J]. Accounts of Chemical Research, 2015, 48(5): 1403-1412. |
| [55] | Villa A, Campisi S, Mohammed K M H, et al. Tailoring the selectivity of glycerol oxidation by tuning the acid–base properties of Au catalysts[J]. Catalysis Science & Technology, 2015, 5(2): 1126-1132. |
| [56] | Zhang X Y, Yang P F, Liu Y N, et al. Support morphology effect on the selective oxidation of glycerol over AuPt/CeO2 catalysts[J]. Journal of Catalysis, 2020, 385: 146-159. |
| [57] | Xu C L, Du Y Q, Li C, et al. Insight into effect of acid/base nature of supports on selectivity of glycerol oxidation over supported Au-Pt bimetallic catalysts[J]. Applied Catalysis B: Environmental, 2015, 164: 334-343. |
| [58] | Shen L Q, Chen X Y, Chen X J, et al. The support influence of Au-based catalysts in glycerin selective oxidation to glyceric acid[J]. Journal of Chemical Technology & Biotechnology, 2023, 98(1): 179-187. |
| [59] | Evans C D, Bartley J K, Taylor S H, et al. Perovskite supported catalysts for the selective oxidation of glycerol to tartronic acid[J]. Catalysis Letters, 2023, 153(7): 2026-2035. |
| [60] | Jiang K H, Li Z Y, Zhang Z H, et al. Stable and active Au catalyst supported on CeMnO3 perovskite for selective oxidation of glycerol[J]. Inorganic Chemistry, 2023, 62(21): 8145-8157. |
| [61] | Purushothaman R K P, van Haveren J, Mayoral A, et al. Exploratory catalyst screening studies on the base free conversion of glycerol to lactic acid and glyceric acid in water using bimetallic Au–Pt nanoparticles on acidic zeolites[J]. Topics in Catalysis, 2014, 57(17): 1445-1453. |
| [62] | Cherni D, Moussa N, Nsib M F, et al. Base-free glycerol oxidation over N-TiO2 supported Au–Pt catalysts[J]. Reaction Kinetics, Mechanisms and Catalysis, 2019, 128(2): 979-990. |
| [63] | Ma H, Nie X, Cai J Y, et al. Au/Mg(OH)2: Highly efficient for selective oxidation of 1, 2-propanediol to lactic acid with molecular oxygen[J]. Science China Chemistry, 2010, 53(7): 1497-1501. |
| [64] | Griffin M B, Rodriguez A A, Montemore M M, et al. The selective oxidation of ethylene glycol and 1, 2-propanediol on Au, Pd, and Au–Pd bimetallic catalysts[J]. Journal of Catalysis, 2013, 307: 111-120. |
| [65] | Olmos C M, Chinchilla L E, Rodrigues E G, et al. Synergistic effect of bimetallic Au-Pd supported on ceria-zirconia mixed oxide catalysts for selective oxidation of glycerol[J]. Applied Catalysis B: Environmental, 2016, 197: 222-235. |
| [66] | Kaskow I, Decyk P, Sobczak I. The effect of copper and silver on the properties of Au-ZnO catalyst and its activity in glycerol oxidation[J]. Applied Surface Science, 2018, 444: 197-207. |
| [67] | Chinchilla L E, Olmos C M, Villa A, et al. Ru-modified Au catalysts supported on ceria–zirconia for the selective oxidation of glycerol[J]. Catalysis Today, 2015, 253: 178-189. |
| [68] | Xue W P, Yin H B, Lu Z P, et al. Catalytic oxidation of 1, 2-propanediol over bimetallic Cu@Au core/shell nanoparticles[J]. Catalysis Letters, 2016, 146(6): 1139-1152. |
| [69] | Zhao Z, Arentz J, Pretzer L A, et al. Volcano-shape glycerol oxidation activity of palladium-decorated gold nanoparticles[J]. Chemical Science, 2014, 5(10): 3715-3728. |
| [70] | Tian J, Liu H, Li P, et al. Selective oxidation of 1,2-propanediol to lactic acid over Cu-modified Au/hydrotalcite catalysts[J]. New Journal of Chemistry, 2020, 44(38): 16311-16319. |
| [71] | Ketchie W C, Murayama M, Davis R J. Selective oxidation of glycerol over carbon-supported AuPd catalysts[J]. Journal of Catalysis, 2007, 250(2): 264-273. |
| [72] | Villa A, Veith G, Prati L. Selective oxidation of glycerol under acidic conditions using gold catalysts[J]. Angewandte Chemie International Edition, 2010, 49(26): 4499-4502. |
| [73] | Brett G L, He Q, Hammond C, et al. Selective oxidation of glycerol by highly active bimetallic catalysts at ambient temperature under base-free conditions[J]. Angewandte Chemie International Edition, 2011, 50(43): 10136-10139. |
| [74] | Hirasawa S, Watanabe H, Kizuka T, et al. Performance, structure and mechanism of Pd–Ag alloy catalyst for selective oxidation of glycerol to dihydroxyacetone[J]. Journal of Catalysis, 2013, 300: 205-216. |
| [75] | Liu S S, Sun K Q, Xu B Q. Specific selectivity of Au-catalyzed oxidation of glycerol and other C3-polyols in water without the presence of a base[J]. ACS Catalysis, 2014, 4(7): 2226-2230. |
| [76] | Cui L, Wang F, Zhang X L, et al. Plant-mediated biosynthesized Au/CuO catalysts for efficient glycerol oxidation to 1, 3-dihydroxyacetone: effect of biomass component on catalytic activity[J]. New Journal of Chemistry, 2024, 48(45): 19206-19219. |
| [77] | Yuan Z F, Gao Z K, Xu B Q. Acid-base property of the supporting material controls the selectivity of Au catalyst for glycerol oxidation in base-free water[J]. Chinese Journal of Catalysis, 2015, 36(9): 1543-1551. |
| [78] | Xiong Z L, Zhang X L, Huang J L, et al. Flower-shaped zinc oxide nanostructures loaded with Au nanoparticles for efficient and highly stable production of dihydroxyacetone from glycerol oxidation[J]. Advanced Sustainable Systems, 2025, 9(4): 2400947. |
| [79] | Yuan Z, Wang Y M, Xie W D, et al. Manipulating the interfacial integration mode of a bio-templated porous ZSM-5 platform with an Au/CuZnOx catalyst for enhanced efficiency and recycling stability in glycerol conversion to 1, 3-dihydroxyacetone[J]. Nanoscale, 2025, 17(9): 5259-5269. |
| [80] | Zhang X L, Liu H, Ke Y H, et al. Biomimetic flower-petal-architected Zn-based nanocatalysts for selective oxidation of glycerol to 1, 3-dihydroxyacetone[J]. ACS Applied Nano Materials, 2025, 8(28): 14052-14064. |
| [81] | An Z, Ma H H, Han H B, et al. Insights into the multiple synergies of supports in the selective oxidation of glycerol to dihydroxyacetone: layered double hydroxide supported Au[J]. ACS Catalysis, 2020, 10(21): 12437-12453. |
| [82] | Yan H, Zhao M Y, Cao Y Q, et al. Crystal-facet-dependent, electron sink effect for the enhanced selective oxidation of polyols at the secondary hydroxyl position[J]. Journal of Catalysis, 2024, 431: 115401. |
| [83] | Yin Y R, Tang T, Xu C L. Au/CuMgAl-hydrotalcite catalysts promoted by Cu+ and basic sites for selective oxidation of glycerol to dihydroxyacetone[J]. Gold Bulletin, 2017, 50(4): 319-326. |
| [84] | Tan H, Yao C J, Zhan T, et al. Selective oxidation of glycerol to dihydroxyacetone over N-doped porous carbon stabilized CuxO supported Au catalysts[J]. Molecular Catalysis, 2020, 498: 111243. |
| [85] | Wang Y X, Pu Y F, Yuan D P, et al. Selective oxidation of glycerol to dihydroxyacetone over Au/CuxZr1-xOy catalysts in base-free conditions[J]. ACS Applied Materials & Interfaces, 2019, 11(47): 44058-44068 |
| [86] | Ke Y H, Qin H Y, Wang X, et al. Facile conversion of glycerol to 1, 3-dihydroxyacetone by using mesoporous CuO–SnO2 composite oxide supported Au catalysts[J]. Journal of Porous Materials, 2023, 30(3): 723-737. |
| [87] | Ke Y H, Wang X, Qin H Y, et al. Cu–Al composite oxides: a highly efficient support for the selective oxidation of glycerol to 1, 3-dihydroxyacetone[J]. New Journal of Chemistry, 2020, 44(42): 18173-18184. |
| [88] | Villa A, Campisi S, Chan-Thaw C E, et al. Bismuth modified Au-Pt bimetallic catalysts for dihydroxyacetone production[J]. Catalysis Today, 2015, 249: 103-108. |
| [89] | Feng Y M, Bi Y P, Wang Y F, et al. Enhanced glycerol oxidation toward dihydroxyacetone over gold/palladium binary nanocatalysts by structure control[J]. Chemistry – A European Journal, 2025, 31(23): e202500601 |
| [90] | Zhao G Q, Wu G D, Liu Y N, et al. Preparation of AuPd/ZnO–CuO for the directional oxidation of glycerol to DHA[J]. Catalysis Science & Technology, 2020, 10(18): 6223-6234. |
| [91] | Ryabenkova Y, Miedziak P J, Dummer N F, et al. The selective oxidation of 1, 2-propanediol by supported gold-based nanoparticulate catalysts[J]. Topics in Catalysis, 2012, 55(19): 1283-1288. |
| [92] | Ryabenkova Y, Miedziak P J, Knight D W, et al. Heterogeneously catalyzed oxidation of butanediols in base free aqueous media[J]. Tetrahedron, 2014, 70(36): 6055-6058. |
| [93] | Sarangapany S, Mohanty K. Facile green synthesis of magnetically separable Au–Pt@TiO2 nanocomposite for efficient catalytic reduction of organic pollutants and selective oxidation of glycerol[J]. Journal of Alloys and Compounds, 2020, 830: 154636. |
| [94] | Zaid S, Skrzyńska E, Addad A, et al. Development of silver based catalysts promoted by noble metal M (M = Au, Pd or Pt) for glycerol oxidation in liquid phase[J]. Topics in Catalysis, 2017, 60(15): 1072-1081. |
| [95] | Yan H, Liu B W, Zhou X, et al. Enhancing polyol/sugar cascade oxidation to formic acid with defect rich MnO2 catalysts[J]. Nature Communications, 2023, 14: 4509. |
| [96] | Bianchi C L, Canton P, Dimitratos N, et al. Selective oxidation of glycerol with oxygen using mono and bimetallic catalysts based on Au, Pd and Pt metals[J]. Catalysis Today, 2005, 102: 203-212. |
| [97] | Dimitratos N, Messi C, Porta F, et al. Investigation on the behaviour of Pt(0)/carbon and Pt(0),Au(0)/carbon catalysts employed in the oxidation of glycerol with molecular oxygen in water[J]. Journal of Molecular Catalysis A: Chemical, 2006, 256(1/2): 21-28. |
| [98] | Takagaki A, Tsuji A, Nishimura S, et al. Genesis of catalytically active gold nanoparticles supported on hydrotalcite for base-free selective oxidation of glycerol in water with molecular oxygen[J]. Chemistry Letters, 2011, 40(2): 150-152. |
| [1] | 孙云龙, 徐肖肖, 黄永方, 郭纪超, 陈卫卫. 水平光滑管内CO2流动沸腾的非绝热可视化研究[J]. 化工学报, 2025, 76(S1): 230-236. |
| [2] | 郭纪超, 徐肖肖, 孙云龙. 基于植物工厂中的CO2浓度气流模拟及优化研究[J]. 化工学报, 2025, 76(S1): 237-245. |
| [3] | 孔繁臣, 张硕, 唐明生, 邹慧明, 胡舟航, 田长青. 二氧化碳直线压缩机气体轴承模拟[J]. 化工学报, 2025, 76(S1): 281-288. |
| [4] | 何婷, 张开, 林文胜, 陈利琼, 陈家富. 沼气超临界压力低温脱碳-液化耦合流程研究[J]. 化工学报, 2025, 76(S1): 418-425. |
| [5] | 周怀荣, 伊嘉伟, 曹阿波, 郭奥雪, 王东亮, 杨勇, 杨思宇. 共电解耦合CO2间接加氢制甲醇工艺集成设计与性能评价[J]. 化工学报, 2025, 76(9): 4586-4600. |
| [6] | 张彬怡, 孙少东, 姚谦, 蔡文河, 张惠宇, 李成新. 煤制甲醇耦合固体氧化物燃料电池混合系统研究[J]. 化工学报, 2025, 76(9): 4658-4669. |
| [7] | 张建民, 何美贵, 贾万鑫, 赵静, 金万勤. 聚氧化乙烯/冠醚共混膜及其二氧化碳分离性能[J]. 化工学报, 2025, 76(9): 4862-4871. |
| [8] | 王一飞, 李玉星, 欧阳欣, 赵雪峰, 孟岚, 胡其会, 殷布泽, 郭雅琦. 基于裂尖减压特性的CO2管道断裂扩展数值计算[J]. 化工学报, 2025, 76(9): 4683-4693. |
| [9] | 廖兵, 祝鑫宇, 黄倩倩, 胥雯, 寇梦瑶, 郭娜. 盐酸羟胺强化芬顿体系在近中性条件下去除2,4-DCP的性能及机理研究[J]. 化工学报, 2025, 76(8): 4273-4283. |
| [10] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| [11] | 刘沁雯, 叶恒冰, 张逸伟, 朱法华, 钟文琪. 煤与禽类粪便混合燃料的加压富氧燃烧特性研究[J]. 化工学报, 2025, 76(7): 3487-3497. |
| [12] | 丁宏鑫, 干文翔, 赵雍洋, 贾润泽, 康子祺, 赵玉隆, 向勇. X65钢焊接接头在超临界CO2相及富H2O相中的腐蚀机理研究[J]. 化工学报, 2025, 76(7): 3426-3435. |
| [13] | 赵美, 甘雨欣, 赵绍磊, 杨令, 王亭杰. 硅橡胶用纳米二氧化硅表面有机修饰及补强机理研究进展[J]. 化工学报, 2025, 76(7): 3125-3136. |
| [14] | 吴雷, 胡紫璇, 高渊, 刘长波, 曹虎生, 刘田田, 朱瑞玉, 周军. 微波联合生物炭活化过硫酸盐氧化修复多环芳烃污染土壤研究[J]. 化工学报, 2025, 76(7): 3659-3670. |
| [15] | 卢煦旸, 徐强, 康浩鹏, 史健, 曹泽水, 郭烈锦. 化学链制氢系统中磁铁矿氧载体的CO还原特性研究[J]. 化工学报, 2025, 76(7): 3286-3294. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号