化工学报 ›› 2021, Vol. 72 ›› Issue (4): 2309-2316.DOI: 10.11949/0438-1157.20201053
收稿日期:2020-07-30
修回日期:2020-12-09
出版日期:2021-04-05
发布日期:2021-04-05
通讯作者:
车黎明
作者简介:茹绍青(1993—),男,硕士研究生,基金资助:
RU Shaoqing1( ),WU Yafei1,2,CHE Liming1(
),WU Yafei1,2,CHE Liming1( )
)
Received:2020-07-30
Revised:2020-12-09
Online:2021-04-05
Published:2021-04-05
Contact:
CHE Liming
摘要:
石蜡乳液是集储热与传热于一体的新型功能流体,具有广阔的应用前景。但是石蜡乳液在降温时会出现明显的过冷现象,降低了其储热与传热的性能。针对这个问题,用镁-铝层状双金属氢氧化物(Mg-Al LDHs)代替化学表面活性剂,采用高速剪切乳化法,制备了石蜡含量为30%(质量)的O/W型Pickering乳液;分别采用扫描电子显微镜、激光粒度分析仪、流变仪、全能稳定性分析仪和差示扫描量热仪对所制得的石蜡Pickering乳液的微观形貌、粒度分布、黏度、稳定性和热力学性质进行了表征。结果表明,当Mg-Al LDHs的含量从1%(质量)增加到5%(质量)时,石蜡Pickering乳液的粒度减小,黏度升高,稳定性得到改善;在石蜡Pickering乳液中,Mg-Al LDHs纳米颗粒吸附在石蜡液滴表面,诱导石蜡在降温时异相成核结晶,从而抑制了其过冷现象;所制备的石蜡Pickering乳液的相变焓值约为56.6 J·g-1,在测试的温度范围内(30~70℃)的平均表观比热容为6.08 J·g-1·K-1,是纯水的1.45倍,具有良好的储热性能。
中图分类号:
茹绍青, 武亚飞, 车黎明. 低过冷度石蜡Pickering乳液的制备和表征[J]. 化工学报, 2021, 72(4): 2309-2316.
RU Shaoqing, WU Yafei, CHE Liming. Preparation and characterization of paraffin Pickering emulsion without subcooling phenomenon[J]. CIESC Journal, 2021, 72(4): 2309-2316.
| 名称 | 规格 | 生产厂家 |
|---|---|---|
| 石蜡 | 熔点58~60℃ | 上海永华石蜡有限公司 |
| MgCl2·6H2O | AR | 国药集团化学试剂有限公司 |
| AlCl3·6H2O | AR | 国药集团化学试剂有限公司 |
| 氨水 | AR | 广东光华科技股份有限公司 |
| NaHCO3 | AR | 国药集团化学试剂有限公司 |
| Na2CO3 | AR | 国药集团化学试剂有限公司 |
| NaCl | AR | 国药集团化学试剂有限公司 |
| 去离子水 | 电阻率不小于18.2 MΩ·cm-1 | 自制 |
表1 原材料与试剂
Table 1 Materials and reagents used in this work
| 名称 | 规格 | 生产厂家 |
|---|---|---|
| 石蜡 | 熔点58~60℃ | 上海永华石蜡有限公司 |
| MgCl2·6H2O | AR | 国药集团化学试剂有限公司 |
| AlCl3·6H2O | AR | 国药集团化学试剂有限公司 |
| 氨水 | AR | 广东光华科技股份有限公司 |
| NaHCO3 | AR | 国药集团化学试剂有限公司 |
| Na2CO3 | AR | 国药集团化学试剂有限公司 |
| NaCl | AR | 国药集团化学试剂有限公司 |
| 去离子水 | 电阻率不小于18.2 MΩ·cm-1 | 自制 |
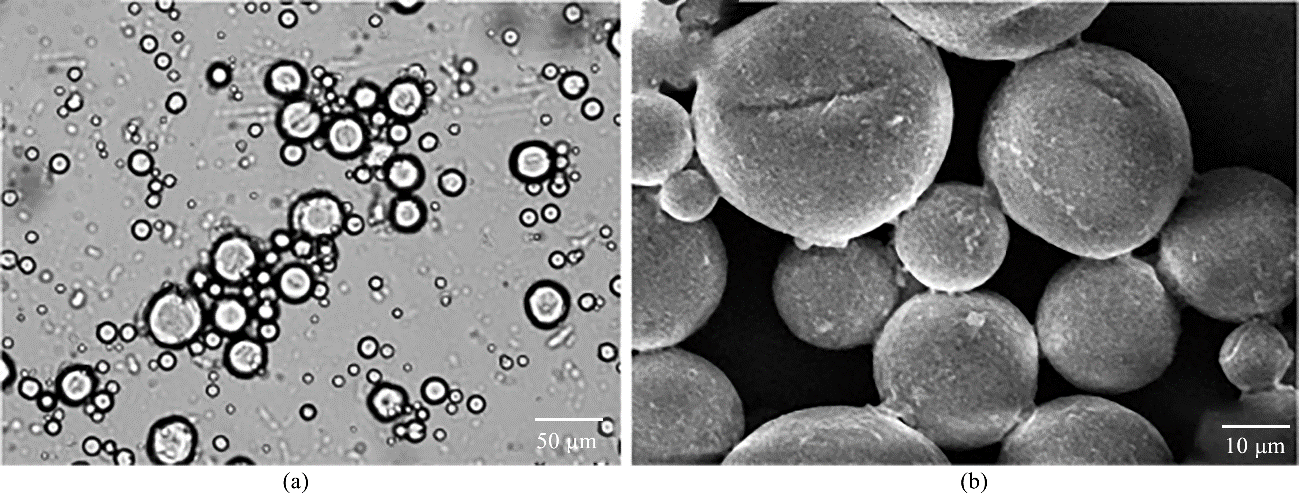
图3 Mg-Al LDHs含量为3%的石蜡Pickering乳液的光学显微照片(a)和扫描电子显微镜照片(b)
Fig.3 Optical microscopic image (a) and scanning electron microscope image (b) of paraffin Pickering emulsion with 3% of Mg-Al LDHs
| 样品 | D3,2平均粒度/μm | D4,3平均粒度/μm |
|---|---|---|
| 1% Mg-Al LDHs | 17.8 | 22.0 |
| 2% Mg-Al LDHs | 26.2 | 33.5 |
| 3% Mg-Al LDHs | 20.2 | 25.1 |
| 4% Mg-Al LDHs | 19.4 | 24.5 |
| 5% Mg-Al LDHs | 16.9 | 21.7 |
表2 不同Mg-Al LDHs含量的石蜡Pickering乳液的平均粒径
Table 2 Average particle sizes of paraffin Pickering emulsions with different amounts of Mg-Al LDHs
| 样品 | D3,2平均粒度/μm | D4,3平均粒度/μm |
|---|---|---|
| 1% Mg-Al LDHs | 17.8 | 22.0 |
| 2% Mg-Al LDHs | 26.2 | 33.5 |
| 3% Mg-Al LDHs | 20.2 | 25.1 |
| 4% Mg-Al LDHs | 19.4 | 24.5 |
| 5% Mg-Al LDHs | 16.9 | 21.7 |
| 样品 | 熔化温度(起始点)/℃ | 凝固温度(起始点)/℃ | 相变焓/(J·g-1) |
|---|---|---|---|
| 石蜡 | 52.4 | 55.0 | 126.6 |
| 1% Mg-Al LDHs | 53.0 | 54.1 | 52.5 |
| 2% Mg-Al LDHs | 52.5 | 54.4 | 52.6 |
| 3% Mg-Al LDHs | 52.4 | 54.8 | 57.3 |
| 4% Mg-Al LDHs | 51.8 | 54.6 | 63.8 |
| 5% Mg-Al LDHs | 52.8 | 54.8 | 56.6 |
表3 石蜡和石蜡Pickering乳液的热力学性质
Table 3 Thermal properties of paraffin and paraffin Pickering emulsions
| 样品 | 熔化温度(起始点)/℃ | 凝固温度(起始点)/℃ | 相变焓/(J·g-1) |
|---|---|---|---|
| 石蜡 | 52.4 | 55.0 | 126.6 |
| 1% Mg-Al LDHs | 53.0 | 54.1 | 52.5 |
| 2% Mg-Al LDHs | 52.5 | 54.4 | 52.6 |
| 3% Mg-Al LDHs | 52.4 | 54.8 | 57.3 |
| 4% Mg-Al LDHs | 51.8 | 54.6 | 63.8 |
| 5% Mg-Al LDHs | 52.8 | 54.8 | 56.6 |
| 1 | 邹得球, 肖睿, 冯自平, 等. 石蜡乳状液高温潜热输送材料的传热特性[J]. 化工学报, 2012, 63(4): 1019-1024. |
| Zou D Q, Xiao R, Feng Z P, et al. Heat transfer characteristics of high melting point paraffin emulsions for latent heat transportation[J]. CIESC Journal, 2012, 63(4): 1019-1024. | |
| 2 | 邹得球, 宋文吉, 肖睿, 等. 石蜡乳状液储热技术研究进展与应用前景[J]. 现代化工, 2008, 28(7): 12-15. |
| Zou D Q, Song W J, Xiao R, et al. Study progress in and wax emulsion as heat storage media its application[J]. Modern Chemical Industry, 2008, 28(7): 12-15. | |
| 3 | 黄莉. 石蜡/水相变乳液的制备与性能[J]. 化工学报, 2018, 69(4): 1749-1757. |
| Huang L. Preparation and properties of paraffin/water phase change emulsion[J]. CIESC Journal, 2018, 69(4): 1749-1757. | |
| 4 | Wang F X, Liu J, Fang X M, et al. Graphite nanoparticles-dispersed paraffin/water emulsion with enhanced thermal-physical property and photo-thermal performance[J]. Solar Energy Materials and Solar Cells, 2016, 147: 101-107. |
| 5 | 毛凌波, 梁志彬, 林敬堂, 等. 纳米材料增强石蜡相变乳状液在太阳能中的应用[J]. 太阳能学报, 2016, 37(1): 142-146. |
| Mao L B, Liang Z B, Lin J T, et al. Nanomaterials enhanced phase change wax emulsions used in the solar energy[J]. Acta Energiae Solaris Sinica, 2016, 37(1): 142-146. | |
| 6 | Qu Y, Wang S, Zhou D, et al. Experimental study on thermal conductivity of paraffin-based shape-stabilized phase change material with hybrid carbon nano-additives[J]. Renewable Energy, 2020, 146: 2637-2645. |
| 7 | Vasile V, Necula H, Badea A, et al. Experimental study of the heat transfer characteristics of a paraffin-in-water emulsion used as a secondary refrigerant[J]. International Journal of Refrigeration, 2018, 88: 1-7. |
| 8 | Huang L, Günther E, Doetsch C, et al. Subcooling in PCM emulsions(1): Experimental[J]. Thermochimica Acta, 2010, 509(1/2): 93-99. |
| 9 | Wu Q, Zhao D, Jiao X, et al. Preparation, properties, and supercooling prevention of phase change material n-octadecane microcapsules with peppermint fragrance scent[J]. Industrial & Engineering Chemistry Research, 2015, 54(33): 8130-8136. |
| 10 | 陈琳, 武亚飞, 车黎明. 一种测量石蜡相变乳液过冷度的新方法: 平衡态比容法[J]. 化工学报, 2019, 70(9): 3370-3376. |
| Chen L, Wu Y F, Che L M. A new approach to determine the degree of subcooling in phase-change paraffin emulsion: equilibrium volumetry[J]. CIESC Journal, 2019, 70(9): 3370-3376. | |
| 11 | Shao J J, Darkwa J, Kokogiannakis G. Review of phase change emulsions (PCMEs) and their applications in HVAC systems[J]. Energy and Buildings, 2015, 94: 200-217. |
| 12 | Huang L, Petermann M, Doetsch C. Evaluation of paraffin/water emulsion as a phase change slurry for cooling applications[J]. Energy, 2009, 34(9): 1145-1155. |
| 13 | Zhang X Y, Niu J L, Zhang S, et al. PCM in water emulsions: supercooling reduction effects of nano-additives, viscosity effects of surfactants and stability[J]. Advanced Engineering Materials, 2015, 17(2): 181-188. |
| 14 | Günther E, Huang L, Mehling H, et al. Subcooling in PCM emulsions (2): Interpretation in terms of nucleation theory[J]. Thermochimica Acta, 2011, 522(1/2): 199-204. |
| 15 | Hong Y, Wu W, Hu J J, et al. Controlling supercooling of encapsulated phase change nanoparticles for enhanced heat transfer[J]. Chemical Physics Letters, 2011, 504(4/5/6): 180-184. |
| 16 | Wang H, Zhao L, Song G L, et al. Organic-inorganic hybrid shell microencapsulated phase change materials prepared from SiO2/TiC-stabilized Pickering emulsion polymerization[J]. Solar Energy Materials and Solar Cells, 2018, 175: 102-110. |
| 17 | 田鹏飞, 刘温霞. Pickering石蜡乳状液的研制[J]. 造纸化学品, 2008, 20(5): 6-8, 30. |
| Tian P F, Liu W X. Preparation of paraffin Pickering emulsions[J]. Paper Chemicals, 2008, 20(5): 6-8, 30. | |
| 18 | Chatterjee P, Sowiak G A, Underhill P T. Effect of phase change on the rheology and stability of paraffin wax-in-water Pickering emulsions[J]. Rheologica Acta, 2017, 56(7/8): 601-613. |
| 19 | Sadeghpour A, Pirolt F, Glatter O. Submicrometer-sized Pickering emulsions stabilized by silica nanoparticles with adsorbed oleic acid[J]. Langmuir, 2013, 29(20): 6004-6012. |
| 20 | Zhang Q, Bai R X, Guo T, et al. Switchable Pickering emulsions stabilized by awakened TiO2 nanoparticle emulsifiers using UV/dark actuation[J]. ACS Applied Materials & Interfaces, 2015, 7(33): 18240-18246. |
| 21 | Xiao Z X, Cao H Y, Jiang X B, et al. Pickering emulsion formation of paraffin wax in an ethanol-water mixture stabilized by primary polymer particles and wax microspheres thereof[J]. Langmuir, 2018, 34(6): 2282-2289. |
| 22 | 杨衡. 镁铝层状双金属氢氧化物作为杀菌剂载体及其表面修饰杂化物的制备与表征[D]. 青岛: 青岛科技大学, 2009. |
| Yang H. Synthesis and characterization of layered double hydroxides as bacteridides delivery system and surface modification nanohybirds[D]. Qingdao: Qingdao University of Science & Technology, 2009. | |
| 23 | Yang F, Liu S Y, Xu J, et al. Pickering emulsions stabilized solely by layered double hydroxides particles: The effect of salt on emulsion formation and stability[J]. Journal of Colloid and Interface Science, 2006, 302(1): 159-169. |
| 24 | Yang F, Niu Q, Lan Q, et al. Effect of dispersion pH on the formation and stability of Pickering emulsions stabilized by layered double hydroxides particles[J]. Journal of Colloid and Interface Science, 2007, 306(2): 285-295. |
| 25 | Pourfaraj R, Fatemi S J, Kazemi S Y, et al. Synthesis of hexagonal mesoporous MgAl LDH nanoplatelets adsorbent for the effective adsorption of Brilliant Yellow[J]. Journal of Colloid and Interface Science, 2017, 508: 65-74. |
| 26 | 杨衡, 全贞兰. 镁铝层状双金属氢氧化物的表面修饰及表征[J]. 青岛科技大学学报(自然科学版), 2010, 31(1): 16-18. |
| Yang H, Quan Z L. Surface modification and characterization of Mg-Al-CO3-LDHs[J]. Journal of Qingdao University of Science and Technology (Natural Science Edition), 2010, 31(1): 16-18. | |
| 27 | 武亚飞. 石蜡相变乳液的制备及其过冷现象的机理研究[D]. 厦门: 厦门大学, 2018. |
| Wu Y F. Preparation of phase-change paraffin emulsion and the mechanism of subcooling[D]. Xiamen: Xiamen University, 2018. | |
| 28 | 奚羽, 郑国源, 吴彩红, 等. 高分散镁铝层状双氢氧化物的煅烧改性及其对甲基橙的吸附性能研究[J]. 人工晶体学报, 2020, 49(3): 532-541. |
| Xi Y, Zheng G Y, Wu C H, et al. Calcination modification of highly dispersed magnesium-aluminum layered double hydroxides and its adsorption properties for methyl orange[J]. Journal of Synthetic Crystals, 2020, 49(3): 532-541. | |
| 29 | 胡小平, 衡惠敏, 卢涛, 等. 镁铝层状双金属氢氧化物的制备及表征[J]. 中国粉体技术, 2012, 18(3): 31-35. |
| Hu X P, Heng H M, Lu T, et al. Synthesis and characterization of Mg-Al layered double hydroxides[J]. China Powder Science and Technology, 2012, 18(3): 31-35. | |
| 30 | 秦华, 马晓燕, 徐岩, 等. 以煤矸石为铝源制备镁铝层状双金属氢氧化物[J]. 硅酸盐通报, 2017, 36(3): 833-838. |
| Qin H, Ma X Y, Xu Y, et al. Preparation of mgnesia-alumina double-layered metal hydroxides with coal gangue as aluminum source[J]. Bulletin of the Chinese Ceramic Society, 2017, 36(3): 833-838. | |
| 31 | Machado J P E, de Freitas R A, Wypych F. Layered clay minerals, synthetic layered double hydroxides and hydroxide salts applied as Pickering emulsifiers[J]. Applied Clay Science, 2019, 169: 10-20. |
| 32 | Kraack H, Deutsch M, Sirota E B. n-Alkane homogeneous nucleation: crossover to polymer behavior[J]. Macromolecules, 2000, 33(16): 6174-6184. |
| [1] | 张双星, 刘舫辰, 张义飞, 杜文静. R-134a脉动热管相变蓄放热实验研究[J]. 化工学报, 2023, 74(S1): 165-171. |
| [2] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [3] | 林肯, 许肖永, 李强, 胡定华. 石蜡-膨胀石墨复合相变材料热导率研究[J]. 化工学报, 2021, 72(8): 4425-4432. |
| [4] | 罗东琴, 孙宁, 李秋红, 隋鹏亮, 姜秋艳, 隋晓飞, 李爱香. 非共价改性纳米颗粒稳定Pickering乳液的制备及可逆调控[J]. 化工学报, 2020, 71(4): 1859-1870. |
| [5] | 陈琳, 武亚飞, 车黎明. 一种测量石蜡相变乳液过冷度的新方法:平衡态比容法[J]. 化工学报, 2019, 70(9): 3370-3376. |
| [6] | 王伟浩, 杨鑫, 李飞, 孙梦梦, 王垚磊, 孟涛. 载酶海藻酸钙复合微球稳定水包油型Pickering乳液及其强化界面酶催化反应[J]. 化工学报, 2019, 70(12): 4777-4786. |
| [7] | 施尚, 余建祖, 陈梦东, 高红霞, 谢永奇. 基于泡沫铜/石蜡的锂电池热管理系统性能[J]. 化工学报, 2017, 68(7): 2678-2683. |
| [8] | 康亚盟, 刁彦华, 赵耀华, 汪顺. 纳米复合相变蓄热材料的制备与特性[J]. 化工学报, 2016, 67(S1): 372-378. |
| [9] | 翟俊菱, 沈敏敏, 孙淳宁, 王俊凤, 哈成勇. 纳米SiO2/OTAC协同稳定Pickering乳液聚合制备水基硅橡胶[J]. 化工学报, 2015, 66(4): 1585-1592. |
| [10] | 贺兴, 谭世语, 郑小刚, 董立春, 李少波, 陈红梅, 罗自萍. 石蜡乳液中直接沉淀制备单分散性的纳米氧化镁[J]. 化工学报, 2015, 66(2): 843-847. |
| [11] | 张方, 史铁钧, 周讯, 周海鸥, 吴竟. 二氧化钛纳米管稳定Pickering乳液制备PS/TiO2纳米管复合微球[J]. 化工学报, 2014, 65(4): 1526-1530. |
| [12] | 林兆云, 于得海, 李友明. 纳米Fe3O4稳定的Pickering型ASA乳液[J]. 化工学报, 2014, 65(2): 641-646. |
| [13] | 杜湖泽,常群羚,王胜杰,李春喜. 氯化石蜡-70脱氯制备石蜡烯及性能表征[J]. 化工进展, 2013, 32(12): 2842-2845. |
| [14] | 胡小冬, 高学农, 李得伦, 陈思婷. 石蜡/膨胀石墨定形相变材料的性能[J]. 化工学报, 2013, 64(10): 3831-3837. |
| [15] | 邹得球1,肖睿2,冯自平2,郭江荣1. 石蜡乳状液高温潜热输送材料的传热特性[J]. 化工学报, 2012, 63(4): 1019-1024. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号