• •
收稿日期:2025-12-10
修回日期:2026-01-12
出版日期:2026-01-23
通讯作者:
赵晓辉
作者简介:马小琴(1995—),女,博士,maxiaoqin@nwepdi.com
基金资助:
Xiaoqin MA( ), Xiaohui ZHAO(
), Xiaohui ZHAO( ), Lin FU, Ye QIANG, Qingchuan BAI
), Lin FU, Ye QIANG, Qingchuan BAI
Received:2025-12-10
Revised:2026-01-12
Online:2026-01-23
Contact:
Xiaohui ZHAO
摘要:
阴离子交换膜(AEM)是燃料电池与电解水制氢系统的核心组件,其离子电导率直接决定了器件的转换效率。针对传统 AEM 存在的离子传导率低的使用瓶颈,本文设计并制备了基于共价有机框架材料(COF)的高性能阴离子交换膜。通过门舒特金反应将功能化改性后的 COF 材料和聚芳基哌啶聚合物(PTP)化学键合,得到QPTP-COF AEM。多孔COF材料的引入大幅提升了膜内的自由体积,构建连续离子传输通道,降低离子传输阻力。结果表明,与QPTP AEM相比,QPTP-COF AEM的离子电导率可达 177.2 mS cm-1。将其应用于燃料电池与电解水测试,均展现出优异的能量转换效率,为高性能 AEM 的设计提供了新策略。
中图分类号:
马小琴, 赵晓辉, 付林, 强也, 白清川. 高性能共价有机框架阴离子交换膜及其燃料电池与电解水应用[J]. 化工学报, DOI: 10.11949/0438-1157.20251392.
Xiaoqin MA, Xiaohui ZHAO, Lin FU, Ye QIANG, Qingchuan BAI. High-performance covalent organic framework anion exchange membranes for fuel cells and water electrolysis[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251392.
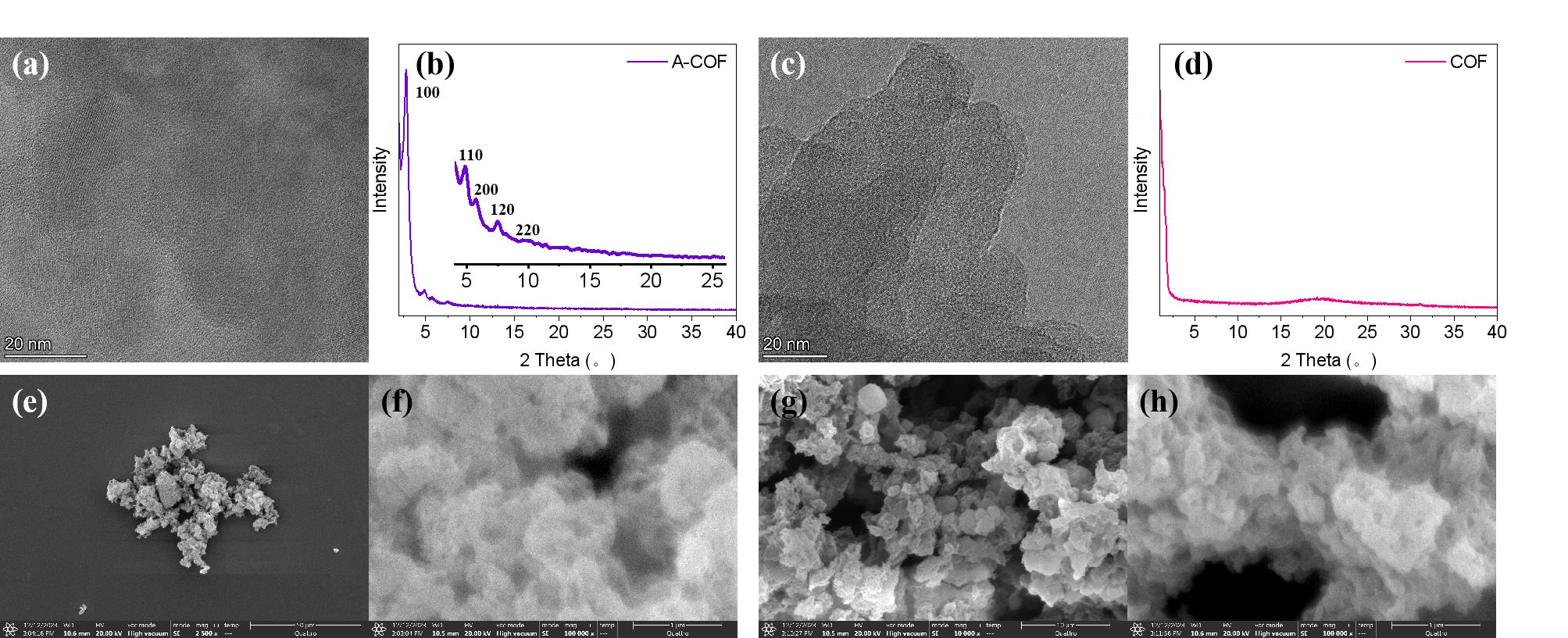
图3 A-COF材料的(a)TEM谱图,(b)XRD谱图以及(e)(f)SEM谱图,修饰后的COF 材料的(c)TEM谱图,(d)XRD谱图以及(g)(h)SEM谱图
Fig. 3 (a) TEM spectrum, (b) XRD spectrum, and (e)(f) SEM spectrum of A-COF material; (c) TEM spectrum, (d) XRD spectrum, and (g)(h) SEM spectrum of modified COF material
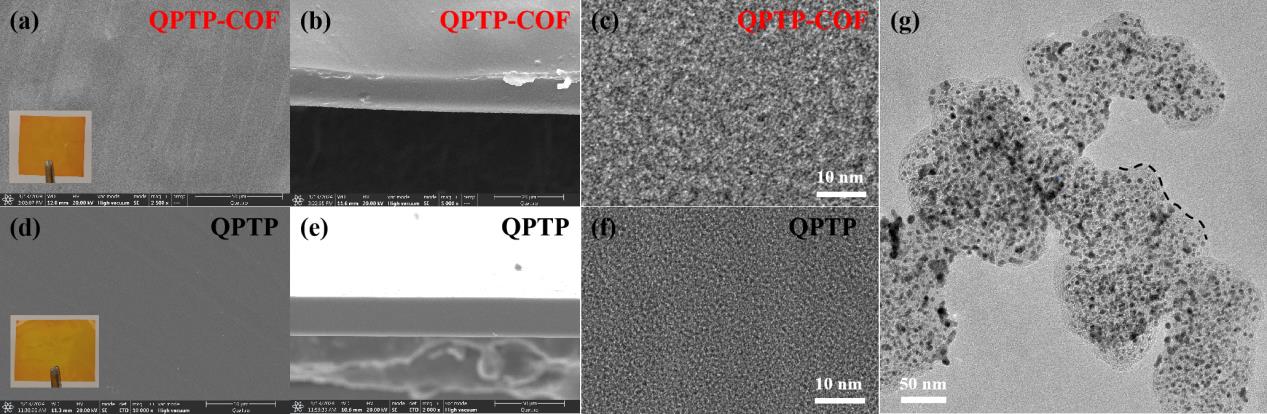
图6 QPTP-COF AEM的(a)SEM表面图和膜照片,(b)SEM截面图,(c)TEM图,QPTP AEM的(d)SEM表面图和膜照片,(e)SEM截面图和(f)TEM图,(g)QPTP-COF作为离聚物配置的催化剂浆料的TEM图
Fig. 6 (a) SEM surface image and membrane photograph, (b) SEM cross-sectional image and (c) TEM image of QPTP-COF AEM, (d) SEM surface image and membrane photograph, (e) SEM cross-sectional image and (f) TEM image of QPTP AEM, (g) TEM image of catalyst slurry configured with QPTP-COF as the ionomer
| 聚合物 | 吸水(%)a | 溶胀(%)a | 拉伸(MPa)b | 电导率(mS/cm)a |
|---|---|---|---|---|
| QPTP | 120.2±5.4 | 17.1±0.7 | 12.2±2.3 | 148.1±4.5 |
| QPTP-COF | 245.3±9.1 | 21.3±0.9 | 11.6±1.6 | 177.2±2.6 |
表1 QPTP AEM 和QPTP-COF AEM的性能
Table 1 The properties of QPTP AEM and QPTP-COF AEM
| 聚合物 | 吸水(%)a | 溶胀(%)a | 拉伸(MPa)b | 电导率(mS/cm)a |
|---|---|---|---|---|
| QPTP | 120.2±5.4 | 17.1±0.7 | 12.2±2.3 | 148.1±4.5 |
| QPTP-COF | 245.3±9.1 | 21.3±0.9 | 11.6±1.6 | 177.2±2.6 |
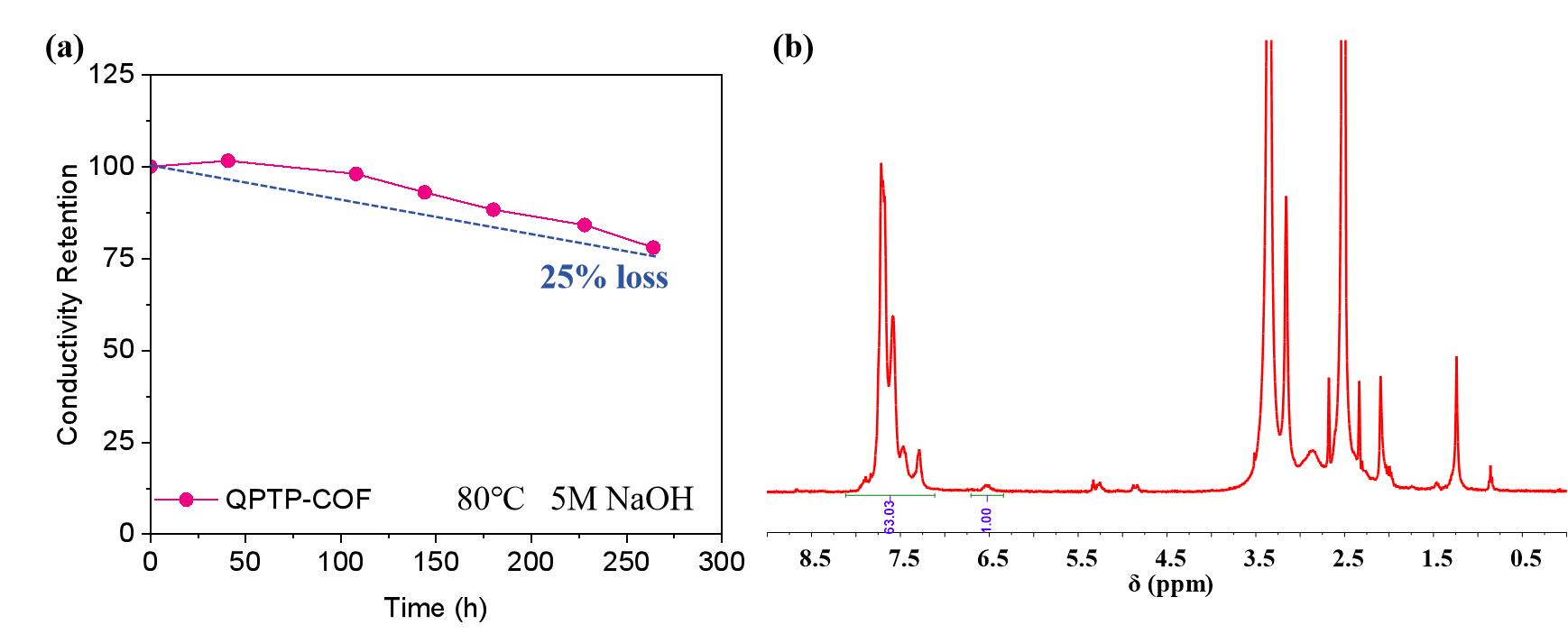
图10 QPTP-COF AEM耐碱测试后的(a)OH-电导率的保留率和(b)核磁谱图
Fig. 10 QPTP-COF AEM after alkali resistance test: (a) retention rate of OH- conductivity and (b) NMR spectrum
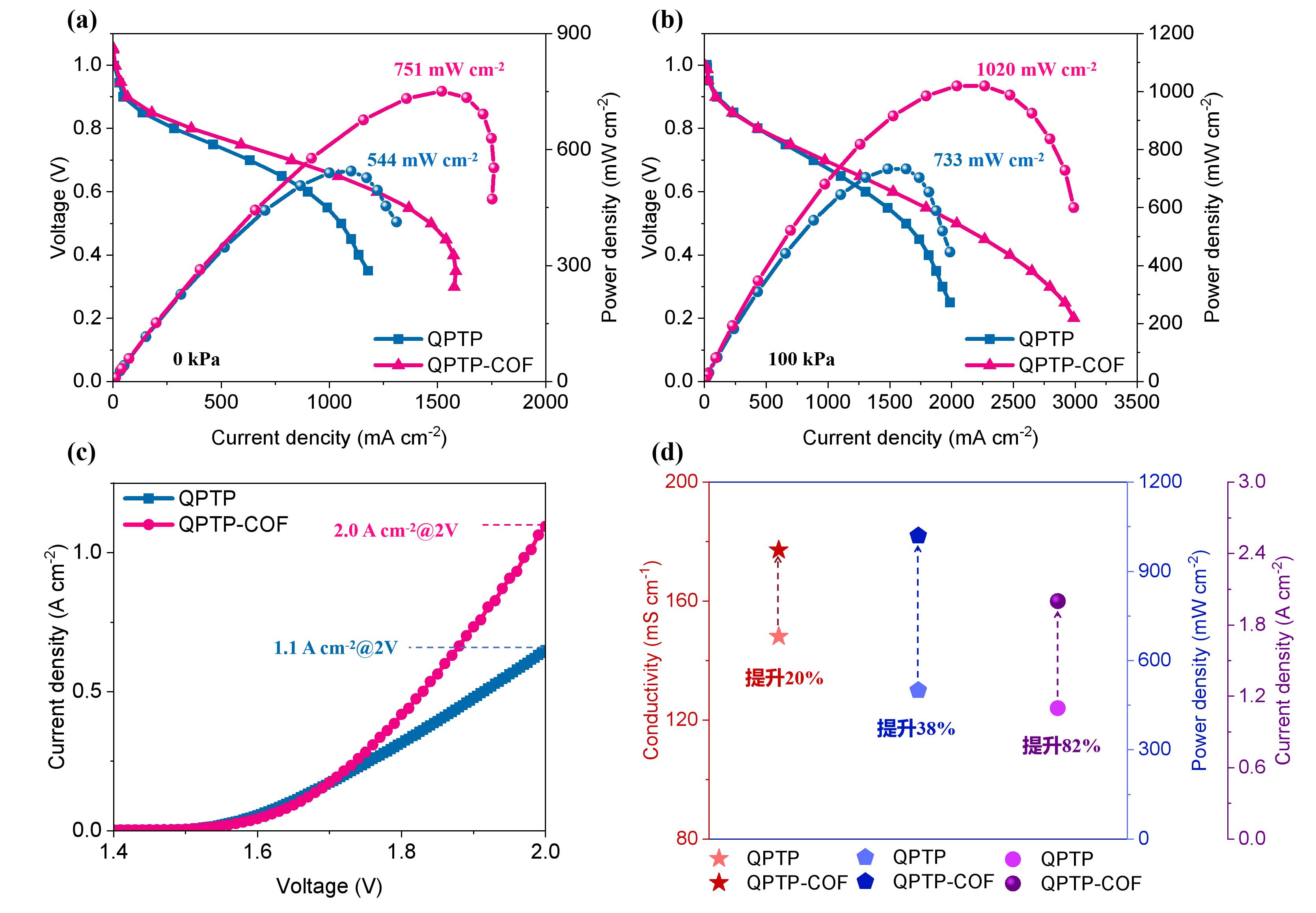
图11 QPTP-COF AEM 和QPTP AEM的(a)燃料电池性能(0 kPa),(b)燃料电池性能(100 kPa),(c)电解水性能和(d)相关性能对比图。
Fig. 11 QPTP-COF AEM and QPTP AEM (a) fuel cell performance (0 kPa), (b)fuel cell performance (100 kPa), (c) water electrolysis performance and (d) Comparative performance diagram
| [1] | Zeng G H, Liu M B, Lei Z X, et al. Optimal configuration of hydrogen energy storage in an integrated energy system considering variable hydrogen production efficiency[J]. Journal of Energy Storage, 2024, 98: 113044. |
| [2] | Shu K Y, Guan B, Zhuang Z Q, et al. Reshaping the energy landscape: Explorations and strategic perspectives on hydrogen energy preparation, efficient storage, safe transportation and wide applications[J]. International Journal of Hydrogen Energy, 2025, 97: 160-213. |
| [3] | Wei X Y, Sharma S, Waeber A, et al. Environmental implications of solid oxide fuel cell system for hydrogen sustainability[J]. Resources, Conservation and Recycling, 2025, 215: 108134. |
| [4] | Navinkumar T M, Bharatiraja C. Sustainable hydrogen energy fuel cell electric vehicles: A critical review of system components and innovative development recommendations[J]. Renewable and Sustainable Energy Reviews, 2025, 215: 115601. |
| [5] | Laleh S S, Rezaei Mousavi H S, Rabet S, et al. Solar thermal assisted proton exchange membrane electrolyzer and solid oxide fuel cell system based on biomass gasification for green power and hydrogen production: Multi-objective optimization and exergoeconomic analysis[J]. Energy Conversion and Management, 2025, 337: 119900. |
| [6] | Wang Z Y, Sun G, Lewis N H C, et al. Water-mediated ion transport in an anion exchange membrane[J]. Nature Communications, 2025, 16: 1099. |
| [7] | Kim H, Jeon S, Choi J, et al. Interfacially Assembled Anion Exchange Membranes for Water Electrolysis[J]. ACS Nano, 2024, 18(47): 32694-32704. |
| [8] | Muhyuddin M, Santoro C, Osmieri L, et al. Anion-Exchange-Membrane Electrolysis with Alkali-Free Water Feed[J]. Chemical Reviews, 2025(15), 125: 6906-6976. |
| [9] | Altinisik H, Celebi C, Ozden A, et al. A review on membranes for anion exchange membrane water electrolyzers[J]. Renewable and Sustainable Energy Reviews, 2026, 226: 116277. |
| [10] | Song W J, Ge X L, Wu L, et al. Bottlenecks of commercializing anion exchange membranes for energy devices[J]. Joule, 2025, 9(8): 102051. |
| [11] | Moghaddam M S, Kafshgari M S, Bahari A, et al. Types, properties, and applications of non-precious oxygen reduction reaction electrocatalyst: A review[J]. Journal of Energy Chemistry, 2025, 107: 305-344. |
| [12] | Wang N, Ou P F, Miao R K, et al. Doping Shortens the Metal/Metal Distance and Promotes OH Coverage in Non-Noble Acidic Oxygen Evolution Reaction Catalysts[J]. Journal of the American Chemical Society, 2023(14), 145: 7829-7836. |
| [13] | Mao J Y, Wang X C, Nie Y T, et al. Anion exchange ionomers for anion exchange membrane fuel cells: properties, advances, and future directions[J]. International Journal of Hydrogen Energy, 2025, 196: 152593. |
| [14] | Liu L, Deng Y K, Zhang W L, et al. Highly alkali-stable polyolefin-based anion exchange membrane enabled by N-cyclic quaternary ammoniums for alkaline fuel cells[J]. Journal of Membrane Science, 2023, 672: 121441. |
| [15] | Cao D F, Nie F M, Liu M, et al. Crosslinked anion exchange membranes prepared from highly reactive polyethylene and polypropylene intermediates[J]. Journal of Membrane Science, 2022, 661: 120921. |
| [16] | Chen J J, Shen C H, Gao S. Polyepichlorohydrin grafted poly(phenylene oxide) to construct hydrophilic and hydrophobic microphase separation anion exchange membrane[J]. Reactive and Functional Polymers, 2024, 195: 105808. |
| [17] | Ng W K, Wong W Y, Loh K S, et al. A comprehensive overview of polyphenylene oxide-based anion exchange membranes from the perspective of ionic conductivity and alkaline stability[J]. Journal of Industrial and Engineering Chemistry, 2024, 138: 49-71. |
| [18] | Ma X Q, Xiang Q, Yuan W, et al. Localized stacked hyper branched anion exchange membrane for fuel cell[J]. Journal of Membrane Science, 2024, 694: 122432. |
| [19] | Yuan C L, Chen Y H, Lu X L, et al. Reducing hydroxide transport resistance by introducing high fractional free volume into anion exchange membranes[J]. Journal of Membrane Science, 2024, 701: 122769. |
| [20] | Sun S Y, Zhao J L, Wu J Y, et al. Covalent organic frameworks engineered poly(aryl piperidinium) ion-conducting networks via hydrogen-bonding for anion exchange membrane fuel cells[J]. Journal of Membrane Science, 2025, 736: 124623. |
| [21] | Lu X L, Zhang Y, Ma X Q, et al. Hydrogen Bond Network Assisted Ultrafast Ion Transport of Anion Exchange Membrane Grafting with Covalent Organic Frameworks for Hydrogen Conversion[J]. Angewandte Chemie International Edition, 2025, 137(21): e202503372. |
| [22] | Yuan C L, Ma X Q, Shen Z D, et al. Sandwich-Like Free-Standing Covalent Organic Framework Anion Exchange Membranes for Water Electrolysis[J]. Angewandte Chemie International Edition, 2025, 64(48): e202513244. |
| [23] | Wang X Y, Shi B B, Yang H, et al. Assembling covalent organic framework membranes with superior ion exchange capacity[J]. Nature Communications, 2022, 13: 1020. |
| [24] | Ma H P, Liu B L, Li B, et al. Cationic Covalent Organic Frameworks: A Simple Platform of Anionic Exchange for Porosity Tuning and Proton Conduction[J]. Journal of the American Chemical Society, 2016, 138(18): 5897-5903. |
| [25] | Chen W T, Liu Q, Pang B, et al. De Novo Design of Aminopropyl Quaternary Ammonium-Functionalized Covalent Organic Frameworks for Enhanced Polybenzimidazole Anion Exchange Membranes[J]. Small, 2025, 21(1): 2407260. |
| [26] | Li Z L, Li H, Guan X Y, et al. Three-Dimensional Ionic Covalent Organic Frameworks for Rapid, Reversible, and Selective Ion Exchange[J]. Journal of the American Chemical Society, 2017, 139(49): 17771-17774. |
| [27] | Liu Y J, Gao W T, Zhu A M, et al. High-performance di-piperidinium-crosslinked poly(p-terphenyl piperidinium) anion exchange membranes[J]. Journal of Membrane Science, 2023, 687: 122045. |
| [28] | Ma X Q, Lu X L, Liang S M, et al. Achieving ultra-low oxygen transport resistance of fuel cells by microporous covalent organic framework ionomers[J]. Chemical Science, 2025, 16(46): 22111-22118. |
| [29] | Fan C Y, Wu H, Guan J Y, et al. Scalable Fabrication of Crystalline COF Membranes from Amorphous Polymeric Membranes[J]. Angewandte Chemie International Edition, 2021, 60(33): 18051-18058. |
| [30] | Gao W T, Gao X L, Zhang Q G, et al. Durable poly(binaphthyl-co-p-terphenyl piperidinium)-based anion exchange membranes with dual side chains[J]. Journal of Energy Chemistry, 2024, 89: 324-335. |
| [31] | Li Q H, Hu M X, Ge C X, et al. Ionomer degradation in catalyst layers of anion exchange membrane fuel cells[J]. Chemical Science, 2023, 14(38): 10429-10434. |
| [32] | Zhang K Y, Yu W S, Ge X L, et al. DFT insight of hydroxide degradation pathways for heterocyclic quaternary ammonium cations in anion exchange membranes[J]. Journal of Membrane Science, 2023, 678: 121672. |
| [33] | Luo X Y, Rojas-Carbonell S, Yan Y S, et al. Structure-transport relationships of poly(aryl piperidinium) anion-exchange membranes: Eeffect of anions and hydration[J]. Journal of Membrane Science, 2020, 598: 117680. |
| [1] | 邹家庆, 张肇钰, 张建国, 张博宇, 刘定胜, 毛庆, 王挺, 李建军. 碱水制氢电解槽极板通道中气泡的生成及演化性质[J]. 化工学报, 2025, 76(9): 4786-4799. |
| [2] | 张彬怡, 孙少东, 姚谦, 蔡文河, 张惠宇, 李成新. 煤制甲醇耦合固体氧化物燃料电池混合系统研究[J]. 化工学报, 2025, 76(9): 4658-4669. |
| [3] | 周奕彤, 周明熙, 刘若晨, 叶爽, 黄伟光. 光伏与电网协同驱动氢基直接还原铁炼钢的技术经济分析[J]. 化工学报, 2025, 76(8): 4318-4330. |
| [4] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [5] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [6] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [7] | 陆学瑞, 周帼彦, 方琦, 俞孟正, 张秀成, 涂善东. 固体氧化物燃料电池外重整器积炭效应数值模拟研究[J]. 化工学报, 2025, 76(7): 3295-3304. |
| [8] | 王子恒, 李文怀, 周嵬. 图形电极在固体氧化物燃料电池中的应用[J]. 化工学报, 2025, 76(7): 3153-3171. |
| [9] | 陈佳祥, 周伟, 张学伟, 王丽杰, 黄玉明, 于洋, 孙苗婷, 李宛静, 袁骏舒, 张宏博, 孟晓晓, 高继慧, 赵广播. 脉冲电压下二维PEMWE模型的制氢特性仿真研究[J]. 化工学报, 2025, 76(7): 3521-3530. |
| [10] | 王珺仪, 夏章讯, 景粉宁, 王素力. 基于重整气的高温聚合物电解质膜燃料电池电化学阻抗谱弛豫时间分布研究[J]. 化工学报, 2025, 76(7): 3509-3520. |
| [11] | 吴天灏, 叶霆威, 林延, 黄振. 生物质化学链气化原位补氢制H2/CO可控合成气[J]. 化工学报, 2025, 76(7): 3498-3508. |
| [12] | 廖鹏伟, 刘庆辉, 潘安, 王嘉岳, 符小贵, 杨思宇, 余皓. 考虑不确定性的风电制氢系统:多时间尺度运行策略[J]. 化工学报, 2025, 76(6): 2743-2754. |
| [13] | 宋粉红, 王文光, 郭亮, 范晶. C元素修饰g-C3N4对TiO2的调控及复合材料光催化产氢性能研究[J]. 化工学报, 2025, 76(6): 2983-2994. |
| [14] | 梁铣, 张晓燕, 魏亦军, 郑云芳, 高全涵, 徐迈, 王凤武. 碱性膜燃料电池中聚电解质的耐久性研究进展[J]. 化工学报, 2025, 76(4): 1447-1462. |
| [15] | 赵鹏飞, 戚若玫, 郭新锋, 方虎, 徐庐飞, 李潇, 林今. 千标方级碱性水电解制氢系统氧中氢杂质分析[J]. 化工学报, 2025, 76(4): 1765-1778. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号