化工学报 ›› 2023, Vol. 74 ›› Issue (9): 3956-3967.DOI: 10.11949/0438-1157.20230132
吴雷1,2( ), 刘姣1(
), 刘姣1( ), 李长聪1, 周军1,2(
), 李长聪1, 周军1,2( ), 叶干1, 刘田田1, 朱瑞玉1, 张秋利1,2, 宋永辉2,3
), 叶干1, 刘田田1, 朱瑞玉1, 张秋利1,2, 宋永辉2,3
收稿日期:2023-02-21
修回日期:2023-05-02
出版日期:2023-09-25
发布日期:2023-11-20
通讯作者:
周军
作者简介:吴雷(1988—),男,博士,工程师,wulei@xauat.edu.cn基金资助:
Lei WU1,2( ), Jiao LIU1(
), Jiao LIU1( ), Changcong LI1, Jun ZHOU1,2(
), Changcong LI1, Jun ZHOU1,2( ), Gan YE1, Tiantian LIU1, Ruiyu ZHU1, Qiuli ZHANG1,2, Yonghui SONG2,3
), Gan YE1, Tiantian LIU1, Ruiyu ZHU1, Qiuli ZHANG1,2, Yonghui SONG2,3
Received:2023-02-21
Revised:2023-05-02
Online:2023-09-25
Published:2023-11-20
Contact:
Jun ZHOU
摘要:
由于兰炭末粒径小、挥发分低等原因限制了其在工业生产中大规模利用。因此,兰炭末高附加值改性制备是当前极具吸引力和前景的研究课题。通过KOH协助微波热解低阶粉煤制备了含有碳纳米管的高附加值改性兰炭末,研究了KOH添加量(碱碳比)对改性兰炭末的形貌和结构、石墨化度、微晶结构和碳纳米管(CNTs)含量的影响,推测了改性兰炭末中碳纳米管的生成机制。结果表明:当碱碳比为1.0时,改性兰炭末中生成了直径在30~50 nm,长度约为几十微米的碳纳米管,其含量为3.01%(质量)。随着碱碳比增加,K的刻蚀和原煤所含矿物质产生的Fe3C对碳纳米管的生成具有促进作用,改性兰炭末的有序性有所增强,石墨层间距的交互作用增强,Raman光谱中出现碳纳米管的标志特征峰G′,表明生成了含有碳纳米管的高附加值改性兰炭末。此外,FT-IR光谱中改性兰炭末中的C—C、C—H和醚键C—O—C结构强度明显减弱。这种现象的产生可能有两方面原因:一是这些碳结构在催化剂的作用下成为了碳纳米管直接生成的固相碳源;二是这些碳结构热分解释放出CO和CH4,这些气体可作为碳纳米管的气相碳源。高附加值改性兰炭末中碳纳米管的生成可能是 “顶端生成”模型和“粒-线-管生成”模型共同作用的结果。
中图分类号:
吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967.
Lei WU, Jiao LIU, Changcong LI, Jun ZHOU, Gan YE, Tiantian LIU, Ruiyu ZHU, Qiuli ZHANG, Yonghui SONG. Catalytic microwave pyrolysis of low-rank pulverized coal for preparation of high value-added modified bluecoke powders containing carbon nanotubes[J]. CIESC Journal, 2023, 74(9): 3956-3967.
| Sample | Industrial analysis/%(mass) | Elemental analysis/%(mass) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FCad. | Mad. | Aad. | Vdaf. | Cdaf. | Hdaf. | Odaf. | Ndaf. | Sdaf. | |
| coal | 54.68 | 6.71 | 10.85 | 34.26 | 81.84 | 4.23 | 9.87 | 0.95 | 3.11 |
表1 原煤的工业和元素分析
Table 1 Proximate and elemental analysis of raw coal
| Sample | Industrial analysis/%(mass) | Elemental analysis/%(mass) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FCad. | Mad. | Aad. | Vdaf. | Cdaf. | Hdaf. | Odaf. | Ndaf. | Sdaf. | |
| coal | 54.68 | 6.71 | 10.85 | 34.26 | 81.84 | 4.23 | 9.87 | 0.95 | 3.11 |
| Composition | Content/%(mass) |
|---|---|
| Fe2O3 | 29.89 |
| Al2O3 | 7.25 |
| CaO | 25.29 |
| MgO | 1.36 |
| SiO2 | 12.01 |
| K2O | 0.08 |
| Na2O | 0.12 |
表2 原煤的灰分分析
Table 2 Ash analysis of raw coal
| Composition | Content/%(mass) |
|---|---|
| Fe2O3 | 29.89 |
| Al2O3 | 7.25 |
| CaO | 25.29 |
| MgO | 1.36 |
| SiO2 | 12.01 |
| K2O | 0.08 |
| Na2O | 0.12 |

图1 微波热解低阶煤制备富含碳纳米管的兰炭末的示意图
Fig.1 Schematic diagram of the preparation of carbon nanotube-rich bluecoke powders using microwave pyrolysis of low-rank coal
| Samples | ID1/IG | ID2/IG | ID3/IG | ID4/IG | IG/IAll |
|---|---|---|---|---|---|
| SC-0 | 2.145 | 0.284 | 1.519 | 0.543 | 0.151 |
| SC-0.4 | 2.206 | 0.215 | 1.489 | 0.434 | 0.130 |
| SC-0.6 | 1.228 | 0.252 | 1.884 | 0.352 | 0.168 |
| SC-0.8 | 1.156 | 0.174 | 1.279 | 0.774 | 0.245 |
| SC-1.0 | 1.152 | 0.135 | 0.791 | 0.324 | 0.247 |
| SC-1.2 | 1.181 | 0.230 | 0.829 | 0.274 | 0.222 |
| CNT | 1.150 | 0.154 | 1.390 | 0.266 | 0.240 |
表3 不同碱碳比下改性兰炭末的Raman谱图分峰拟合参数
Table 3 Raman spectral fitting parameters for modified bluecoke powders at different alkali-carbon ratios
| Samples | ID1/IG | ID2/IG | ID3/IG | ID4/IG | IG/IAll |
|---|---|---|---|---|---|
| SC-0 | 2.145 | 0.284 | 1.519 | 0.543 | 0.151 |
| SC-0.4 | 2.206 | 0.215 | 1.489 | 0.434 | 0.130 |
| SC-0.6 | 1.228 | 0.252 | 1.884 | 0.352 | 0.168 |
| SC-0.8 | 1.156 | 0.174 | 1.279 | 0.774 | 0.245 |
| SC-1.0 | 1.152 | 0.135 | 0.791 | 0.324 | 0.247 |
| SC-1.2 | 1.181 | 0.230 | 0.829 | 0.274 | 0.222 |
| CNT | 1.150 | 0.154 | 1.390 | 0.266 | 0.240 |
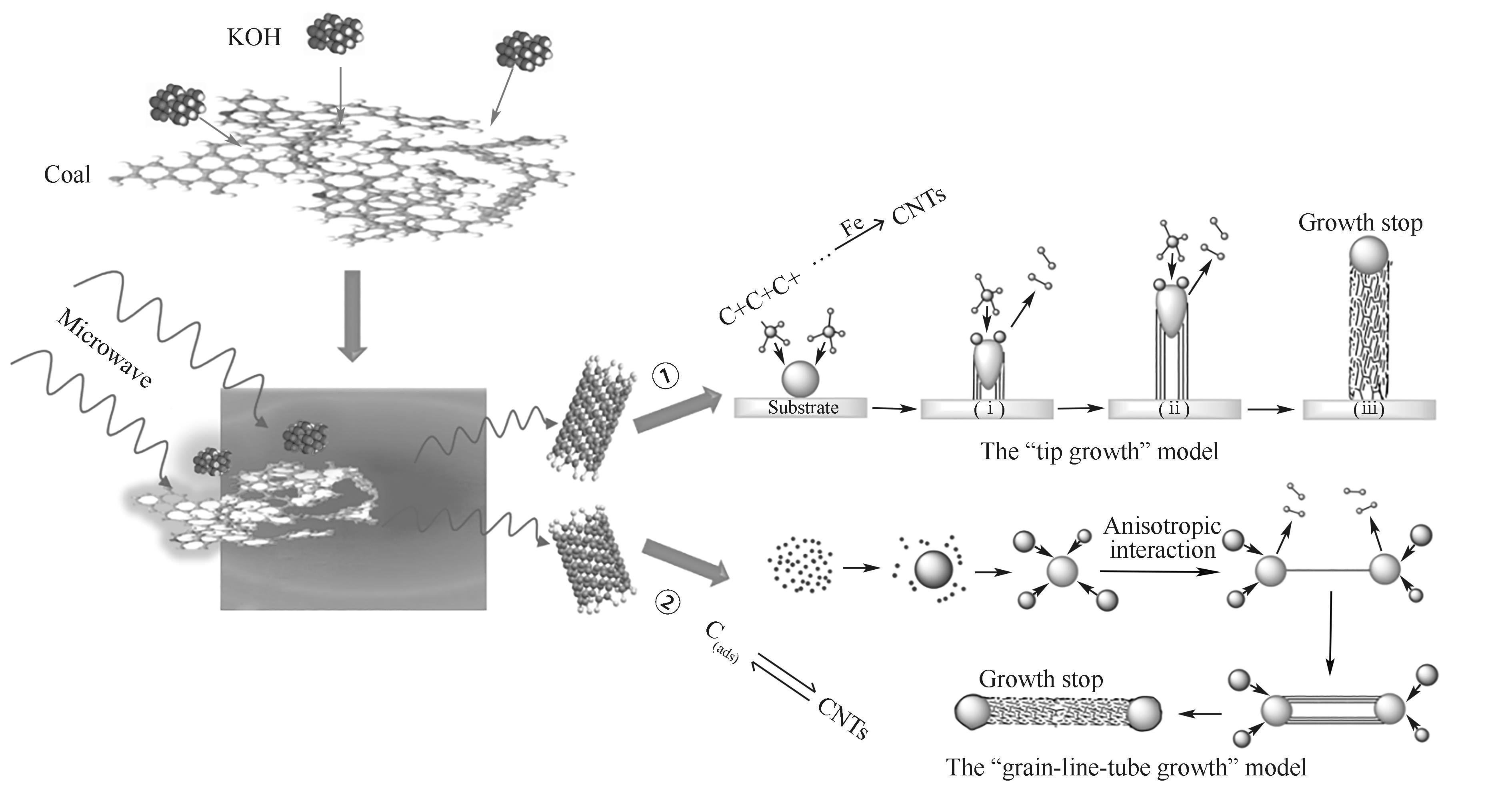
图9 KOH协助低阶煤微波热解所得兰炭末中碳纳米管的生成机制
Fig.9 Generation mechanism of carbon nanotube in bluecoke powders obtained by KOH-assisted microwave pyrolysis of low-rank coal
| 34 | Minutolo P, Commodo M, Santamaria A, et al. Characterization of flame-generated 2-D carbon nano-disks[J]. Carbon, 2014, 68: 138-148. |
| 35 | Brubaker Z E, Langford J J, Kapsimalis R J, et al. Quantitative analysis of Raman spectral parameters for carbon fibers: practical considerations and connection to mechanical properties[J]. Journal of Materials Science, 2021, 56(27): 15087-15121. |
| 36 | Beyssac O, Goffé B, Petitet J P, et al. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2003, 59(10): 2267-2276. |
| 37 | Meng D X, Yue C Y, Wang T, et al. Evolution of carbon structure and functional group during Shenmu lump coal pyrolysis[J]. Fuel, 2021, 287: 119538. |
| 38 | Zhang T K, Zhang Y F, Wang Q, et al. Mechanism of K-catalyzed transformation of solid carbon structure into carbon nanotubes in coal[J]. Fuel Processing Technology, 2020, 204: 106409. |
| 39 | Zhang T K, Wang Q, Li G Q, et al. Formation of carbon nanotubes from potassium catalyzed pyrolysis of bituminous coal[J]. Fuel, 2019, 239: 230-238. |
| 40 | He R Z, Deng J, Deng X L, et al. Effects of alkali and alkaline earth metals of inherent minerals on Fe-catalyzed coal pyrolysis[J]. Energy, 2022, 238: 121985. |
| 41 | Wang Z P, Ogata H, Morimoto S, et al. Nanocarbons from rice husk by microwave plasma irradiation: from graphene and carbon nanotubes to graphenated carbon nanotube hybrids[J]. Carbon, 2015, 94: 479-484. |
| 42 | Sun Y, Wang Y J, Ma H J, et al. Fe3C nanocrystals encapsulated in N-doped carbon nanofibers as high-efficient microwave absorbers with superior oxidation/corrosion resistance[J]. Carbon, 2021, 178: 515-527. |
| 43 | He L M, Hu S, Jiang L, et al. Carbon nanotubes formation and its influence on steam reforming of toluene over Ni/Al2O3 catalysts: roles of catalyst supports[J]. Fuel Processing Technology, 2018, 176: 7-14. |
| 44 | Lv X M, Zhang Y F, Wang Y, et al. Formation of coal-based carbon nanotubes by Fe-K catalyst[J]. Journal of Analytical and Applied Pyrolysis, 2022, 161: 105400. |
| 45 | Yuan J C, Wang Y, Tang M F, et al. Preparation of N, O co-doped carbon nanotubes and activated carbon composites with hierarchical porous structure for CO2 adsorption by coal pyrolysis[J]. Fuel, 2023, 333: 126465. |
| 46 | Zhou J, Wu L, Zhou J J, et al. Advances in coal catalytic microwave pyrolysis and its carbon-based absorbing microwave catalysts[J]. Chemical Industry and Engineering Progress, 2019, 38(9): 4060-4074. |
| 47 | Zhang P, Wu M D, Liang C, et al. In-situ exsolution of Fe-Ni alloy catalysts for H2 and carbon nanotube production from microwave plasma-initiated decomposition of plastic wastes[J]. Journal of Hazardous Materials, 2023, 445: 130609. |
| 1 | Jorschick H, Preuster P, Bösmann A, et al. Hydrogenation of aromatic and heteroaromatic compounds—a key process for future logistics of green hydrogen using liquid organic hydrogen carrier systems[J]. Sustainable Energy & Fuels, 2021, 5(5): 1311-1346. |
| 2 | Yang R R, Zhou J, Wu L, et al. Understanding effects of potassium activator on the porous structure and adsorption performance of bluecoke-based porous powder during microwave heating[J]. Journal of Molecular Liquids, 2022, 366: 120249. |
| 3 | Wu L, Liu J, Reddy B R, et al. Preparation of coal-based carbon nanotubes using catalytical pyrolysis: a brief review[J]. Fuel Processing Technology, 2022, 229: 107171. |
| 4 | Yang R R, Zhou J, Wu L, et al. Fabrication of developed porous carbon derived from bluecoke powder by microwave-assisted KOH activation for simulative organic wastewater treatment[J]. Diamond and Related Materials, 2022, 124: 108929. |
| 5 | Zhang X Y, Sun B K, Fan X, et al. Hierarchical porous carbon derived from coal and biomass for high performance supercapacitors[J]. Fuel, 2022, 311: 122552. |
| 6 | Ma M Y, Chai W C, Cao Y J. Structure and electrochemical property of coal-based activated carbon modified by nitric acid[C]//TMS 2021 150th Annual Meeting & Exhibition Supplemental Proceedings. Cham: Springer, 2021: 15-24. |
| 7 | Im U S, Kim J, Lee S H, et al. Preparation of activated carbon from needle coke via two-stage steam activation process[J]. Materials Letters, 2019, 237: 22-25. |
| 8 | Zhang G J, Qu J W, Du Y N, et al. Hydrogen production from CO2 reforming of methane over high pressure H2O2 modified different semi-cokes[J]. Journal of Industrial and Engineering Chemistry, 2014, 20(5): 2948-2957. |
| 9 | Reddy B R, Ashok I, Vinu R. Preparation of carbon nanostructures from medium and high ash Indian coals via microwave-assisted pyrolysis[J]. Advanced Powder Technology, 2020, 31(3): 1229-1240. |
| 10 | Shen X, Zhao Z, Li H, et al. Microwave-assisted pyrolysis of plastics with iron-based catalysts for hydrogen and carbon nanotubes production[J]. Materials Today Chemistry, 2022, 26: 101166. |
| 11 | Das T, Saikia B K, Baruah B P. Formation of carbon nano-balls and carbon nano-tubes from northeast Indian Tertiary coal: value added products from low grade coal[J]. Gondwana Research, 2016, 31: 295-304. |
| 12 | Deng H, Li G X, Yang H B, et al. Preparation of activated carbons from cotton stalk by microwave assisted KOH and K2CO3 activation[J]. Chemical Engineering Journal, 2010, 163(3): 373-381. |
| 13 | Ashok A, Kumar A, Ponraj J, et al. Synthesis and growth mechanism of bamboo like N-doped CNT/graphene nanostructure incorporated with hybrid metal nanoparticles for overall water splitting[J]. Carbon, 2020, 170: 452-463. |
| 14 | Nie H R, Cui M M, Russell T P. A route to rapid carbon nanotube growth[J]. Chemical Communications, 2013, 49(45): 5159-5161. |
| 15 | Bajpai R, Wagner H D. Fast growth of carbon nanotubes using a microwave oven[J]. Carbon, 2015, 82: 327-336. |
| 16 | Mahmood A, Muhmood T, Ahmad F. Carbon nanotubes heterojunction with graphene like carbon nitride for the enhancement of electrochemical and photocatalytic activity[J]. Materials Chemistry and Physics, 2022, 278: 125640. |
| 17 | Botas J A, Serrano D P, Guil-López R, et al. Methane catalytic decomposition over ordered mesoporous carbons: a promising route for hydrogen production[J]. International Journal of Hydrogen Energy, 2010, 35(18): 9788-9794. |
| 18 | Qiu J S, Wang Z Y, Zhao Z B, et al. Synthesis of double-walled carbon nanotubes from coal in hydrogen-free atmosphere[J]. Fuel, 2007, 86(1/2): 282-286. |
| 19 | Zhao Y, Liu L, Qiu P H, et al. Impacts of chemical fractionation on Zhundong coal’s chemical structure and pyrolysis reactivity[J]. Fuel Processing Technology, 2017, 155: 144-152. |
| 20 | Zhao J Y, Deng J, Chen L, et al. Correlation analysis of the functional groups and exothermic characteristics of bituminous coal molecules during high-temperature oxidation[J]. Energy, 2019, 181: 136-147. |
| 21 | Zhang J B, Jin L J, Cheng J, et al. Hierarchical porous carbons prepared from direct coal liquefaction residue and coal for supercapacitor electrodes[J]. Carbon, 2013, 55: 221-232. |
| 22 | Widayat, Satriadi H, Wibawa L P, et al. Oil and gas characteristics of coal with pyrolysis process[C]//AIP Conference Proceedings. AIP Publishing LLC, 2022, 2453(1): 020077. |
| 23 | Liu H P, Zhang L Y, Chen T P, et al. Experimental study on the fluidization behaviors of the superfine particles[J]. Chemical Engineering Journal, 2015, 262: 579-587. |
| 24 | Xia H Y, Wang K, Yang S H, et al. Formation of graphene flowers during high temperature activation of mesocarbon microbeads with KOH[J]. Microporous and Mesoporous Materials, 2016, 234: 384-391. |
| 25 | Bai Y, Yue H, Wang J, et al. Super-durable ultralong carbon nanotubes[J]. Science, 2020, 369(6507): 1104-1106. |
| 26 | Lin X C, Wang C H, Ideta K, et al. Insights into the functional group transformation of a Chinese brown coal during slow pyrolysis by combining various experiments[J]. Fuel, 2014, 118: 257-264. |
| 27 | Osswald S, Flahaut E, Gogotsi Y. In situ Raman spectroscopy study of oxidation of double-and single-wall carbon nanotubes[J]. Chemistry of Materials, 2006, 18(6): 1525-1533. |
| 28 | Xu L, Liu H Y, Jin Y, et al. Structural order and dielectric properties of coal chars[J]. Fuel, 2014, 137: 164-171. |
| 29 | Mohammadi S, Kolahdouz Z, Mohajerzadeh S. Hydrogenation-assisted unzipping of carbon nanotubes to realize graphene nano-sheets[J]. Journal of Materials Chemistry C, 2013, 1(7): 1309-1316. |
| 30 | Yao L S, Yi B K, Zhao X Q, et al. Microwave-assisted decomposition of waste plastic over Fe/FeAl2O4 to produce hydrogen and carbon nanotubes[J]. Journal of Analytical and Applied Pyrolysis, 2022, 165: 105577. |
| 31 | Lee S H, Park J, Kim H R, et al. Synthesis of carbon nanotube fibers using the direct spinning process based on design of experiment (DOE)[J]. Carbon, 2016, 100: 647-655. |
| 32 | Liu X H, Zheng Y, Liu Z H, et al. Study on the evolution of the char structure during hydrogasification process using Raman spectroscopy[J]. Fuel, 2015, 157: 97-106. |
| 33 | Sheng C D. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity[J]. Fuel, 2007, 86(15): 2316-2324. |
| [1] | 裴仁花, 王永洪, 张新儒, 李晋平. 碳纳米管/环糊精金属有机骨架协同强化混合基质膜的CO2分离[J]. 化工学报, 2022, 73(9): 3904-3914. |
| [2] | 肖皓宇, 杨海平, 张雄, 陈应泉, 王贤华, 陈汉平. 塑料催化热解制备高附加值产品的研究进展[J]. 化工学报, 2022, 73(8): 3461-3471. |
| [3] | 刘学安, 汤丽怡, 覃健, 唐大江, 童张法, 曲慧颖. 热解Ni/Co-ZIF-8制备碳纳米管桥连多孔碳及其在超级电容器中的应用[J]. 化工学报, 2022, 73(7): 3287-3297. |
| [4] | 蔡楚玥, 方晓明, 张正国, 凌子夜. CNTs阵列增强石蜡/硅橡胶复合相变垫片的散热性能研究[J]. 化工学报, 2022, 73(7): 2874-2884. |
| [5] | 石兴达, 陈华艳, 戈亚南, 武春瑞, 贾红友, 吕晓龙. 低界面热阻改性氮化铝和多壁碳纳米管充填PVDF构建杂化三维网络及其导热性能强化[J]. 化工学报, 2022, 73(5): 2262-2269. |
| [6] | 韩雪, 高生旺, 王国英, 夏训峰. 铈掺杂强化碳纳米管活化过一硫酸盐实验研究[J]. 化工学报, 2022, 73(4): 1743-1753. |
| [7] | 徐欢, 柯律, 张生辉, 张子林, 韩广东, 崔金声, 唐道远, 黄东辉, 高杰峰, 何新建. GO表面原位生长CNTs改善聚丙烯导热复合材料分散与界面形态[J]. 化工学报, 2022, 73(11): 5150-5157. |
| [8] | 吴诗德, 易峰, 平丹, 张逸飞, 郝健, 刘国际, 方少明. NH4Cl辅助热解制备镍-氮-碳纳米管催化剂及其电还原CO2性能[J]. 化工学报, 2022, 73(10): 4484-4497. |
| [9] | 周石杰, 任祯, 杨宇森, 卫敏. 不同形貌金属氧化物的制备及其在工业催化反应中的应用[J]. 化工学报, 2021, 72(6): 2972-3001. |
| [10] | 赵露, 宁国庆, 李兴洵. 掺硫碳纳米管作导电添加剂改进磷酸锰铁锂电化学性能[J]. 化工学报, 2021, 72(12): 6388-6398. |
| [11] | 石晓飞, 姜沁源, 李润, 崔一鸣, 刘青雄, 魏飞, 张如范. 碳纳米管水平阵列的结构控制生长:进展与展望[J]. 化工学报, 2021, 72(1): 86-115. |
| [12] | 赵惠忠, 雷敏, 黄天厚, 刘涛, 张敏. 复合吸附剂MWCNT/MgCl2的水蒸气吸附性能[J]. 化工学报, 2020, 71(S1): 272-281. |
| [13] | 陈志远, 颜冬, 钱凡, 李文翠. 高导电三明治状MnO2/CNTs/MnO2介孔材料的制备及其赝电容性能[J]. 化工学报, 2019, 70(12): 4864-4871. |
| [14] | 黄金, 李晓朋, 王婷, 胡艳鑫, 盛鑫鑫. 基于MWCNTs/PA复合材料铜表面处理的传热性能[J]. 化工学报, 2018, 69(7): 2956-2963. |
| [15] | 周丰, 黄慧敏, 钱飞跃, 沈耀良, 周晓吉, 李欣, 夏雪. 碳纳米管负载层结构对复合膜分离性能的影响研究[J]. 化工学报, 2018, 69(5): 2318-2326. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号