化工学报 ›› 2025, Vol. 76 ›› Issue (8): 4297-4309.DOI: 10.11949/0438-1157.20250054
王小令1,2( ), 王绍清2(
), 王绍清2( ), 赵云刚2,3, 常方哲2,4, 穆瑞峰2
), 赵云刚2,3, 常方哲2,4, 穆瑞峰2
收稿日期:2025-01-13
修回日期:2025-03-19
出版日期:2025-08-25
发布日期:2025-09-17
通讯作者:
王绍清
作者简介:王小令(1994—),男,博士,讲师,wangxiaoling@xju.edu.cn
基金资助:
Xiaoling WANG1,2( ), Shaoqing WANG2(
), Shaoqing WANG2( ), Yungang ZHAO2,3, Fangzhe CHANG2,4, Ruifeng MU2
), Yungang ZHAO2,3, Fangzhe CHANG2,4, Ruifeng MU2
Received:2025-01-13
Revised:2025-03-19
Online:2025-08-25
Published:2025-09-17
Contact:
Shaoqing WANG
摘要:
为揭示煤中有机Ca在煤加氢热解过程中的迁移转化机制,构建了含羧酸Ca的煤大分子结构模型,采用反应分子动力学(ReaxFF MD)模拟手段,基于不同升温模式从原子水平追踪了Ca在煤加氢热解过程中的运动轨迹;通过人为加载Ca型煤进行加氢热解实验,采用电感耦合等离子体光谱仪(ICP-OES)测定了产物中Ca元素的含量。模拟结果表明,在升温模拟过程中,有机Ca在高温下向无机Ca、气态烃Ca、焦油Ca迁变。原子Ca很难单独从焦炭中释放,并且极易和生成的H2O分子结合。在恒温模拟过程中,随着温度的升高,煤加氢热解过程中有机Ca迁变行为加剧,焦炭Ca大量向无机Ca和气态烃中的Ca转化。在较低温度下(1600~2200 K),需要持续一定时间有机Ca才会发生迁变,而在较高温度下(2500~2800 K)相应发生迁变过程需要的时间更短。通过分析热解实验中Ca的演化规律,初步验证模拟结果。此外,还探讨了显微组分对有机Ca迁移行为的影响,镜质体含有较多脂肪链,能热分解更多小分子碎片与Ca结合,而惰质体因芳香结构致密且活性位点较少,有机Ca的结合能力略弱。最终,从原子水平揭示了有机Ca在煤加氢热解的迁变机制:有机Ca的结合能力很强,极易与分解后的小分子物质结合。有机Ca可以直接向无机小分子、气态烃与焦油分子中转化。其中,Ca首先倾向于进入无机小分子,其次是气态烃,最后是焦油。同时,赋存在三者之间的Ca会在高温下持续迁移转化。研究结果可为高碱煤在中低温热解产业中污染控制与产物优化提供科学参考和理论支持。
中图分类号:
王小令, 王绍清, 赵云刚, 常方哲, 穆瑞峰. 基于ReaxFF MD模拟的煤加氢热解有机Ca转化机制研究[J]. 化工学报, 2025, 76(8): 4297-4309.
Xiaoling WANG, Shaoqing WANG, Yungang ZHAO, Fangzhe CHANG, Ruifeng MU. Mechanism of organic Ca transformation during coal hydropyrolysis: insights from ReaxFF molecular dynamics simulations[J]. CIESC Journal, 2025, 76(8): 4297-4309.
| 样品 | 工业分析/% | Ro/% | 元素分析/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | Cdaf | Hdaf | Odaf① | Ndaf | St,d | ||
| ZDV | 15.14 | 2.93 | 48.57 | 0.54 | 72.92 | 4.27 | 21.57 | 0.61 | 0.63 |
| ZDI | 14.33 | 3.95 | 31.73 | 0.37 | 78.66 | 3.10 | 17.01 | 0.63 | 0.60 |
表1 煤样的工业分析、元素分析和最大镜质体反射率
Table 1 The proximate analysis, ultimate analysis, and mean maximum vitrinite reflectance (Ro) of samples
| 样品 | 工业分析/% | Ro/% | 元素分析/% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | Cdaf | Hdaf | Odaf① | Ndaf | St,d | ||
| ZDV | 15.14 | 2.93 | 48.57 | 0.54 | 72.92 | 4.27 | 21.57 | 0.61 | 0.63 |
| ZDI | 14.33 | 3.95 | 31.73 | 0.37 | 78.66 | 3.10 | 17.01 | 0.63 | 0.60 |
| 样品 | Ca的含量/% | |
|---|---|---|
| 实验加载Ca | 模型中的Ca | |
| ZDV-Ca | 1.98 | 1.53 |
| ZDI-Ca | 2.02 | 1.61 |
表2 加载Ca型煤实验和分子模型中Ca的含量
Table 2 Content of Ca in loading Ca experiment and molecular model of coal
| 样品 | Ca的含量/% | |
|---|---|---|
| 实验加载Ca | 模型中的Ca | |
| ZDV-Ca | 1.98 | 1.53 |
| ZDI-Ca | 2.02 | 1.61 |
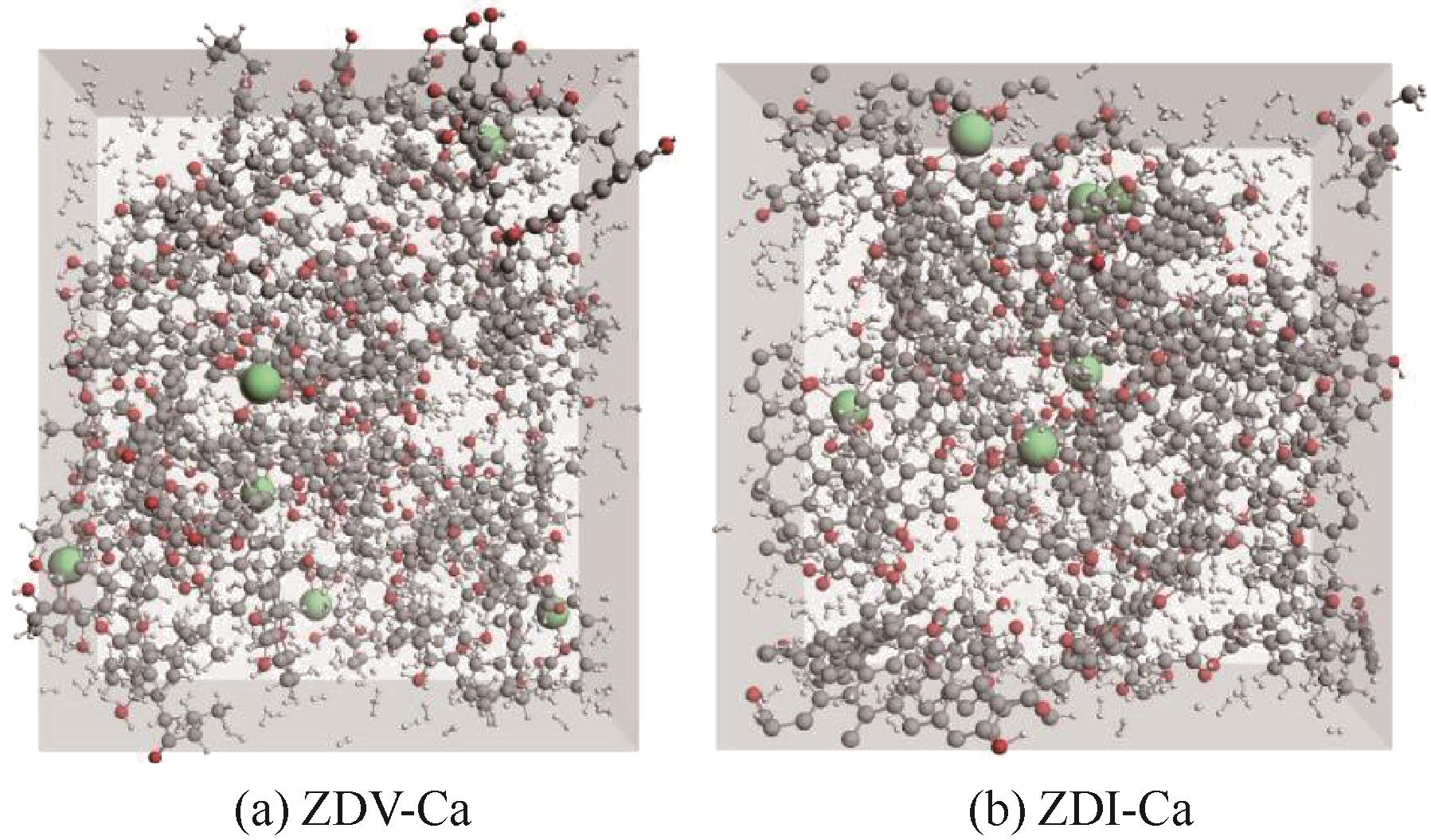
图2 含Ca体系煤(ZDV-Ca与ZDI-Ca)聚集态结构模型(包含600个H2分子)
Fig.2 Aggregate structural model of coal containing calcium-system (ZDV-Ca and ZDI-Ca, containing 600 H2 molecules)
| 样品与产物 | Ca的含量/(mg/kg) | ||
|---|---|---|---|
| Char(焦炭基准) | Char(原煤基准) | Tar(原煤基准) | |
| ZDV-Ca | 19716.5 | 19716.5 | — |
| ZDV-Ca-500 | 27262.0 | 17338.6 | 43.3 |
| ZDV-Ca-600 | 27313.2 | 15732.4 | 2.0 |
| ZDV-Ca-700 | 33422.3 | 17780.7 | 57.2 |
表3 ZDV-Ca在500、600、700℃下加氢热解焦炭、焦油产物中Ca的含量
Table 3 The content of Ca in char and tar products of ZDV-Ca hydropyrolysis at 500, 600, 700℃
| 样品与产物 | Ca的含量/(mg/kg) | ||
|---|---|---|---|
| Char(焦炭基准) | Char(原煤基准) | Tar(原煤基准) | |
| ZDV-Ca | 19716.5 | 19716.5 | — |
| ZDV-Ca-500 | 27262.0 | 17338.6 | 43.3 |
| ZDV-Ca-600 | 27313.2 | 15732.4 | 2.0 |
| ZDV-Ca-700 | 33422.3 | 17780.7 | 57.2 |
| [1] | 尚建选, 牛犇, 牛梦龙, 等. 以煤热解为龙头的煤分质利用技术: 回顾与展望[J]. 洁净煤技术, 2023, 29(7): 1-20. |
| Shang J X, Niu B, Niu M L, et al. Coal grading utilization technologies based on coal pyrolysis: review and prospect[J]. Clean Coal Technology, 2023, 29(7): 1-20. | |
| [2] | Wu L, Guan Y N, Li C C, et al. Free-radical behaviors of co-pyrolysis of low-rank coal and different solid hydrogen-rich donors: a critical review[J]. Chemical Engineering Journal, 2023, 474: 145900. |
| [3] | Zhou J B, Zhuang X G, Alastuey A, et al. Geochemistry and mineralogy of coal in the recently explored Zhundong large coal field in the Junggar basin, Xinjiang province, China[J]. International Journal of Coal Geology, 2010, 82(1/2): 51-67. |
| [4] | Zhu C, Qu S J, Zhang J, et al. Distribution, occurrence and leaching dynamic behavior of sodium in Zhundong coal[J]. Fuel, 2017, 190: 189-197. |
| [5] | Zhao Y, Liu L, Qiu P H, et al. Impacts of chemical fractionation on Zhundong coal's chemical structure and pyrolysis reactivity[J]. Fuel Processing Technology, 2017, 155: 144-152. |
| [6] | Liang D C, Xie Q, Wei Z, et al. Transformation of alkali and alkaline earth metals in Zhundong coal during pyrolysis in an entrained flow bed reactor[J]. Journal of Analytical and Applied Pyrolysis, 2019, 142: 104661. |
| [7] | Shi H, Wu Y X, Zhang M, et al. Ash deposition of Zhundong coal in a 350 MW pulverized coal furnace: influence of sulfation[J]. Fuel, 2020, 260: 116317. |
| [8] | Vainio E, Vänskä K, Laurén T, et al. Impact of boiler load and limestone addition on SO3 and corrosive cold-end deposits in a coal-fired CFB boiler[J]. Fuel, 2021, 304: 121313. |
| [9] | Huffman G P, Huggins F E. Analysis of the inorganic constituents in low-rank coals[M]//The Chemistry of Low-Rank Coals. Washington, D.C.: American Chemical Society, 1984: 159-174. |
| [10] | Benson S A, Holm P L. Comparison of inorganics in three low-rank coals[J]. Industrial & Engineering Chemistry Product Research and Development, 1985, 24(1): 145-149. |
| [11] | 马岩, 黄镇宇, 唐慧儒, 等. 准东煤灰化过程中的矿物演变及矿物添加剂对其灰熔融特性的影响[J]. 燃料化学学报, 2014, 42(1): 20-25. |
| Ma Y, Huang Z Y, Tang H R, et al. Mineral conversion of Zhundong coal during ashing process and the effect of mineral additives on its ash fusion characteristics[J]. Journal of Fuel Chemistry and Technology, 2014, 42(1): 20-25. | |
| [12] | Li G D, Li S Q, Huang Q, et al. Fine particulate formation and ash deposition during pulverized coal combustion of high-sodium lignite in a down-fired furnace[J]. Fuel, 2015, 143: 430-437. |
| [13] | 赵冰, 王嘉瑞, 陈凡敏, 等. 高钠煤水热脱钠处理及其对燃烧特性的影响[J]. 燃料化学学报, 2014, 42(12): 1416-1422. |
| Zhao B, Wang J R, Chen F M, et al. Hydrothermal treatment to remove sodium from high sodium coal and its influence on combustion characteristics[J]. Journal of Fuel Chemistry and Technology, 2014, 42(12): 1416-1422. | |
| [14] | Yang X H, Lv P, Zhu S H, et al. Release of Ca during coal pyrolysis and char gasification in H2O, CO2 and their mixtures[J]. Journal of Analytical and Applied Pyrolysis, 2018, 132: 217-224. |
| [15] | Wang C A, Zhao L, Han T, et al. Release and transformation behaviors of sodium, calcium, and iron during oxy-fuel combustion of Zhundong coals[J]. Energy & Fuels, 2018, 32(2): 1242-1254. |
| [16] | Quyn D M, Wu H W, Li C Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal (part Ⅰ): Volatilisation of Na and Cl from a set of NaCl-loaded samples[J]. Fuel, 2002, 81(2): 143-149. |
| [17] | Li C Z, Sathe C, Kershaw J R, et al. Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal[J]. Fuel, 2000, 79(3/4): 427-438. |
| [18] | Hong D K, Cao Z, Guo X. Effect of calcium on the secondary reactions of tar from Zhundong coal pyrolysis: a molecular dynamics simulation using ReaxFF[J]. Journal of Analytical and Applied Pyrolysis, 2019, 137: 246-252. |
| [19] | Shadman F, Sams D A, Punjak W A. Significance of the reduction of alkali carbonates in catalytic carbon gasification[J]. Fuel, 1987, 66(12): 1658-1663. |
| [20] | Yao Q, Li S Q, Xu H W, et al. Studies on formation and control of combustion particulate matter in China: a review[J]. Energy, 2009, 34(9): 1296-1309. |
| [21] | Li X J, Li C Z. Volatilisation and catalytic effects of alkali and alkaline earth metallic species during the pyrolysis and gasification of Victorian brown coal (part Ⅷ): Catalysis and changes in char structure during gasification in steam[J]. Fuel, 2006, 85(10/11): 1518-1525. |
| [22] | Wang X L, Wang S Q, Zhao Y G, et al. Occurrence modes of AAEMs (Na+ and Ca2+) and the effect on the molecular structures of Zhundong coal via quantum chemistry[J]. ACS Omega, 2023, 8(49): 46528-46539. |
| [23] | Du C S, Liu L, Qiu P H. Variation of char reactivity during catalytic gasification with steam: comparison among catalytic gasification by ion-exchangeable Na, Ca, and Na/Ca mixture[J]. Energy & Fuels, 2018, 32(1): 142-153. |
| [24] | Wang X L, Wang S Q, Zhao Y G, et al. Construction and verification of vitrinite-rich and inertinite-rich Zhundong coal models at the aggregate level: new insights from the spatial arrangement and thermal behavior perspective[J]. RSC Advances, 2023, 13(11): 7569-7584. |
| [25] | Li G Y, Ding J X, Zhang H, et al. ReaxFF simulations of hydrothermal treatment of lignite and its impact on chemical structures[J]. Fuel, 2015, 154: 243-251. |
| [26] | Zheng M, Li X X, Liu J, et al. Pyrolysis of Liulin coal simulated by GPU-based ReaxFF MD with cheminformatics analysis[J]. Energy & Fuels, 2014, 28(1): 522-534. |
| [27] | Zhang T T, Li X X, Qiao X J, et al. Initial mechanisms for an overall behavior of lignin pyrolysis through large-scale ReaxFF molecular dynamics simulations[J]. Energy & Fuels, 2016, 30(4): 3140-3150. |
| [28] | Zheng M, Li X X, Liu J, et al. Initial chemical reaction simulation of coal pyrolysis via ReaxFF molecular dynamics[J]. Energy & Fuels, 2013, 27(6): 2942-2951. |
| [29] | Zhou Z J, Guo L Z, Chen L P, et al. Study of pyrolysis of brown coal and gasification of coal-water slurry using the ReaxFF reactive force field[J]. International Journal of Energy Research, 2018, 42(7): 2465-2480. |
| [30] | Guo L Z, Zhou Z J, Chen L P, et al. Study of the pyrolysis of coals of different rank using the ReaxFF reactive force field[J]. Journal of Molecular Modeling, 2019, 25(6): 174. |
| [31] | Salmon E, van Duin A C T, Lorant F, et al. Thermal decomposition process in algaenan of Botryococcus braunii race L(part 2): Molecular dynamics simulations using the ReaxFF reactive force field[J]. Organic Geochemistry, 2009, 40(3): 416-427. |
| [32] | Cheng X M, Wang Q D, Li J Q, et al. ReaxFF molecular dynamics simulations of oxidation of toluene at high temperatures[J]. The Journal of Physical Chemistry A, 2012, 116(40): 9811-9818. |
| [33] | Sun C, Zhu A X, Xu T, et al. Atomic-level analysis of migration and transformation of organic sodium in high-alkali coal pyrolysis using reactive molecular dynamics simulations[J]. Journal of Environmental Chemical Engineering, 2023, 11(3): 110189. |
| [34] | Liang Y H, Wang F, Zhang H, et al. A ReaxFF molecular dynamics study on the mechanism of organic sulfur transformation in the hydropyrolysis process of lignite[J]. Fuel Processing Technology, 2016, 147: 32-40. |
| [35] | Sun C, Wei X L, Kang R N, et al. Intrinsic sodium occurrence in Zhundong coal: experimental observations and molecular modeling[J]. Fuel, 2021, 305: 121491. |
| [36] | Si T, Hong D K, Li P, et al. The migration and transformation of sodium carboxylate during Zhundong coal pyrolysis and combustion: a ReaxFF simulation study[J]. Journal of Analytical and Applied Pyrolysis, 2021, 156: 105098. |
| [1] | 密晓光, 孙国刚, 程昊, 张晓慧. 印刷电路板式天然气冷却器性能仿真模型和验证[J]. 化工学报, 2025, 76(S1): 426-434. |
| [2] | 任现超, 谷雅秀, 段少斌, 贾文竹, 李汉林. 翅片式椭圆套管蒸发式冷凝器传热传质性能实验研究[J]. 化工学报, 2025, 76(S1): 75-83. |
| [3] | 沙鑫权, 胡然, 丁磊, 蒋珍华, 吴亦农. 空间用单机两级有阀线性压缩机研制及测试[J]. 化工学报, 2025, 76(S1): 114-122. |
| [4] | 孙浩然, 吴成云, 王艳蒙, 孙静楠, 胡仞与, 段钟弟. 热对流影响下液滴蒸发特性模型与实验研究[J]. 化工学报, 2025, 76(S1): 123-132. |
| [5] | 燕子腾, 詹飞龙, 丁国良. 空调用套管式分流器结构设计及分流效果验证[J]. 化工学报, 2025, 76(S1): 152-159. |
| [6] | 袁琳慧, 王瑜. 单服务器浸没射流式液冷系统散热性能[J]. 化工学报, 2025, 76(S1): 160-169. |
| [7] | 吴迪, 胡斌, 姜佳彤. R1233zd(E)高温热泵实验研究与应用分析[J]. 化工学报, 2025, 76(S1): 377-383. |
| [8] | 黄国瑞, 赵耀, 谢明熹, 陈尔健, 代彦军. 一种新型数据中心余热回收系统实验与分析[J]. 化工学报, 2025, 76(S1): 409-417. |
| [9] | 林嘉豪, 付芳忠, 叶昊辉, 胡金, 姚明灿, 范鹤林, 王旭, 王瑞祥, 徐志峰. NdF3含量对NdF3-LiF熔盐局域结构和输运性质的影响[J]. 化工学报, 2025, 76(8): 3834-3841. |
| [10] | 高正, 汪辉, 屈治国. 数据驱动辅助高通量筛选阴离子柱撑金属有机框架储氢[J]. 化工学报, 2025, 76(8): 4259-4272. |
| [11] | 黄荣廷, 陶奕淳, 陈江林, 李世航, 杨子系, 王仕远, 罗祥轩. 矿井除湿溶液再生速率预测研究[J]. 化工学报, 2025, 76(7): 3572-3584. |
| [12] | 李姿睿, 齐凯, 王军, 夏国栋. 基于Janus纳米通道的脱盐过程分子动力学模拟研究[J]. 化工学报, 2025, 76(7): 3531-3538. |
| [13] | 乔亮, 李尚, 刘新亮, 王明, 张沛, 侯影飞. 三元共聚物稠油降黏剂的合成及分子模拟研究[J]. 化工学报, 2025, 76(7): 3686-3695. |
| [14] | 范振宁, 梁海宁, 房茂立, 赫一凡, 于帅, 闫兴清, 安佳然, 乔帆帆, 喻健良. CO2管道不同相态节流放空特性研究与对比[J]. 化工学报, 2025, 76(7): 3742-3751. |
| [15] | 齐昊, 王玉杰, 李申辉, 邹琦, 刘轶群, 赵之平. 双金属Co/Zn-ZIFs中C3H6和C3H8吸附和扩散行为分子模拟研究[J]. 化工学报, 2025, 76(5): 2313-2326. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号