化工学报 ›› 2025, Vol. 76 ›› Issue (8): 4081-4094.DOI: 10.11949/0438-1157.20241525
杨宁1,2( ), 李皓男1, LIN Xiao3(
), 李皓男1, LIN Xiao3( ), GEORGIADOU Stella2, LIN Wen-Feng2,4,5
), GEORGIADOU Stella2, LIN Wen-Feng2,4,5
收稿日期:2024-12-30
修回日期:2025-04-17
出版日期:2025-08-25
发布日期:2025-09-17
通讯作者:
LIN Xiao
作者简介:杨宁 (1989—),男,博士,副教授,1020219438@qq.com
基金资助:
Ning YANG1,2( ), Haonan LI1, Xiao LIN3(
), Haonan LI1, Xiao LIN3( ), Stella GEORGIADOU2, Wen-Feng LIN2,4,5
), Stella GEORGIADOU2, Wen-Feng LIN2,4,5
Received:2024-12-30
Revised:2025-04-17
Online:2025-08-25
Published:2025-09-17
Contact:
Xiao LIN
摘要:
采用模板碳化和水热法将塑料矿泉水瓶转化为氮掺杂多孔碳材料(PNAC),然后在此基础上利用水热法和退火法成功制备了负载在塑料衍生氮掺杂碳上的CoMoO4纳米颗粒(CoMoO4@PNAC)复合材料,并用于电解水析氢催化剂。合成的CoMoO4@PNAC复合材料在1 mol/L KOH的碱性电解液中表现出良好的析氢催化活性,仅需要162 mV的过电位就可以达到10 mA/cm2的电流密度,并表现出良好的稳定性。PNAC提供了良好的电子转移通道,其卷曲的纳米片形状可以对CoMoO4纳米颗粒附近的电解液产生扰动,加快电解液与活性位点之间的质量交换,促进气泡产物的快速脱离。DFT计算表明,与CoMoO4相比,CoMoO4@PNAC具有更低的ΔGH*,DOS曲线显示,PNAC的引入填充了纯CoMoO4之中的态密度间隙,重新调整了电子分布,费米能级附近的态密度增加,提高了可用电子数量,释放出CoMoO4的析氢催化潜力,进而使得CoMoO4@PNAC具有良好的析氢催化性能。
中图分类号:
杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094.
Ning YANG, Haonan LI, Xiao LIN, Stella GEORGIADOU, Wen-Feng LIN. Application of plastic-derived carbon@CoMoO4 composites as an efficient electrocatalyst for hydrogen evolution reaction in water electrolysis[J]. CIESC Journal, 2025, 76(8): 4081-4094.
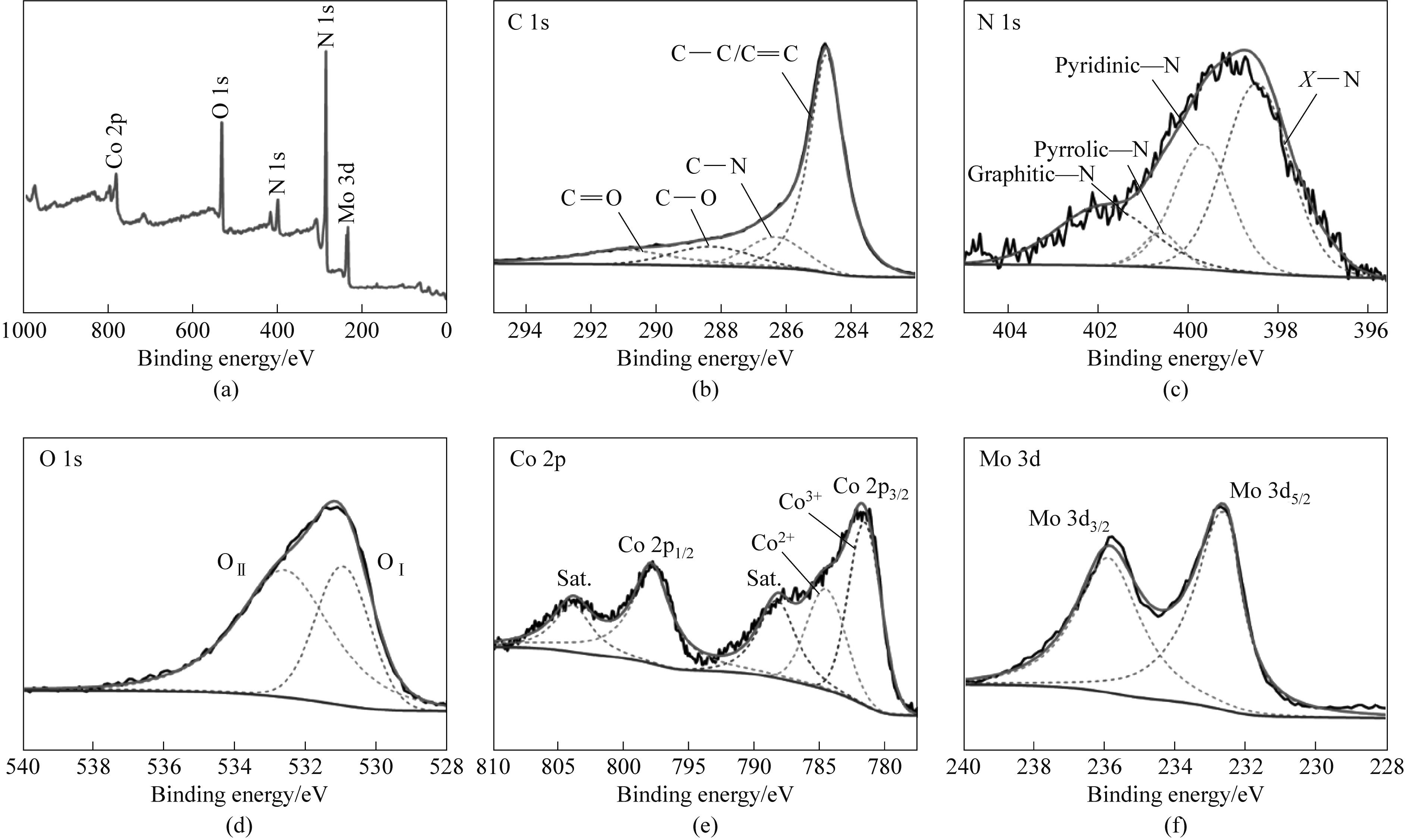
图7 CoMoO4@PNAC的XPS全谱(a)和C 1s、N 1s、O 1s、Co 2p、Mo 3d的XPS精细谱[(b)~(f)]
Fig.7 Survey XPS spectra (a) and high-resolution XPS spectra of C 1s,N 1s,O 1s,Co 2p,Mo 3d [(b)—(f)] of CoMoO4@PNAC
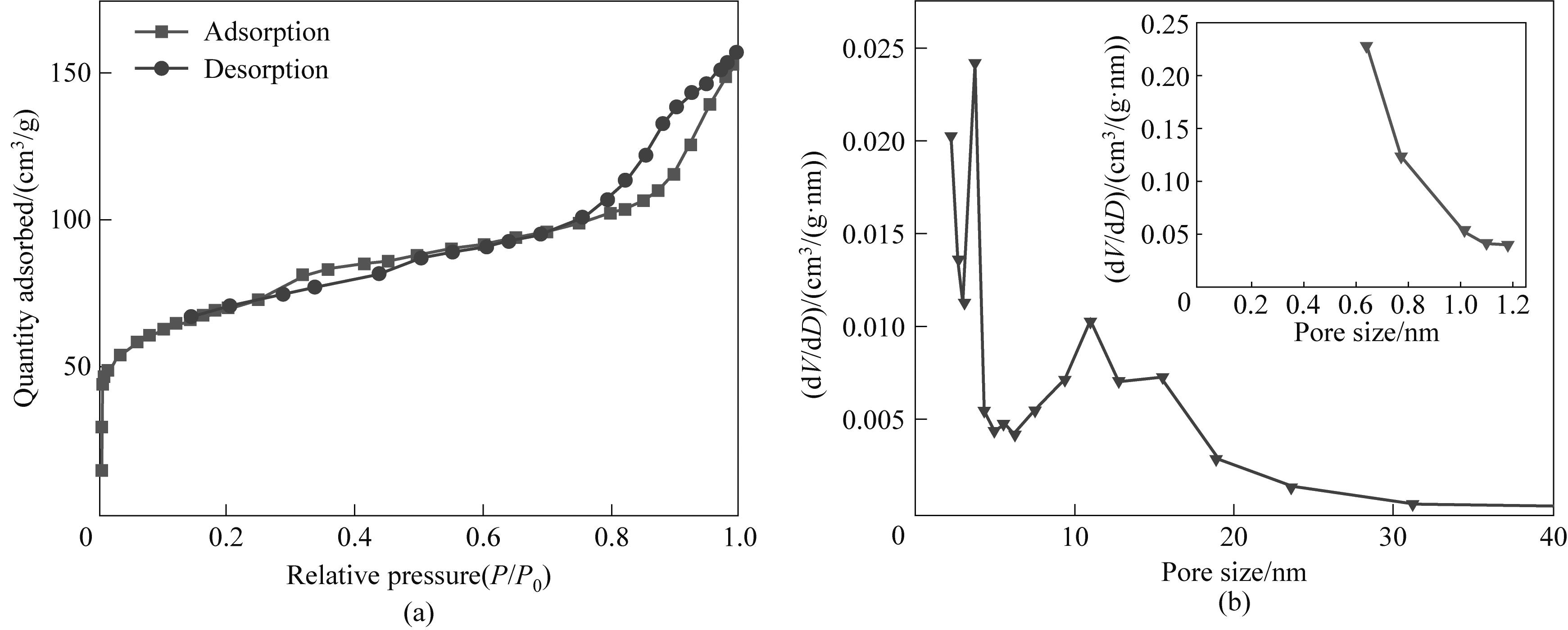
图8 CoMoO4@PNAC的氮气吸附-脱附等温线(a)和孔径分布曲线(b)
Fig.8 Nitrogen adsorption-desorption isotherm (a) and the corresponding pore size distribution curve (b) obtained on CoMoO4@PNAC
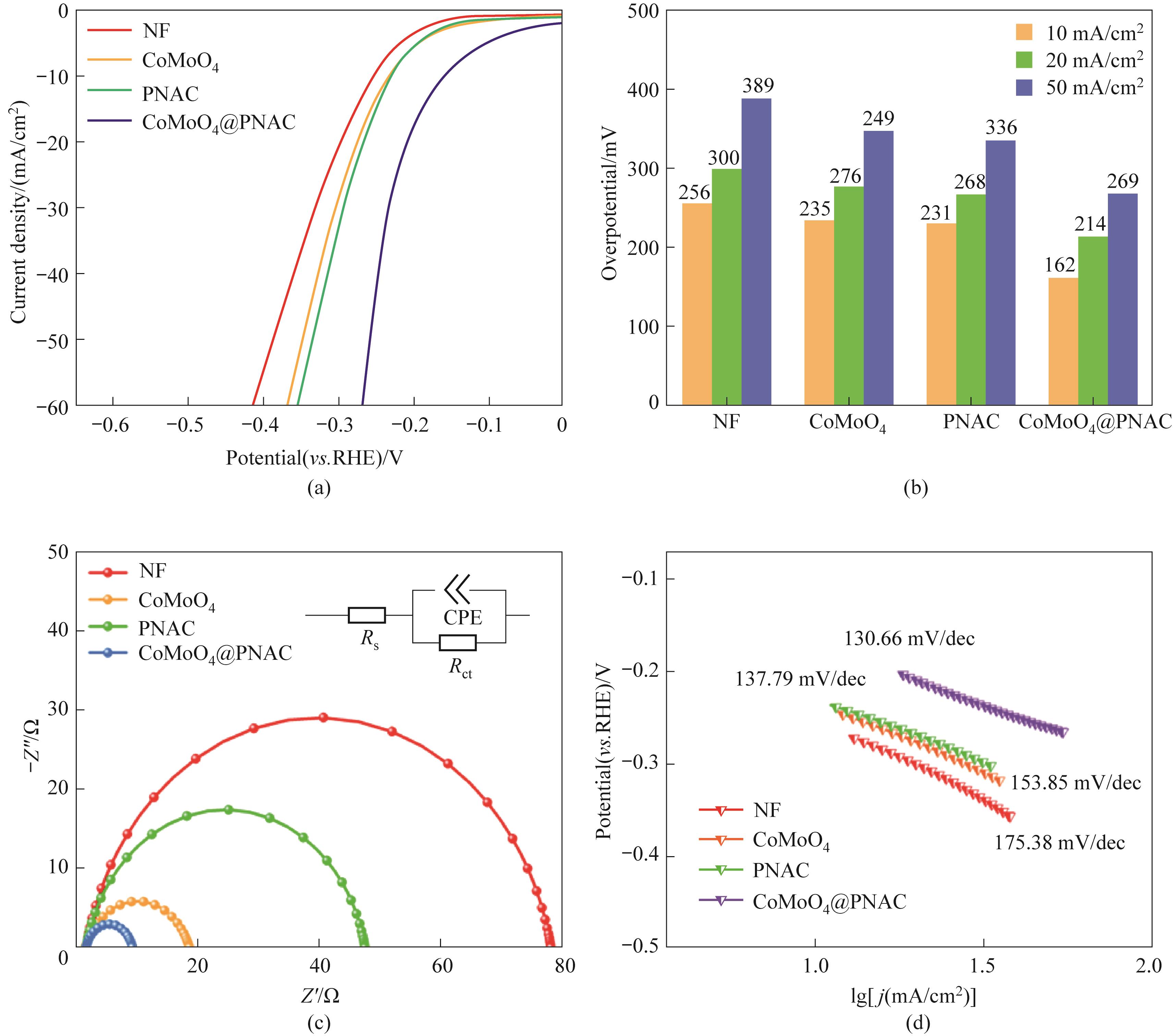
图9 制备的样品在1 mol/L KOH 的碱性电解液中进行电化学测试:(a)样品的极化曲线; (b)过电位; (c)EIS曲线; (d)Tafel斜率
Fig.9 Electrochemical measurements of the as-prepared samples for hydrogen evolution reaction in 1 mol/L KOH aqueous solution: (a) polarization curves; (b) overpotential; (c) EIS curves; (d) Tafel plots
| 催化剂 | 电解液 | η10/mV | Tafel斜率/(mV/dec) | 文献 |
|---|---|---|---|---|
| CMO x CNT | 1 mol/L KOH | 171 | 69.9 | [ |
| CoP@CoMoO4 HNTs | 1 mol/L KOH | 120 | 34 | [ |
| CoMoO4/N, S co-doped carbon | 1 mol/L KOH | 89 | 32 | [ |
| Co0.5Zn0.5MoO4 | 1 mol/L KOH | 204 | 162.7 | [ |
| N,S-NCO@CMO | 1 mol/L KOH | 100 | 64 | [ |
| CoMoO4@PNAC | 1 mol/L KOH | 162 | 130.66 | 本工作 |
表1 CoMoO4基催化剂的部分研究
Table 1 Partial studies on CoMoO4-based catalysts
| 催化剂 | 电解液 | η10/mV | Tafel斜率/(mV/dec) | 文献 |
|---|---|---|---|---|
| CMO x CNT | 1 mol/L KOH | 171 | 69.9 | [ |
| CoP@CoMoO4 HNTs | 1 mol/L KOH | 120 | 34 | [ |
| CoMoO4/N, S co-doped carbon | 1 mol/L KOH | 89 | 32 | [ |
| Co0.5Zn0.5MoO4 | 1 mol/L KOH | 204 | 162.7 | [ |
| N,S-NCO@CMO | 1 mol/L KOH | 100 | 64 | [ |
| CoMoO4@PNAC | 1 mol/L KOH | 162 | 130.66 | 本工作 |
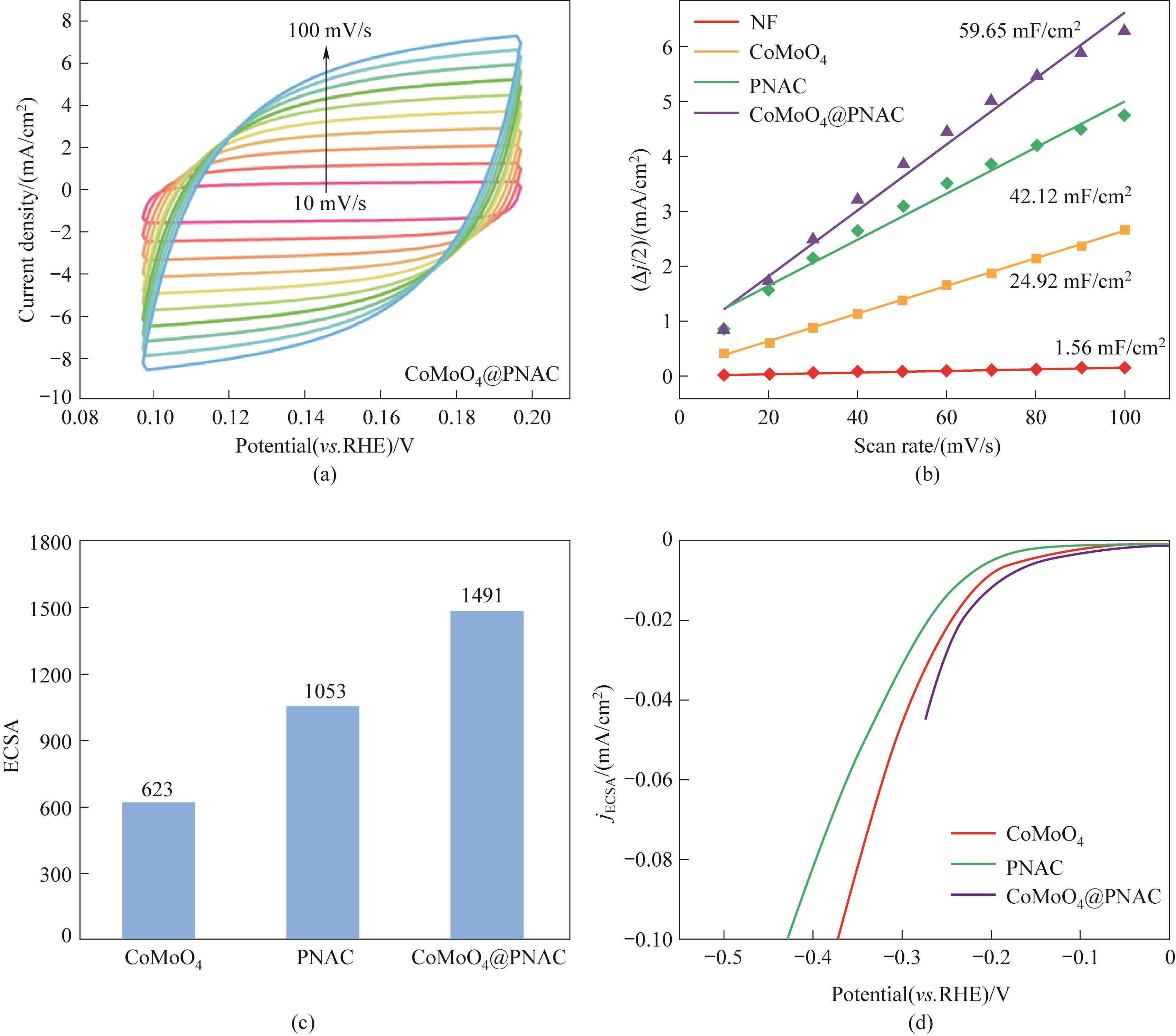
图10 CoMoO4@PNAC的CV曲线(a); 样品的Cdl(b); CoMoO4、PNAC与CoMoO4@PNAC的ECSA(c); 归一化HER极化曲线(d)
Fig.10 CV curve obtained on CoMoO4@PNAC (a); Cdl of the sample (b); ECSA of CoMoO4, PNAC and CoMoO4@PNAC (c); Normalized HER polarization curve (d)
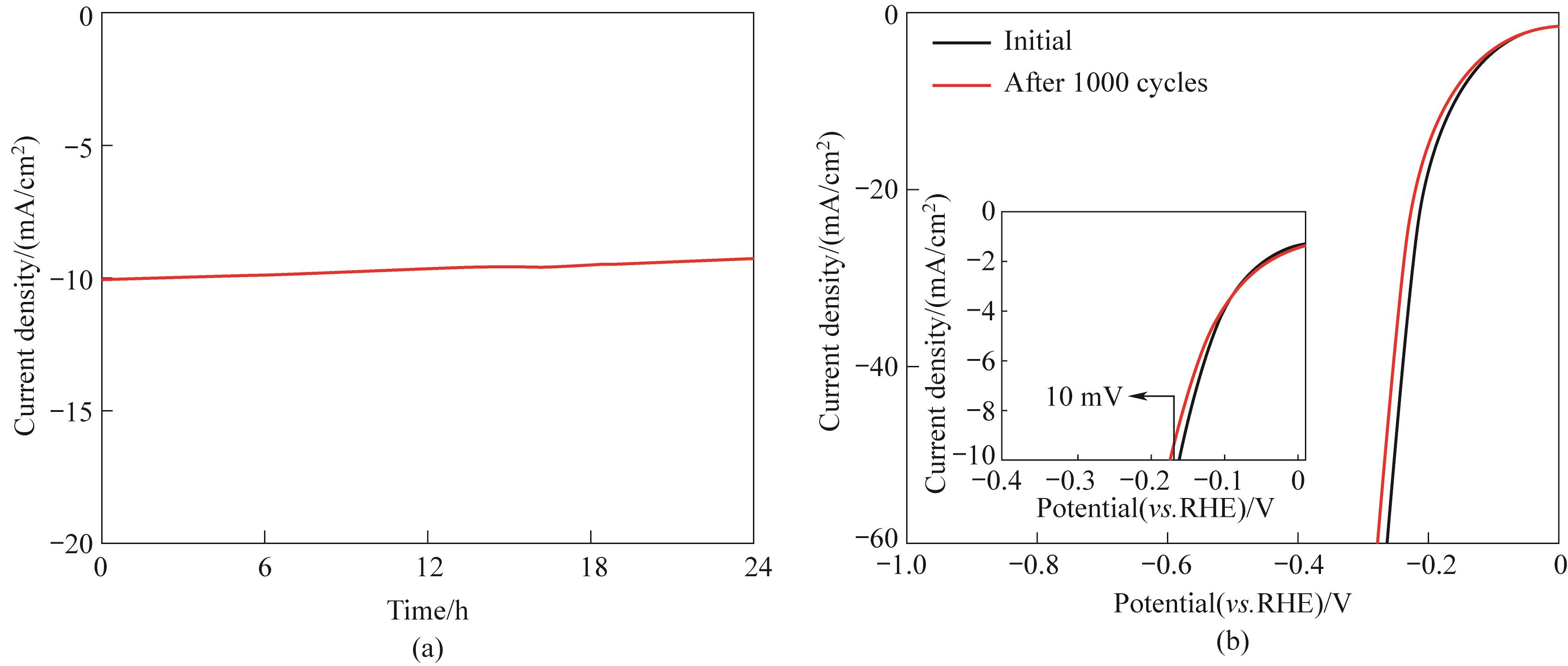
图11 CoMoO4@PNAC在1 mol/L KOH中的i-t曲线(a); CoMoO4@PNAC经过1000次循环前后的极化曲线(b)
Fig.11 The i-t curves obtained on CoMoO4@PNAC in 1 mol/L KOH (a) and polarization curves obtained before and after 1000 CV cycles (b)

图12 样品表面的吸附模型(a);氢吸附自由能(b); DOS曲线(c)
Fig.12 Models of the structures for the adsorption on the sample surface (a); hydrogen adsorption free energy (b); DOS curve (c)
| [1] | Truong H B, Tran N T, Do H H. Recent advancements and perspectives of hydrogen evolution reaction electrocatalysts based on molybdenum phosphides[J]. International Journal of Hydrogen Energy, 2024, 80: 696-711. |
| [2] | 张正, 宋凌珺. 电解水制氢技术: 进展、挑战与未来展望[J]. 工程科学学报, 2025, 47(2): 282-295. |
| Zhang Z, Song L J. Hydrogen production by water electrolysis: advances, challenges and future prospects[J]. Chinese Journal of Engineering, 2025, 47(2): 282-295. | |
| [3] | Zhao G Q, Rui K, Dou S X, et al. Heterostructures for electrochemical hydrogen evolution reaction: a review[J]. Advanced Functional Materials, 2018, 28(43): 1803291. |
| [4] | Strmcnik D, Lopes P P, Genorio B, et al. Design principles for hydrogen evolution reaction catalyst materials[J]. Nano Energy, 2016, 29: 29-36. |
| [5] | Gao G L, Zhao G Z, Zhu G, et al. Recent advancements in noble-metal electrocatalysts for alkaline hydrogen evolution reaction[J]. Chinese Chemical Letters, 2025, 36(1): 109557. |
| [6] | Chen J Y C, Miller J T, Gerken J B, et al. Inverse spinel NiFeAlO4 as a highly active oxygen evolution electrocatalyst: promotion of activity by a redox-inert metal ion[J]. Energy & Environmental Science, 2014, 7(4): 1382-1386. |
| [7] | Upadhyay S, Ahmad Mir R, Kumar N, et al. Reusing waste plastic bottle to reduce MoO3 into carbon-supported MoO2 nanoparticles for efficient water electrolysis[J]. Surfaces and Interfaces, 2023, 42: 103297. |
| [8] | Jin Y S, Wang H T, Li J J, et al. Porous MoO2 nanosheets as non-noble bifunctional electrocatalysts for overall water splitting[J]. Advanced Materials, 2016, 28(19): 3785-3790. |
| [9] | Zhang C, Liu Y, Wang J M, et al. A well-designed fencelike Co3O4@MoO3 derived from Co foam for enhanced electrocatalytic HER[J]. Applied Surface Science, 2022, 595: 153532. |
| [10] | Jiang M H, Hu Z C, Zou Y J, et al. NiSe-modified CoMoO4 nanosheets as bifunctional electrocatalysts for hydrogen and oxygen evolution reactions[J]. Journal of Alloys and Compounds, 2024, 978: 173495. |
| [11] | Dashtian K, Ganjali M R, Albo J, et al. CoMoO4 nano-architecture-based supercapacitors: tunable properties, performance optimization, and prospective applications[J]. Journal of Energy Storage, 2024, 102: 114063. |
| [12] | Li W X, Wang X W, Hu Y C, et al. Hydrothermal synthesized of CoMoO4 microspheres as excellent electrode material for supercapacitor[J]. Nanoscale Research Letters, 2018, 13(1): 120. |
| [13] | You N, Cao S, Huang M Q, et al. Constructing P-CoMoO4@NiCoP heterostructure nanoarrays on Ni foam as efficient bifunctional electrocatalysts for overall water splitting[J]. Nano Materials Science, 2023, 5(3): 278-286. |
| [14] | Ray S K, Bastakoti B P. Improved supercapacitor and oxygen evolution reaction performances of morphology-controlled cobalt molybdate[J]. International Journal of Hydrogen Energy, 2024, 51: 1109-1118. |
| [15] | Li T Z, Dong Y Y, Zhang J J, et al. Carbon dots-based composites electrocatalysts in hydrogen evolution reaction and oxygen evolution reaction: a mini review[J]. International Journal of Hydrogen Energy, 2024, 77: 359-372. |
| [16] | Keivanimehr F, Habibzadeh S, Baghban A, et al. Electrocatalytic hydrogen evolution on the noble metal-free MoS2/carbon nanotube heterostructure: a theoretical study[J]. Scientific Reports, 2021, 11(1): 3958. |
| [17] | Reddy S, Song L, Kang L X, et al. Preparation of Mo2C-carbon nanomaterials for hydrogen evolution reaction[J]. Carbon Letters, 2019, 29(3): 225-232. |
| [18] | 杨妮娜, 左剑恶, 张艳艳, 等. 塑料老化过程及其环境危害研究进展[J]. 环境科学, 2025, 46(3): 1850-1860. |
| Yang N N, Zuo J E, Zhang Y Y, et al. Research progress on plastic aging processes and their environmental hazards[J]. Environmental Science, 2025, 46(3): 1850-1860. | |
| [19] | Lian Y M, Ni M, Huang Z H, et al. Polyethylene waste carbons with a mesoporous network towards highly efficient supercapacitors[J]. Chemical Engineering Journal, 2019, 366: 313-320. |
| [20] | Yu W L, Chen Z, Yu S T, et al. Highly dispersed Pt catalyst supported on nanoporous carbon derived from waste PET bottles for reductive alkylation[J]. RSC Advances, 2019, 9(53): 31092-31101. |
| [21] | Ahmad Mir R, Kaur G, Pandey O P. Facile process to utilize carbonaceous waste as a carbon source for the synthesis of low cost electrocatalyst for hydrogen production[J]. International Journal of Hydrogen Energy, 2020, 45(44): 23908-23919. |
| [22] | Yang Y X, An X Q, Wang D X, et al. Selenium-inducing activates molybdenum phosphide/nitrogen-doped porous carbon nanoparticles for boosting hydrogen generation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2023, 666: 131307. |
| [23] | Sui D, Luo R S, Xie S M, et al. Atomic ruthenium doping in collaboration with oxygen vacancy engineering boosts the hydrogen evolution reaction by optimizing H absorption[J]. Chemical Engineering Journal, 2024, 480: 148007. |
| [24] | Pi C R, Huang C, Yang Y X, et al. In situ formation of N-doped carbon-coated porous MoP nanowires: a highly efficient electrocatalyst for hydrogen evolution reaction in a wide pH range[J]. Applied Catalysis B: Environmental, 2020, 263: 118358. |
| [25] | Wu A P, Xie Y, Ma H, et al. Integrating the active OER and HER components as the heterostructures for the efficient overall water splitting[J]. Nano Energy, 2018, 44: 353-363. |
| [26] | Homayounfard A M, Maleki M, Ghanbari H, et al. Growth of few-layer flower-like MoS2 on heteroatom-doped activated carbon as a hydrogen evolution reaction electrode[J]. International Journal of Hydrogen Energy, 2024, 55: 1360-1370. |
| [27] | Tao Y H, Wang X G, Yue S N, et al. Molybdenum carbide nanoparticles supported on nitrogen-doped carbon as efficient electrocatalysts for hydrogen evolution reaction[J]. Journal of Electroanalytical Chemistry, 2019, 842: 89-97. |
| [28] | He X, Zeng K, Xie Y P, et al. The effects of temperature and molten salt on solar pyrolysis of lignite[J]. Energy, 2019, 181: 407-416. |
| [29] | Nti F, Anang D A, Han J I. Facilely synthesized NiMoO4/CoMoO4 nanorods as electrode material for high performance supercapacitor[J]. Journal of Alloys and Compounds, 2018, 742: 342-350. |
| [30] | Lv J L, Yang M, Suzuki K, et al. Synthesis of CoMoO4@RGO nanocomposites as high-performance supercapacitor electrodes[J]. Microporous and Mesoporous Materials, 2017, 242: 264-270. |
| [31] | Fioravanti F, Martínez S, Delgado S, et al. Effect of MoS2 in doped-reduced graphene oxide composites. Enhanced electrocatalysis for HER[J]. Electrochimica Acta, 2023, 441: 141781. |
| [32] | Zhang B, Liu G J, Jin B, et al. CoMoO4/rGO hybrid structure embellished with Cu nanoparticles: an electrocatalyst rich in oxygen vacancies towards enhanced oxygen evolution reaction[J]. Materials Letters, 2021, 293: 129741. |
| [33] | Yan D G, Feng D X, Khan D S U, et al. Polyoxometalate and resin-derived P-doped Mo2C@N-doped carbon as a highly efficient hydrogen-evolution reaction catalyst at all pH values[J]. Chemistry - An Asian Journal, 2018, 13(2): 158-163. |
| [34] | Zuo P, Liu Y F, Liu X L, et al. N, P-codoped molybdenum carbide nanoparticles loaded into N, P-codoped graphene for the enhanced electrocatalytic hydrogen evolution[J]. International Journal of Hydrogen Energy, 2022, 47(69): 29730-29740. |
| [35] | Chen C, Xin X, Cheng T, et al. The synergistic benefits of hydrate CoMoO4 and carbon nanotubes culminate in the creation of highly efficient electrocatalysts for hydrogen evolution[J]. Alexandria Engineering Journal, 2024, 87: 93-106. |
| [36] | Gao Z F, Zeng Z F, Xu X W, et al. Hollow nanotube arrays of CoP@CoMoO4 as advanced electrocatalyst for overall water splitting[J]. International Journal of Hydrogen Energy, 2024, 49: 260-271. |
| [37] | Zhang Z F, Ran J X, Fan E Z, et al. Mesoporous CoMoO4 hollow tubes derived from POMOFs as efficient electrocatalyst for overall water splitting[J]. Journal of Alloys and Compounds, 2023, 968: 172169. |
| [38] | Li J S, Zhao C X, Yang Y X, et al. Synthesis of monodispersed CoMoO4 nanoclusters on the ordered mesoporous carbons for environment-friendly supercapacitors[J]. Journal of Alloys and Compounds, 2019, 810: 151841. |
| [39] | Meng L X, Liu W W, Lu Y, et al. Lamellar-stacked cobalt-based nanopiles integrated with nitrogen/sulfur Co-doped graphene as a bifunctional electrocatalyst for ultralong-term zinc-air batteries[J]. Journal of Energy Chemistry, 2023, 81: 633-641. |
| [40] | Asen P. Cobalt molybdenum oxide/sponge-like nitrogen and sulfur Co-doped carbon composite as a bifunctional electrocatalyst for overall water splitting in alkaline media[J]. International Journal of Hydrogen Energy, 2025, 115: 1-9. |
| [41] | Chamani S, Sadeghi E, Unal U, et al. Tuning electrochemical hydrogen-evolution activity of CoMoO4 through Zn incorporation[J]. Catalysts, 2023, 13(5): 798. |
| [42] | Wang J, Xuan H C, Meng L X, et al. N, S Co-doped NiCo2O4@CoMoO4/NF hierarchical heterostructure as an efficient bifunctional electrocatalyst for overall water splitting[J]. International Journal of Hydrogen Energy, 2023, 48(22): 8144-8155. |
| [43] | Gao X C, Lu K X, Chen J J, et al. NiCoP–CoP heterostructural nanowires grown on hierarchical Ni foam as a novel electrocatalyst for efficient hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2021, 46(45): 23205-23213. |
| [44] | Yang X, Qiu R, Lan M J, et al. Direct synthesis of binder-free Ni-Fe-S on Ni foam as superior electrocatalysts for hydrogen evolution reaction[J]. International Journal of Hydrogen Energy, 2022, 47(86): 36556-36565. |
| [45] | Zhang L, Hu Z H, Huang J T, et al. Experimental and DFT studies of flower-like Ni-doped Mo2C on carbon fiber paper: a highly efficient and robust HER electrocatalyst modulated by Ni(NO3)2 concentration[J]. Journal of Advanced Ceramics, 2022, 11(8): 1294-1306. |
| [46] | Liu H C, Tan Z X, Niu Y X, et al. Ir-decorated MoS2 monolayer as a promising candidate to detect dissolved gas in transformer oil: a DFT study[J]. Chemical Physics Letters, 2023, 818: 140410. |
| [1] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [2] | 刘世昌, 李一白, 王靖, 刘永忠. 氢气驱动电化学捕碳系统的模块化设计与优化[J]. 化工学报, 2025, 76(8): 4108-4118. |
| [3] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| [4] | 刘建海, 王磊, 鲁朝金, 白志山, 张平雨. 耦合电化学与多相流模型的电解槽性能研究[J]. 化工学报, 2025, 76(8): 3885-3893. |
| [5] | 罗佳欣, 袁艳. 压电材料在固态金属二次电池中的研究进展[J]. 化工学报, 2025, 76(8): 3822-3833. |
| [6] | 周奕彤, 周明熙, 刘若晨, 叶爽, 黄伟光. 光伏与电网协同驱动氢基直接还原铁炼钢的技术经济分析[J]. 化工学报, 2025, 76(8): 4318-4330. |
| [7] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [8] | 李欣然, 常龙娇, 罗绍华, 李永兵, 杨瑞芬, 侯增磊, 邹杰. Ho掺杂诱导NCM622局域电子重构抑制阳离子混排的改性机制研究[J]. 化工学报, 2025, 76(7): 3733-3741. |
| [9] | 陈培强, 郑群, 姜玉廷, 熊春华, 陈今茂, 王旭东, 黄龙, 阮曼, 徐万里. 电液流量及电流密度对海水激活电池输出特性的影响[J]. 化工学报, 2025, 76(7): 3235-3245. |
| [10] | 王珺仪, 夏章讯, 景粉宁, 王素力. 基于重整气的高温聚合物电解质膜燃料电池电化学阻抗谱弛豫时间分布研究[J]. 化工学报, 2025, 76(7): 3509-3520. |
| [11] | 王子恒, 李文怀, 周嵬. 图形电极在固体氧化物燃料电池中的应用[J]. 化工学报, 2025, 76(7): 3153-3171. |
| [12] | 陈佳祥, 周伟, 张学伟, 王丽杰, 黄玉明, 于洋, 孙苗婷, 李宛静, 袁骏舒, 张宏博, 孟晓晓, 高继慧, 赵广播. 脉冲电压下二维PEMWE模型的制氢特性仿真研究[J]. 化工学报, 2025, 76(7): 3521-3530. |
| [13] | 吴天灏, 叶霆威, 林延, 黄振. 生物质化学链气化原位补氢制H2/CO可控合成气[J]. 化工学报, 2025, 76(7): 3498-3508. |
| [14] | 赵世颖, 左志帅, 贺梦颖, 安华良, 赵新强, 王延吉. Co-Pt/HAP的制备及其催化1,2-丙二醇氨化反应[J]. 化工学报, 2025, 76(7): 3305-3315. |
| [15] | 吴鹂霄, 燕溪溪, 张素娜, 徐一鸣, 钱佳颖, 乔永民, 王利军. 磷掺杂微晶石墨的制备及其在锂离子电池负极材料中的电化学性能研究[J]. 化工学报, 2025, 76(7): 3615-3625. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号