化工学报 ›› 2019, Vol. 70 ›› Issue (6): 2182-2191.DOI: 10.11949/0438-1157.20190021
收稿日期:2019-01-09
修回日期:2019-03-21
出版日期:2019-06-05
发布日期:2019-06-05
通讯作者:
郭洪臣
作者简介:<named-content content-type="corresp-name">赵云</named-content>(1983—),男,博士研究生,<email>zhyun119@163.com</email>
基金资助:
Yun ZHAO( ),Chunyan LIU,Jiaxu LIU,Ning HE,Hongchen GUO(
),Chunyan LIU,Jiaxu LIU,Ning HE,Hongchen GUO( )
)
Received:2019-01-09
Revised:2019-03-21
Online:2019-06-05
Published:2019-06-05
Contact:
Hongchen GUO
摘要:
采用氨气程序升温脱附(NH3-TPD)、紫外-可见光谱和小型固定床反应器,重点研究了碱金属钠离子和过渡金属锌离子改性对纳米H-ZSM-5沸石表面酸性及其催化C5~C8链烷烃芳构化反应性能的影响。结果表明,用碱金属钠离子改性能够有效地消除沸石表面的强酸中心,适当降低催化剂表面酸量,从而减少甲烷、乙烷和丙烷等低值副产物的生成,优化芳构化反应产物分布。适宜的Zn2+负载量为3%(质量分数),过高的Zn2+负载量会使产物中干气生成量增大,不利于芳烃收率的提高。C5~C8链烷烃芳构化的适宜反应温度为530℃。
中图分类号:
赵云, 刘春燕, 刘家旭, 贺宁, 郭洪臣. Zn/NaHZSM-5沸石在C5~C8链烷烃芳构化反应中的催化性能[J]. 化工学报, 2019, 70(6): 2182-2191.
Yun ZHAO, Chunyan LIU, Jiaxu LIU, Ning HE, Hongchen GUO. Catalytic performance of Zn/NaHZSM-5 zeolite for C5-C8 chain-alkanes aromatization[J]. CIESC Journal, 2019, 70(6): 2182-2191.
| 组成 | 正构烷烃 | 异构烷烃 | 烯烃 | 环烷烃 | 芳烃 | 总和 |
|---|---|---|---|---|---|---|
| C4 | 0.01 | 0 | 0 | 0 | 0 | 0.01 |
| C5 | 3.66 | 1.46 | 0.12 | 3.41 | 0 | 8.65 |
| C6 | 12.43 | 30.65 | 2.35 | 1.77 | 0 | 47.20 |
| C7 | 6.59 | 25.40 | 0.25 | 0.67 | 0.27 | 33.18 |
| C8 | 1.67 | 8.01 | 0.01 | 0.56 | 0 | 10.25 |
| C9 | 0 | 0.71 | 0 | 0 | 0 | 0.71 |
| 总和 | 24.36 | 66.23 | 2.73 | 6.41 | 0.27 | 100 |
表1 重整抽余油的组成
Table 1 Composition of raffinate oil/%(mass)
| 组成 | 正构烷烃 | 异构烷烃 | 烯烃 | 环烷烃 | 芳烃 | 总和 |
|---|---|---|---|---|---|---|
| C4 | 0.01 | 0 | 0 | 0 | 0 | 0.01 |
| C5 | 3.66 | 1.46 | 0.12 | 3.41 | 0 | 8.65 |
| C6 | 12.43 | 30.65 | 2.35 | 1.77 | 0 | 47.20 |
| C7 | 6.59 | 25.40 | 0.25 | 0.67 | 0.27 | 33.18 |
| C8 | 1.67 | 8.01 | 0.01 | 0.56 | 0 | 10.25 |
| C9 | 0 | 0.71 | 0 | 0 | 0 | 0.71 |
| 总和 | 24.36 | 66.23 | 2.73 | 6.41 | 0.27 | 100 |
| 样品 | 弱酸 | 强酸 | 总酸 |
|---|---|---|---|
| HZSM-5 | 0.72 | 0.28 | 1 |
| Zn3 | 0.75 | 0.24 | 0.99 |
| Na0.2 | 0.81 | 0.18 | 0.99 |
| Na0.8 | 0.95 | 0.05 | 1 |
| Na1.5 | 0.96 | 0.04 | 1 |
| Na2.0 | 0.92 | 0.01 | 0.93 |
| Na0.2Zn3 | 0.86 | 0.12 | 0.98 |
| Na0.8Zn3 | 0.87 | 0.10 | 0.97 |
| Na1.5Zn3 | 0.86 | 0.09 | 0.95 |
| Na2.0Zn3 | 0.80 | 0.07 | 0.87 |
| Zn3Na0.2 | 0.78 | 0.11 | 0.89 |
| Zn3Na0.8 | 0.76 | 0.08 | 0.84 |
| Zn3Na1.5 | 0.74 | 0.05 | 0.79 |
| Zn3Na2.0 | 0.64 | 0.04 | 0.68 |
表2 Na+和Zn2+改性纳米H-ZSM-5沸石的相对酸量分布
Table 2 Relative acid amount of nano-sized H-ZSM-5 zeolites modified by Na+ and Zn2+
| 样品 | 弱酸 | 强酸 | 总酸 |
|---|---|---|---|
| HZSM-5 | 0.72 | 0.28 | 1 |
| Zn3 | 0.75 | 0.24 | 0.99 |
| Na0.2 | 0.81 | 0.18 | 0.99 |
| Na0.8 | 0.95 | 0.05 | 1 |
| Na1.5 | 0.96 | 0.04 | 1 |
| Na2.0 | 0.92 | 0.01 | 0.93 |
| Na0.2Zn3 | 0.86 | 0.12 | 0.98 |
| Na0.8Zn3 | 0.87 | 0.10 | 0.97 |
| Na1.5Zn3 | 0.86 | 0.09 | 0.95 |
| Na2.0Zn3 | 0.80 | 0.07 | 0.87 |
| Zn3Na0.2 | 0.78 | 0.11 | 0.89 |
| Zn3Na0.8 | 0.76 | 0.08 | 0.84 |
| Zn3Na1.5 | 0.74 | 0.05 | 0.79 |
| Zn3Na2.0 | 0.64 | 0.04 | 0.68 |
| Item | HZSM-5 | Zn3 | Na0.8Zn3 | Na1.5Zn3 | Na2.0Zn3 | Zn3Na0.8 | Zn3Na1.5 | Zn3Na2.0 |
|---|---|---|---|---|---|---|---|---|
| X | 99.89 | 98.98 | 95.97 | 93.05 | 92.38 | 97.89 | 85.30 | 76.83 |
| Y(A) | 47.83 | 51.57 | 47.49 | 47.58 | 47.5 | 51.63 | 43.24 | 37.34 |
| S(A) | 47.88 | 52.10 | 49.48 | 51.13 | 51.42 | 52.74 | 50.69 | 48.60 |
| S(C1~C2) | 27.71 | 25.95 | 23.09 | 16.80 | 14.98 | 13.09 | 12.53 | 12.35 |
| S(C30) | 21.82 | 19.23 | 19.66 | 18.27 | 17.66 | 20.04 | 17.06 | 14.97 |
| S(A)/[S(C1~C2) + S( | 0.97 | 1.15 | 1.16 | 1.46 | 1.58 | 1.59 | 1.71 | 1.78 |
| 产物分布 | ||||||||
| 甲烷 | 13.23 | 11.91 | 8.40 | 7.61 | 5.72 | 5.52 | 4.14 | 3.67 |
| 乙烷 | 14.27 | 13.48 | 13.32 | 7.15 | 7.06 | 6.18 | 5.01 | 3.90 |
| 乙烯 | 0.18 | 0.30 | 0.44 | 0.87 | 1.06 | 1.11 | 1.54 | 1.92 |
| 丙烷 | 21.80 | 19.03 | 18.87 | 17.00 | 16.31 | 19.62 | 14.55 | 11.50 |
| 丙烯 | 0.10 | 0.35 | 0.79 | 1.58 | 1.82 | 1.68 | 2.84 | 3.88 |
| 丁烷 | 2.07 | 1.60 | 4.49 | 8.79 | 9.81 | 8.98 | 9.89 | 7.57 |
| 丁烯 | 0.05 | 0.18 | 0.29 | 0.69 | 0.90 | 0.67 | 1.18 | 1.61 |
| 苯 | 7.18 | 10.30 | 8.24 | 7.04 | 6.36 | 6.72 | 4.66 | 3.45 |
| 甲苯 | 22.32 | 24.31 | 21.28 | 21.59 | 20.19 | 24.69 | 17.82 | 15.06 |
| C8芳烃 | 12.75 | 12.49 | 13.96 | 14.23 | 15.48 | 14.38 | 14.34 | 12.65 |
| C9+芳烃 | 5.58 | 4.47 | 4.01 | 4.72 | 5.47 | 5.84 | 6.42 | 6.18 |
| C5+非芳烃 | 0.48 | 1.58 | 5.90 | 8.74 | 9.83 | 4.60 | 17.62 | 28.60 |
| 总和 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
表3 Na+、Zn2+改性纳米H-ZSM-5沸石上C5~C8链烷烃的芳构化反应产物分布
Table 3 Products distribution of aromatization of C5-C8 chain alkanes over
| Item | HZSM-5 | Zn3 | Na0.8Zn3 | Na1.5Zn3 | Na2.0Zn3 | Zn3Na0.8 | Zn3Na1.5 | Zn3Na2.0 |
|---|---|---|---|---|---|---|---|---|
| X | 99.89 | 98.98 | 95.97 | 93.05 | 92.38 | 97.89 | 85.30 | 76.83 |
| Y(A) | 47.83 | 51.57 | 47.49 | 47.58 | 47.5 | 51.63 | 43.24 | 37.34 |
| S(A) | 47.88 | 52.10 | 49.48 | 51.13 | 51.42 | 52.74 | 50.69 | 48.60 |
| S(C1~C2) | 27.71 | 25.95 | 23.09 | 16.80 | 14.98 | 13.09 | 12.53 | 12.35 |
| S(C30) | 21.82 | 19.23 | 19.66 | 18.27 | 17.66 | 20.04 | 17.06 | 14.97 |
| S(A)/[S(C1~C2) + S( | 0.97 | 1.15 | 1.16 | 1.46 | 1.58 | 1.59 | 1.71 | 1.78 |
| 产物分布 | ||||||||
| 甲烷 | 13.23 | 11.91 | 8.40 | 7.61 | 5.72 | 5.52 | 4.14 | 3.67 |
| 乙烷 | 14.27 | 13.48 | 13.32 | 7.15 | 7.06 | 6.18 | 5.01 | 3.90 |
| 乙烯 | 0.18 | 0.30 | 0.44 | 0.87 | 1.06 | 1.11 | 1.54 | 1.92 |
| 丙烷 | 21.80 | 19.03 | 18.87 | 17.00 | 16.31 | 19.62 | 14.55 | 11.50 |
| 丙烯 | 0.10 | 0.35 | 0.79 | 1.58 | 1.82 | 1.68 | 2.84 | 3.88 |
| 丁烷 | 2.07 | 1.60 | 4.49 | 8.79 | 9.81 | 8.98 | 9.89 | 7.57 |
| 丁烯 | 0.05 | 0.18 | 0.29 | 0.69 | 0.90 | 0.67 | 1.18 | 1.61 |
| 苯 | 7.18 | 10.30 | 8.24 | 7.04 | 6.36 | 6.72 | 4.66 | 3.45 |
| 甲苯 | 22.32 | 24.31 | 21.28 | 21.59 | 20.19 | 24.69 | 17.82 | 15.06 |
| C8芳烃 | 12.75 | 12.49 | 13.96 | 14.23 | 15.48 | 14.38 | 14.34 | 12.65 |
| C9+芳烃 | 5.58 | 4.47 | 4.01 | 4.72 | 5.47 | 5.84 | 6.42 | 6.18 |
| C5+非芳烃 | 0.48 | 1.58 | 5.90 | 8.74 | 9.83 | 4.60 | 17.62 | 28.60 |
| 总和 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
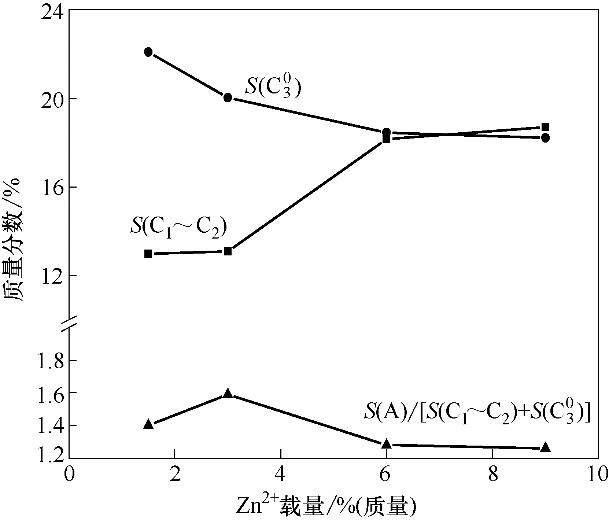
图5 改性纳米H-ZSM-5沸石(ZnyNa0.8)的反应性能与Zn2+负载量的关联
Fig.5 Correlation between reaction performance and Zn2+ loading of modified nano-sized H-ZSM-5 zeolite (ZnyNa0.8)
| Item | Zn1.5Na0.8 | Zn3Na0.8 | Zn6Na0.8 | Zn9Na0.8 |
|---|---|---|---|---|
| X | 95.28 | 97.89 | 95.33 | 94.85 |
| Y(A) | 46.95 | 51.63 | 44.78 | 44.16 |
| S(A) | 49.28 | 52.74 | 46.97 | 46.56 |
| S(C1~C2) | 12.98 | 13.09 | 18.16 | 18.70 |
| S(C03) | 22.09 | 20.04 | 18.46 | 18.22 |
| S(A)/[S(C1~C2) + S( | 1.40 | 1.59 | 1.28 | 1.26 |
| 产物分布 | ||||
| 甲烷 | 5.10 | 5.52 | 6.47 | 6.35 |
| 乙烷 | 6.07 | 6.18 | 10.02 | 10.65 |
| 乙烯 | 1.20 | 1.11 | 0.82 | 0.74 |
| 丙烷 | 21.05 | 19.62 | 17.60 | 17.28 |
| 丙烯 | 1.79 | 1.68 | 1.60 | 1.66 |
| 丁烷 | 7.79 | 8.98 | 8.78 | 8.55 |
| 丁烯 | 0.58 | 0.67 | 0.64 | 0.71 |
| 苯 | 6.68 | 6.72 | 6.57 | 6.31 |
| 甲苯 | 21.21 | 24.69 | 18.87 | 18.11 |
| C8芳烃 | 13.42 | 14.38 | 14.43 | 14.91 |
| C9+芳烃 | 5.64 | 5.84 | 4.91 | 4.83 |
| C5+非芳烃 | 9.47 | 4.60 | 9.29 | 9.90 |
| 总和 | 100 | 100 | 100 | 100 |
表4 不同Zn2+负载量纳米H-ZSM-5沸石上C5~C8链烷烃芳构化反应的产物分布
Table 4 Products distribution of aromatization of C5-C8 chain alkanes over modified nano-sized H-ZSM-5 zeolite with different Zn2+ loading/%(mass)
| Item | Zn1.5Na0.8 | Zn3Na0.8 | Zn6Na0.8 | Zn9Na0.8 |
|---|---|---|---|---|
| X | 95.28 | 97.89 | 95.33 | 94.85 |
| Y(A) | 46.95 | 51.63 | 44.78 | 44.16 |
| S(A) | 49.28 | 52.74 | 46.97 | 46.56 |
| S(C1~C2) | 12.98 | 13.09 | 18.16 | 18.70 |
| S(C03) | 22.09 | 20.04 | 18.46 | 18.22 |
| S(A)/[S(C1~C2) + S( | 1.40 | 1.59 | 1.28 | 1.26 |
| 产物分布 | ||||
| 甲烷 | 5.10 | 5.52 | 6.47 | 6.35 |
| 乙烷 | 6.07 | 6.18 | 10.02 | 10.65 |
| 乙烯 | 1.20 | 1.11 | 0.82 | 0.74 |
| 丙烷 | 21.05 | 19.62 | 17.60 | 17.28 |
| 丙烯 | 1.79 | 1.68 | 1.60 | 1.66 |
| 丁烷 | 7.79 | 8.98 | 8.78 | 8.55 |
| 丁烯 | 0.58 | 0.67 | 0.64 | 0.71 |
| 苯 | 6.68 | 6.72 | 6.57 | 6.31 |
| 甲苯 | 21.21 | 24.69 | 18.87 | 18.11 |
| C8芳烃 | 13.42 | 14.38 | 14.43 | 14.91 |
| C9+芳烃 | 5.64 | 5.84 | 4.91 | 4.83 |
| C5+非芳烃 | 9.47 | 4.60 | 9.29 | 9.90 |
| 总和 | 100 | 100 | 100 | 100 |
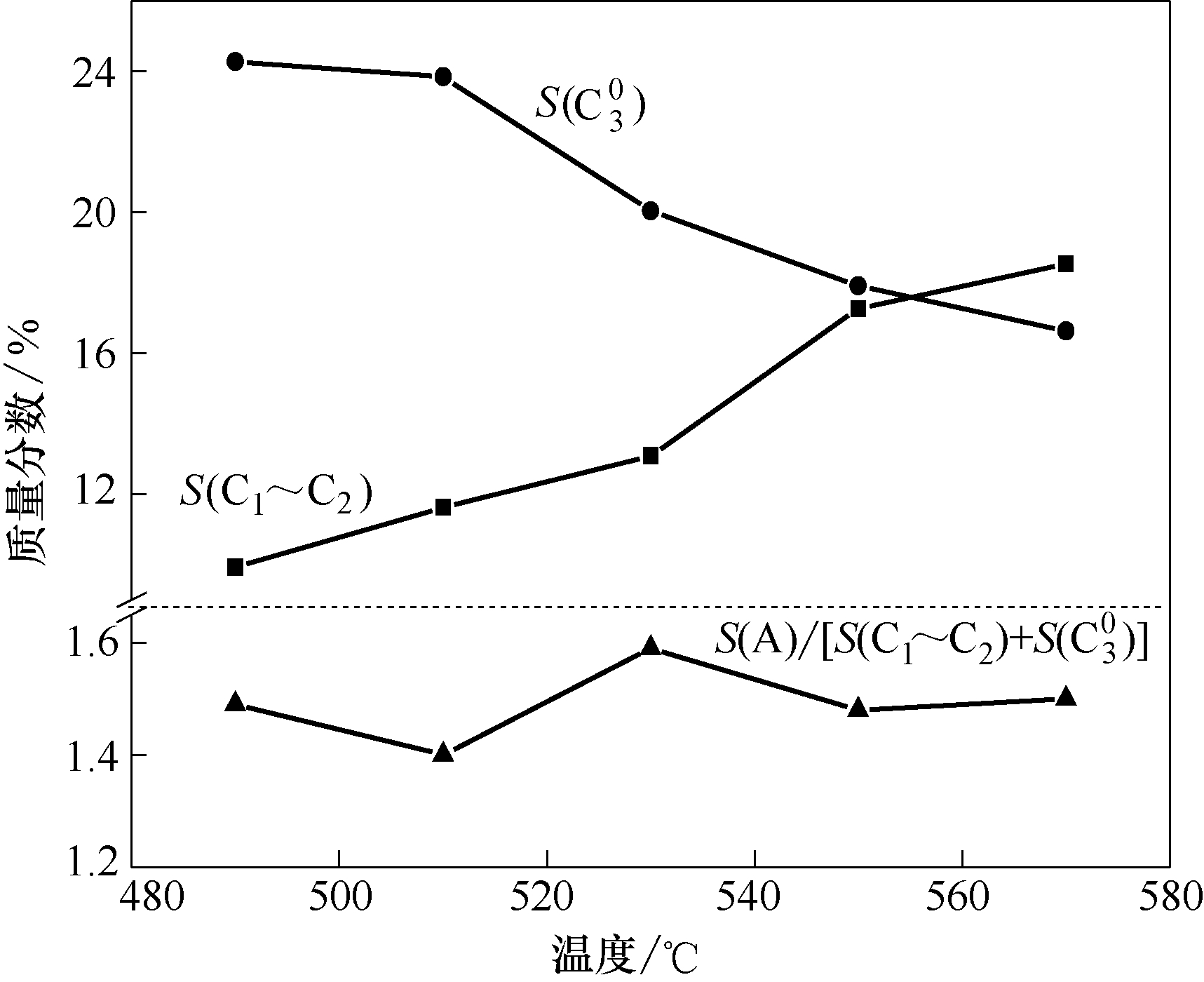
图6 改性纳米H-ZSM-5沸石(Zn3Na0.8)的反应性能与反应温度的关联
Fig.6 Correlation between reaction performance of modified nano-sized H-ZSM-5 (Zn3Na0.8) and reaction temperatures
| Item | 490℃ | 510℃ | 530℃ | 550℃ | 570℃ |
|---|---|---|---|---|---|
| X | 93.25 | 94.59 | 97.89 | 98.02 | 98.44 |
| Y(A) | 47.58 | 47.05 | 51.63 | 51.11 | 51.92 |
| S(A) | 51.02 | 49.74 | 52.74 | 52.14 | 52.74 |
| S(C1~C2) | 9.93 | 11.62 | 13.09 | 17.27 | 18.53 |
| S(C30) | 24.28 | 23.85 | 20.04 | 17.92 | 16.63 |
| S(A)/[S(C1~C2) + S( | 1.49 | 1.40 | 1.59 | 1.48 | 1.50 |
| 产物分布 | |||||
| 甲烷 | 3.90 | 4.89 | 5.52 | 7.01 | 7.66 |
| 乙烷 | 4.66 | 5.22 | 6.18 | 8.42 | 9.04 |
| 乙烯 | 0.70 | 0.88 | 1.11 | 1.50 | 1.54 |
| 丙烷 | 22.64 | 22.56 | 19.62 | 17.57 | 16.37 |
| 丙烯 | 1.17 | 1.35 | 1.68 | 2.33 | 2.41 |
| 丁烷 | 8.96 | 10.33 | 8.98 | 7.96 | 6.95 |
| 丁烯 | 0.43 | 0.48 | 0.67 | 0.98 | 0.72 |
| 苯 | 4.74 | 5.68 | 6.72 | 8.46 | 9.92 |
| 甲苯 | 20.52 | 21.49 | 24.69 | 23.70 | 23.42 |
| C8芳烃 | 15.19 | 14.30 | 14.38 | 14.06 | 14.10 |
| C9+芳烃 | 7.13 | 5.58 | 5.84 | 4.89 | 4.48 |
| C5+非芳烃 | 9.96 | 7.25 | 4.60 | 3.11 | 3.39 |
| 总和 | 100 | 100 | 100 | 100 | 100 |
表5 不同反应温度下改性纳米H-ZSM-5(Zn3Na0.8)沸石上C5~C8链烷烃芳构化反应的产物分布
Table 5 Products distribution of aromatization of C5-C8 chain alkanes over modified nano-sized H-ZSM-5 zeolite (Zn3Na0.8) at different temperatures/%(mass)
| Item | 490℃ | 510℃ | 530℃ | 550℃ | 570℃ |
|---|---|---|---|---|---|
| X | 93.25 | 94.59 | 97.89 | 98.02 | 98.44 |
| Y(A) | 47.58 | 47.05 | 51.63 | 51.11 | 51.92 |
| S(A) | 51.02 | 49.74 | 52.74 | 52.14 | 52.74 |
| S(C1~C2) | 9.93 | 11.62 | 13.09 | 17.27 | 18.53 |
| S(C30) | 24.28 | 23.85 | 20.04 | 17.92 | 16.63 |
| S(A)/[S(C1~C2) + S( | 1.49 | 1.40 | 1.59 | 1.48 | 1.50 |
| 产物分布 | |||||
| 甲烷 | 3.90 | 4.89 | 5.52 | 7.01 | 7.66 |
| 乙烷 | 4.66 | 5.22 | 6.18 | 8.42 | 9.04 |
| 乙烯 | 0.70 | 0.88 | 1.11 | 1.50 | 1.54 |
| 丙烷 | 22.64 | 22.56 | 19.62 | 17.57 | 16.37 |
| 丙烯 | 1.17 | 1.35 | 1.68 | 2.33 | 2.41 |
| 丁烷 | 8.96 | 10.33 | 8.98 | 7.96 | 6.95 |
| 丁烯 | 0.43 | 0.48 | 0.67 | 0.98 | 0.72 |
| 苯 | 4.74 | 5.68 | 6.72 | 8.46 | 9.92 |
| 甲苯 | 20.52 | 21.49 | 24.69 | 23.70 | 23.42 |
| C8芳烃 | 15.19 | 14.30 | 14.38 | 14.06 | 14.10 |
| C9+芳烃 | 7.13 | 5.58 | 5.84 | 4.89 | 4.48 |
| C5+非芳烃 | 9.96 | 7.25 | 4.60 | 3.11 | 3.39 |
| 总和 | 100 | 100 | 100 | 100 | 100 |
| 1 | TetsuyaF, HisashiK. Halogen-promoted Pt/KL zeolite catalyst for the production of aromatic hydrocarbons from light naphtha [J]. Catal. Surv. Asia, 2010, 14: 96-102. |
| 2 | DerouaneE G, VandervekenD J. Structural recognition and preorganization in zeolite catalysis: direct aromatization of n-hexane on zeolite L-based catalysts[J]. Appl. Catal., 1988, 45(1): 15-22. |
| 3 | SantilliD S, LongJ J, LewisR T. Highly active and highly selective aromatization catalyst: US4698322A[P]. 1987-10-06. |
| 4 | BlessingC D, BrownS H, CheungT P, et al. Method of enhancing an aromatization catalyst: US7932452B2[P]. 2011-04-26. |
| 5 | LukyanovD B, GnepN S, GuisnetM R. Kinetic modeling of propane aromatization reaction over HZSM-5 and GaHZSM-5[J]. Ind. Eng. Chem. Res., 1995, 34: 516-523. |
| 6 | BiscardiJ A, MeitznerG D, IglesiaE. Structure and density of active Zn species in Zn/H-ZSM5 propane aromatization catalysts[J]. J. Catal., 1998, 179: 192-202. |
| 7 | ChoudharyV R, SivadinarayanaC, KinageA K, et al. H-gallosilicate(MFI) propane aromatization catalyst influence of calcination temperature on acidity, activity and deactivation due to coking[J]. Appl. Catal. A, 1996, 136: 125-142. |
| 8 | ChoudharyV R, KinageA K, SivadinarayanaC, et al. Initial activity/selectivity of H-gallosilicate (MFI) in propane aromatization: influence of H+ exchange and thermal/ hydrothermal pretreatments[J]. Catal. Lett., 1995, 33: 401-422. |
| 9 | ChoudharyV R, DevadasP, KinageA K, et al. Acidity, catalytic activity, and deactivation of H-gallosilicate (MFI) in propane aromatization: influence of hydrothermal pretreatments[J]. J. Catal., 1996, 158: 537-550. |
| 10 | ChoudharyV R, DevadasP. Influence of space velocity on product selectivity and distribution of aromatics and xylenes in propane aromatization over H-GaMFI zeolite[J]. J. Catal., 1997, 172: 475-478. |
| 11 | SongC, LiuK F, ZhangD Z, et al. Effect of cofeeding n-butane with methanol on aromatization performance and coke formation over a Zn loaded ZSM-5/ZSM-11 zeolite[J]. Appl. Catal. A, 2014, 470: 15-23. |
| 12 | IshaqM, KhanM A, YashimaT. Aromatization of butane over gallosilicates[J]. Fuel Process. Technol., 1999, 60: 173-184. |
| 13 | Le Van MaoR, YaoJ H, DufresneL A, et al. Hybrid catalysts containing zeolite ZSM-5 and supported gallium oxide in the aromatization of n-butane[J]. Catal. Today, 1996, 31: 247-255. |
| 14 | Le Van MaoR, CarliR, YaoJ H, et al. Catalysts for the aromatization of n-butane prepared by surface-contact transfer of gallium onto ZSM-5 zeolite particles[J]. Catal. Lett., 1992, 16: 43-52. |
| 15 | FukaseS, IgarashiN, AimotoK, et al. A new process of light naphtha aromatization using a zeolite-based catalyst with long-time stability[J]. Stud. Surf. Sci. Catal., 1997, 105: 885-892. |
| 16 | FukaseS, IgarashiN, KatoK, et al. Development of light naphtha aromatization process using a conventional fixed bed unit[J]. Stud. Surf. Sci. Catal., 1995, 100: 455-464. |
| 17 | SaitoS, HirabayashiK, ShibataS, et al. Paper presented at 1992 NRPA annual meeting[C]// New Orleans, 1992: AM-92-38. |
| 18 | GuisnetM, GnepN S, AittalebD. Conversion of light alkanes into aromatic-hydrocarbons. 6. Aromatization of C2—C4 alkanes on H-ZSM-5 reaction mechanisms[J]. Appl. Catal. A, 1992, 87: 255-270. |
| 19 | GuisnetM, GnepN S. Mechanism of short-chain alkane transformation over protonic zeolites. Alkylation, disproportionation and aromatization[J]. Appl. Catal. A, 1996, 146: 33-64. |
| 20 | MilasI, NascimentoM A C. The dehydrogenation and cracking reactions of isobutane over the ZSM-5 zeolite[J]. Chem. Phys. Lett., 2003, 373: 379-384. |
| 21 | 赵云, 贺宁, 郭洪臣. 石脑油在芳构化反应中活化途径的热力学分析[J]. 科技通报, 2016, 32: 5-15. |
| ZhaoY, HeN, GuoH C. Thermodynamic study on the activation pathways of naphtha fraction in aromatization[J]. Bull. Sci. Technol., 2016, 32: 5-15. | |
| 22 | 徐瑞芳, 刘家旭, 梁翠翠, 等. 碱金属离子改性对纳米HZSM-5沸石丁烯裂解催化性能的影响[J]. 燃料化学学报, 2011, 39: 449-454. |
| XuR F, LiuJ X, LiangC C, et al. Effect of alkali metal ion modification on the catalytic performance of nano-HZSM-5 zeolite in butane cracking[J]. J. Fuel Chem. Technol., 2011, 39: 449-454. | |
| 23 | 刘家旭, 梁翠翠, 徐瑞芳, 等. 碳四液化气在离子浸渍改性纳米HZSM-5沸石上的转化反应研究[J]. 分子催化, 2010, 24: 208-216. |
| LiuJ X, LiangC C, XuR F, et al. Effect of ion-impregnation treatment on the transformation of C4 LPG over modified nano-sized HZSM-5 zeolite[J]. J. Mol. Catal., 2010, 24: 208-216. | |
| 24 | KolyaginY G, OrdomskyV V, KhimyakY Z, et al. Initial stages of propane activation over Zn/MFI catalyst studied by in situ NMR and IR spectroscopic techniques[J]. J. Catal., 2006, 238: 122-133. |
| 25 | AlmutairiS M, MezariB, MagusinP C, et al. Structure and reactivity of Zn-modified ZSM-5 zeolites: the importance of clustered cationic Zn complexes[J]. ACS Catalysis, 2012, 2: 71-83. |
| 26 | XuJ, ZhengA M, WangX M, et al. Room temperature activation of methane over Zn modified H-ZSM-5 zeolite: Insight from solid-state NMR and theoretical calculations[J]. Chem. Sci., 2012, 3: 2932. |
| 27 | WangX M, QiG D, XuJ, et al. NMR-Spectroscopic evidence of intermediate dependent pathways for acetic acid formation from methane and carbon monoxide over a ZiiZSM-5 zeolite catalyst[J]. Angew. Chemie, 2012, 124: 3916-3919. |
| 28 | QIG D, XUJ, SUJ H, et al. Low-temperature reactivity of Zn+ ions confined in ZSM-5 zeolite toward carbon monoxide oxidation: insight from in situ DRIFT and ESR spectroscopy[J]. J. Am. Chem. Soc., 2013, 135: 6762-6765. |
| [1] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [2] | 董茂林, 陈李栋, 黄六莲, 吴伟兵, 戴红旗, 卞辉洋. 酸性助水溶剂制备木质纳米纤维素及功能应用研究进展[J]. 化工学报, 2023, 74(6): 2281-2295. |
| [3] | 王敏, 程金兰, 李鑫, 陆晶晶, 尹崇鑫, 戴红旗. 酸性助水溶剂脱除木质素机理分析[J]. 化工学报, 2022, 73(5): 2206-2221. |
| [4] | 王宏梅, 王挥遒, 宋文龙, 崔超婕, 陈兆辉, 张晨曦, 骞伟中, 魏飞. 多段流化床强化甲醇或烷烃芳构化研究进展[J]. 化工学报, 2021, 72(12): 6002-6015. |
| [5] | 韩建年, 王刚, 杨梅, 刘美佳, 高成地, 高金森. 费托蜡催化裂化反应生产清洁汽油的热力学分析[J]. 化工学报, 2020, 71(S1): 38-45. |
| [6] | 金放,张鹏,吴桂英,吴迪. Brönsted方程动力学模型研究ZSM-5催化乙烯齐聚及芳构化活性和酸强度分布之间的定量关系[J]. 化工学报, 2020, 71(5): 2076-2087. |
| [7] | 严正琦, 高江姗, 张鑫韬, 南非, 何燕. 氧化石墨烯的酸性还原及其超级电容性能[J]. 化工学报, 2019, 70(12): 4881-4888. |
| [8] | 王闻年, 袁德林, 李浩, 任申勇, 郭巧霞, 申宝剑. β沸石结构及其催化性能的调控[J]. 化工学报, 2016, 67(8): 3429-3435. |
| [9] | 马通, 耿祖豹, 李冰, 赵琦, 巩雁军. 不同模板制备ZSM-5分子筛的酸性特征及催化裂解性能差异[J]. 化工学报, 2016, 67(8): 3374-3379. |
| [10] | 李晓慧, 郑庆庆, 米硕, 申宝剑. 磷铝复合改性ZSM-5分子筛及其催化性能[J]. 化工学报, 2016, 67(8): 3357-3362. |
| [11] | 卢丹, 赵国英, 任保增, 江振西, 张锁江. 醚基功能化离子液体合成及催化烷基化反应[J]. 化工学报, 2015, 66(7): 2481-2487. |
| [12] | 施梅勤, 郑慧新, 魏爱平, 马淳安. Zn 助剂对WC/HZSM-5催化正己烷芳构化性能影响[J]. 化工学报, 2015, 66(2): 553-560. |
| [13] | 薛琳, 秦松岩, 刘宗瑜, 吴莉莉, 肖菊芳, 解永磊. 含硫酸亚铁废溶液的生物氧化过程中氮源对溶解性Fe(Ⅲ)生成量的影响[J]. 化工学报, 2014, 65(6): 2344-2349. |
| [14] | 应允攀, 曾凡平, 吴平易, 阳庆元, 刘大欢, 兰玲, 王少华, 张轶, 仲崇立. 溶剂化效应对金属-有机骨架材料界面微环境催化性能的影响[J]. 化工学报, 2014, 65(5): 1652-1659. |
| [15] | 罗怡, 周亚松, 魏强, 韩璐, 刘霄, 张超. 磷、柠檬酸改性对MoW/Ni/Al2O3催化剂性质及加氢脱氮性能的影响[J]. 化工学报, 2014, 65(10): 3916-3923. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号