化工学报 ›› 2020, Vol. 71 ›› Issue (5): 2076-2087.DOI: 10.11949/0438-1157.20191536
收稿日期:2019-12-18
修回日期:2020-02-11
出版日期:2020-05-05
发布日期:2020-05-05
通讯作者:
金放
作者简介:金放(1980—),男,博士, 教授,基金资助:
Fang JIN1( ),Peng ZHANG1(
),Peng ZHANG1( ),Guiying WU1,Di WU2
),Guiying WU1,Di WU2
Received:2019-12-18
Revised:2020-02-11
Online:2020-05-05
Published:2020-05-05
Contact:
Fang JIN
摘要:
乙烯催化齐聚和芳构化是烯烃高值化和合成液体燃料的关键过程,采用NH3-TPD谱图分析计算方法得到的探针分子脱附活化能作为催化剂的酸位点强度分布的参数,结合线性自由能理论在Br?nsted方程中引入酸强度对反应的影响因子γ作为动力学方程参数关联探针分子脱附活化能与反应活化能,明确固体酸酸强度的基准在催化动力学方程中的物理意义。建立同为MFI拓扑结构 Si /Al摩尔比为12.5、19、25、60、70的ZSM-5分子筛催化剂上不同酸强度位和乙烯齐聚及芳构化中氢转移、齐聚、芳构化各单元步骤以及对应的烷烃、低碳烯烃和芳烃产物分布的定量关系。根据拟合得到动力学参数γ可以发现不同强度的酸位点对乙烯齐聚及芳构化具有不同的作用系数。氨脱附活化能(DAE)为90 kJ·mol-1的酸位点是氢转移反应的主要活性位点,而对于乙烯齐聚反应中主要的活性酸位点为DAE 90 kJ·mol-1和124 kJ·mol-1,DAE为150 kJ·mol-1的酸位点是芳构化反应的主要活性位点。
中图分类号:
金放,张鹏,吴桂英,吴迪. Brönsted方程动力学模型研究ZSM-5催化乙烯齐聚及芳构化活性和酸强度分布之间的定量关系[J]. 化工学报, 2020, 71(5): 2076-2087.
Fang JIN,Peng ZHANG,Guiying WU,Di WU. Brönsted equation kinetic modeling for quantitative relationship between activity and acidity strength distribution in oligomerization and aromatization of ethylene over ZSM-5 catalyst[J]. CIESC Journal, 2020, 71(5): 2076-2087.
| T0/K | (T0+ΔT)/K | E/(kJ·mol-1) | KE/s-1 |
|---|---|---|---|
| 348 | 398 | 51.9 | 1.1×10-7 |
| 373 | 423 | 62.9 | 1.3×10-8 |
| 398 | 448 | 71.7 | 1.8×10-8 |
| 423 | 473 | 88.9 | 5.1×10-9 |
| 448 | 498 | 105.6 | 9.4×10-11 |
| 473 | 523 | 128.5 | 2.2×10-11 |
| 523 | 573 | 145.5 | 6.8×10-12 |
| 573 | 623 | 176.2 | 1.10×10-13 |
表1 根据式(9)计算单一酸强度位的E和KE
Table 1 E and KEvalues estimated by fitting Eq. (9) from acid sites with uniform acid strength over ZSM-5(Si/Al molar ratio 12.5)
| T0/K | (T0+ΔT)/K | E/(kJ·mol-1) | KE/s-1 |
|---|---|---|---|
| 348 | 398 | 51.9 | 1.1×10-7 |
| 373 | 423 | 62.9 | 1.3×10-8 |
| 398 | 448 | 71.7 | 1.8×10-8 |
| 423 | 473 | 88.9 | 5.1×10-9 |
| 448 | 498 | 105.6 | 9.4×10-11 |
| 473 | 523 | 128.5 | 2.2×10-11 |
| 523 | 573 | 145.5 | 6.8×10-12 |
| 573 | 623 | 176.2 | 1.10×10-13 |
| 样品 | TOF/(mmol·h-1·g-1) | Aci/(mmol·h-1·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| C2 | C3 | C3= | C4= | C6A | C7A | C8A | ||
| 19ZSM-5 | 67.76 | 0.51 | 0.37 | 28.20 | 22.67 | 1.93 | 12.66 | 11.65 |
| 25ZSM-5 | 62.06 | 0.45 | 0.54 | 23.68 | 15.36 | 1.75 | 9.80 | 12.46 |
| 60ZSM-5 | 48.91 | 0.25 | 0.24 | 18.08 | 10.68 | 1.32 | 6.17 | 6.23 |
| 70ZSM-5 | 45.41 | 0.16 | 0.25 | 13.78 | 9.87 | 0.83 | 5.33 | 5.63 |
表2 不同Si /Al摩尔比的ZSM-5 的乙烯齐聚和芳构化活性
Table 2 Activity of xZSM-5 with different Si/Al molar ratio for ethylene oligomerization and aromatization
| 样品 | TOF/(mmol·h-1·g-1) | Aci/(mmol·h-1·g-1) | ||||||
|---|---|---|---|---|---|---|---|---|
| C2 | C3 | C3= | C4= | C6A | C7A | C8A | ||
| 19ZSM-5 | 67.76 | 0.51 | 0.37 | 28.20 | 22.67 | 1.93 | 12.66 | 11.65 |
| 25ZSM-5 | 62.06 | 0.45 | 0.54 | 23.68 | 15.36 | 1.75 | 9.80 | 12.46 |
| 60ZSM-5 | 48.91 | 0.25 | 0.24 | 18.08 | 10.68 | 1.32 | 6.17 | 6.23 |
| 70ZSM-5 | 45.41 | 0.16 | 0.25 | 13.78 | 9.87 | 0.83 | 5.33 | 5.63 |
| 产物 | δ | γ | R2 | RSS | TSS |
|---|---|---|---|---|---|
| C2 | 0.0005 | 0.0031 | 0.95 | 3.36×10-9 | 6.35×10-8 |
| C3 | 0.0003 | 0.0072 | 0.77 | 1.17×10-8 | 5.15×10-8 |
| C3= | 0.0350 | 0.0025 | 0.86 | 1.62×10-5 | 1.20×10-4 |
| C4= | 0.0250 | 0.0021 | 0.85 | 1.15×10-5 | 7.49×10-5 |
| C6A | 0.0026 | 0.0018 | 0.99 | 1.24×10-8 | 1.03×10-6 |
| C7A | 0.0186 | 0.0010 | 0.94 | 2.07×10-6 | 3.40×10-5 |
| C8A | 0.0057 | 0.0100 | 0.98 | 1.29×10-7 | 3.91×10-5 |
表3 拟合不同Si /Al摩尔比的ZSM-5的乙烯齐聚和芳构化反应的催化活性和位点强度分布,计算出在平均强度酸位点上的Br?nsted关系的δ, γ值
Table 3 δ, γ values of Br?nsted relation on average strength acid sites calculated by fitting catalytic activity and site strength distribution for ethylene oligomerization and aromatization on different ZSM-5
| 产物 | δ | γ | R2 | RSS | TSS |
|---|---|---|---|---|---|
| C2 | 0.0005 | 0.0031 | 0.95 | 3.36×10-9 | 6.35×10-8 |
| C3 | 0.0003 | 0.0072 | 0.77 | 1.17×10-8 | 5.15×10-8 |
| C3= | 0.0350 | 0.0025 | 0.86 | 1.62×10-5 | 1.20×10-4 |
| C4= | 0.0250 | 0.0021 | 0.85 | 1.15×10-5 | 7.49×10-5 |
| C6A | 0.0026 | 0.0018 | 0.99 | 1.24×10-8 | 1.03×10-6 |
| C7A | 0.0186 | 0.0010 | 0.94 | 2.07×10-6 | 3.40×10-5 |
| C8A | 0.0057 | 0.0100 | 0.98 | 1.29×10-7 | 3.91×10-5 |
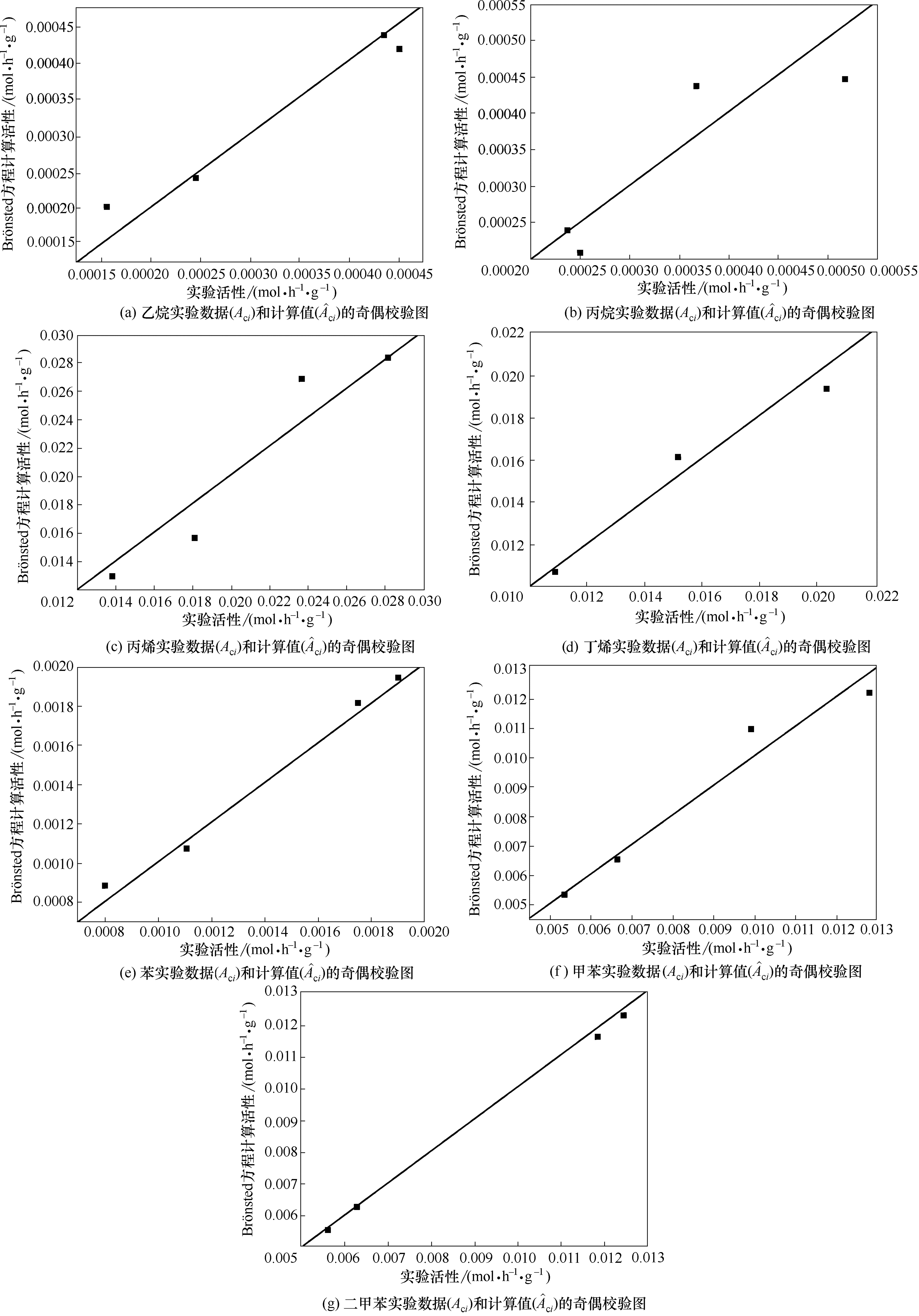
图4 Br?nsted方程拟合乙烷、丙烷、丙烯、丁烯、苯、甲苯、二甲苯实验数据(Aci)和计算值(?ci)的奇偶校验图
Fig.4 Parity plots between experimental Aci and model calculated date for ?ciwith Br?nsted ethane, propane, propylane, butene, benzene, toluene, xylene
| 产物 | 19ZSM-5 | 25ZSM-5 | 60ZSM-5 | 70ZSM-5 | R2 | |
|---|---|---|---|---|---|---|
| C2 | δ | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.99 |
| γ | 0.0030 | 0.0029 | 0.0032 | 0.0031 | ||
| C3 | δ | 0.0003 | 0.0003 | 0.0003 | 0.0003 | 0.99 |
| γ | 0.0060 | 0.0080 | 0.0072 | 0.0085 | ||
| C3= | δ | 0.0350 | 0.0320 | 0.0380 | 0.0360 | 0.99 |
| γ | 0.0025 | 0.0022 | 0.0030 | 0.0028 | ||
| C4= | δ | 0.0250 | 0.0210 | 0.0240 | 0.0270 | 0.99 |
| γ | 0.0025 | 0.0021 | 0.0026 | 0.0025 | ||
| C6A | δ | 0.0026 | 0.0025 | 0.0024 | 0.0026 | 0.99 |
| γ | 0.0017 | 0.0018 | 0.0019 | 0.0016 | ||
| C7A | δ | 0.0186 | 0.0160 | 0.0170 | 0.0170 | 0.99 |
| γ | 0.0010 | 0.0010 | 0.0011 | 0.0012 | ||
| C8A | δ | 0.0057 | 0.0056 | 0.0055 | 0.0056 | 0.99 |
| γ | 0.0100 | 0.0100 | 0.0100 | 0.0095 |
表4 拟合每种不同Si/Al摩尔比的ZSM-5的乙烯齐聚和芳构化反应的催化活性和位点强度分布,计算出平均强度酸位点上Br?nsted关系的δ、γ值
Table 4 δ,γ values of Br?nsted relation on average strength acid site calculated by fitting catalytic activity and site strength distribution for ethylene oligomerization and aromatization with ZSM-5 of different Si/Al molar ratio
| 产物 | 19ZSM-5 | 25ZSM-5 | 60ZSM-5 | 70ZSM-5 | R2 | |
|---|---|---|---|---|---|---|
| C2 | δ | 0.0005 | 0.0005 | 0.0005 | 0.0005 | 0.99 |
| γ | 0.0030 | 0.0029 | 0.0032 | 0.0031 | ||
| C3 | δ | 0.0003 | 0.0003 | 0.0003 | 0.0003 | 0.99 |
| γ | 0.0060 | 0.0080 | 0.0072 | 0.0085 | ||
| C3= | δ | 0.0350 | 0.0320 | 0.0380 | 0.0360 | 0.99 |
| γ | 0.0025 | 0.0022 | 0.0030 | 0.0028 | ||
| C4= | δ | 0.0250 | 0.0210 | 0.0240 | 0.0270 | 0.99 |
| γ | 0.0025 | 0.0021 | 0.0026 | 0.0025 | ||
| C6A | δ | 0.0026 | 0.0025 | 0.0024 | 0.0026 | 0.99 |
| γ | 0.0017 | 0.0018 | 0.0019 | 0.0016 | ||
| C7A | δ | 0.0186 | 0.0160 | 0.0170 | 0.0170 | 0.99 |
| γ | 0.0010 | 0.0010 | 0.0011 | 0.0012 | ||
| C8A | δ | 0.0057 | 0.0056 | 0.0055 | 0.0056 | 0.99 |
| γ | 0.0100 | 0.0100 | 0.0100 | 0.0095 |
| Parameter | Ei/(kJ·mol-1) | C2 | C3 | C3= | C4= | C6A | C7A | C8A |
|---|---|---|---|---|---|---|---|---|
| δ | 0.0004 | 0.00015 | 0.0436 | 0.0119 | 0.0019 | 0.011 | 0.015 | |
| γi | 63 | 0.0008 | -0.0326 | -0.0211 | -0.0243 | -0.0055 | -0.0007 | 0.002 |
| 90 | 0.0324 | 0.0488 | 0.0144 | -0.0161 | 0.0003 | 0.008 | -0.0279 | |
| 124 | -0.0001 | 0.0036 | -0.0043 | 0.0133 | 0.0019 | -0.0031 | -0.0179 | |
| 150 | 0.0064 | 0.0144 | 0.007 | 0.0121 | 0.0084 | 0.01 | 0.003 | |
| 175 | -0.0058 | 0.0021 | -0.0078 | -0.0131 | 0.0021 | -0.0053 | 0.005 | |
| R2 | 0.954 | 0.935 | 0.912 | 0.980 | 0.915 | 0.919 | 0.996 |
表5 拟合不同Si / Al摩尔比的ZSM-5的乙烯齐聚和芳构化反应的催化活性和位点强度分布,计算出在不同强度酸位点上的Br?nsted关系的δ、γi值
Table 5 δ, γi values of Br?nsted relation on different strength acid sites calculated by fitting catalytic activity and site strength distribution for ethylene oligomerization and aromatization on ZSM-5 of different Si/Al molar ratio
| Parameter | Ei/(kJ·mol-1) | C2 | C3 | C3= | C4= | C6A | C7A | C8A |
|---|---|---|---|---|---|---|---|---|
| δ | 0.0004 | 0.00015 | 0.0436 | 0.0119 | 0.0019 | 0.011 | 0.015 | |
| γi | 63 | 0.0008 | -0.0326 | -0.0211 | -0.0243 | -0.0055 | -0.0007 | 0.002 |
| 90 | 0.0324 | 0.0488 | 0.0144 | -0.0161 | 0.0003 | 0.008 | -0.0279 | |
| 124 | -0.0001 | 0.0036 | -0.0043 | 0.0133 | 0.0019 | -0.0031 | -0.0179 | |
| 150 | 0.0064 | 0.0144 | 0.007 | 0.0121 | 0.0084 | 0.01 | 0.003 | |
| 175 | -0.0058 | 0.0021 | -0.0078 | -0.0131 | 0.0021 | -0.0053 | 0.005 | |
| R2 | 0.954 | 0.935 | 0.912 | 0.980 | 0.915 | 0.919 | 0.996 |

图5 改进Br?nsted方程拟合乙烷、丙烷、丙烯、丁烯、苯、甲苯、二甲苯实验数据Aci和计算值?ci的奇偶校验图
Fig.5 Parity plots between experimental Aci and model calculated date for ?ciwith modified Br?nsted equation ethane, propane, propylane, butene, benzene, toluene, xylene
| 1 | 张惠明. 甲醇制低碳烯烃工艺技术新进展[J]. 化学反应工程与工艺, 2008, 24(2): 178-182. |
| Zhang H M. Advances in process research of methanol to light olefins[J]. Chemical Reaction Engineering and Technology, 2008, 24(2): 178-182. | |
| 2 | Chen J, Bozzano A, Glover B, et al. Recent advancements in ethylene and propylene production using the UOP/Hydro MTO process[J]. Catalysis Today, 2005, 106(1/2/3/4): 103-107. |
| 3 | Koempel H, Liebner W. Lurgi’s methanol to propylene (MTP®) report on a successful commercialisation[J]. Studies in Surface Science and Catalysis, 2007, 167: 261-267. |
| 4 | Ding W, Li S, Meitzner G, et al. Methane conversion to aromatics on Mo/H-ZSM5: structure of molybdenum species in working catalysts[J]. The Journal of Physical Chemistry B, 2000, 105(2): 506-513. |
| 5 | Okolie C, Lyu Y, Kovarik L, et al. Coupling of methane to ethane, ethylene and aromatics over nickel on ceria-zirconia at low temperatures[J]. ChemCatChem. , 2018, 10(12): 2700-2708. |
| 6 | 孙晓轩. 生物质气化合成甲醇二甲醚技术现状及展望[J]. 中外能源, 2007, 12(4): 29-36. |
| Sun X X. Status & Prospect of biomass gasfication methnol/dimethyl ether synthesis system[J]. Sino-global Energy, 2007, 12(4): 29-36. | |
| 7 | Bai P T, Rajmohan K S, Prasad P S S, et al. Oxidative dehydrogenation of ethane to ethylene over metal oxide catalysts using carbon dioxide[M]//Winter F, Agarwal R A, Hrdlicka J, et al. CO2 Separation, Purification and Conversion to Chemicals and Fuels. Singapore: Springer Singapore, 2019: 93-117. |
| 8 | Zhang M, Yu Y. Dehydration of ethanol to ethylene[J]. Industrial & Engineering Chemistry Research, 2013, 52(28): 9505-9514. |
| 9 | 钱伯章. 甲醇制汽油路线及其应用[J]. 化工设计通讯, 2009, 35(4): 31-36. |
| Qian B Z. Methanol to gasoline process and application[J]. Chemical Engineering Design Communications, 2009, 35(4): 31-36. | |
| 10 | Chang C, Chu C, Socha R. Methanol conversion to olefins over ZSM-5(I): Effect of temperature and zeolite SiO2/Al2O3 [J]. Journal of Catalysis, 1984, 86(2): 289-296. |
| 11 | Bröonsted J. Acid and basic catalysis[J]. Chem. Rev. , 1928, 5(3): 231-338. |
| 12 | Jin F, Li Y. A FTIR and TPD examination of the distributive properties of acid sites on ZSM-5 zeolite with pyridine as a probe molecule[J]. Catalysis Today, 2009, 145(1/2): 101-107. |
| 13 | Yi X, Liu K, Chen W, et al. Origin and structural characteristics of tri-coordinated extra-framework aluminum species in dealuminated zeolites[J]. Journal of the American Chemical Society, 2018, 140(34): 10764-10774. |
| 14 | Zheng A, Li S, Liu S, et al. Acidic properties and structure-activity correlations of solid acid catalysts revealed by solid-state NMR spectroscopy[J]. Accounts of Chemical Research, 2016, 49(4): 655-663. |
| 15 | Hashimoto K, Masuda T, Mori T. A method for calculating activation energy distribution of desorption from temperature-programmed desorption spectrum of ammonia[J]. Studies in Surface Science and Catalysis, 1986, 28: 503-510. |
| 16 | Auroux A. Calorimetry and Thermal Methods in Catalysis[M]. Hull R, Jagadish C, et al. Berlin: Springer Series in Materials Science, 2013: 131-171. |
| 17 | Smith A, Aranoff S. Thermodesorption of gases from solids[J]. The Journal of Physical Chemistry, 1958, 62(6): 684-686. |
| 18 | Cvetanovic R, Amenomiya Y. Application of a temperature-programmed desorption technique to catalyst studies[J]. Adv. Catal. , 1967, 17: 103-149. |
| 19 | Cvetanovic R, Amenomiya Y. A temperature programmed desorption technique for investigation of practical catalysts[J]. Catalysis Reviews, 1972, 6(1): 21-48. |
| 20 | Konvalinka J A. Analysis of second-order desorption kinetics in temperature-programmed desorption[J]. Journal of Catalysis, 1977, 48(1/2/3): 365-373. |
| 21 | Hunger B, Hoffmann J. Kinetic analysis of NH3 temperature programmed desorption (TPD) on a HZSM-5 zeolite[J]. Thermochimica Acta, 1986, 106: 133-140. |
| 22 | Forni L, Magni E. Temperature-programmed desorption study of ammonia desorption-diffusion in molecular sieves: I. Theory[J]. Journal of Catalysis, 1988, 112(2): 437-443. |
| 23 | Leary K, Michaels J, Stacy A. Temperature‐programmed desorption: multisite and subsurface diffusion models[J]. AIChE Journal, 1988, 34(2): 263-271. |
| 24 | Kuipers H, Leuven H, Visser W. The characterization of heterogeneous catalysts by XPS based on geometrical probability(1): Monometallic catalysts[J]. Surface and Interface Analysis, 1986, 8(6): 235-242. |
| 25 | Bhatia S, Beltramini J, Do D D. Temperature programmed analysis and its applications in catalytic systems[J]. Catalysis Today, 1990, 7(3): 309-438. |
| 26 | Falconer J L, Madix R J. Desorption rate isotherms in flash desorption analysis[J]. Journal of Catalysis, 1977, 48(1/2/3): 262-268. |
| 27 | Yang S R. Shangrun Y. Relationship between peak height, peak temperature and activation energy of desorption in tpd spectrum of first order desorption kinetics[J]. Acta Physico-Chimica Sinica, 1987, 3(3): 313-320. |
| 28 | Xu W X, Tao Y G, Yang S R. Determination of thermal desorption parameters Ed and a by linear analysis method[J]. Chin. J. Catal. , 1993, 14(1): 77-80. |
| 29 | Duan X W Q. A new method of quantitative analysis of TPD spectrograms[J]. Chin. J. Catal. , 1986, 7(2): 169-176. |
| 30 | Sawa M, Niwa M, Murakami Y. One-point method for determining acid strength of zeolite by temperature-programmed desorption of ammonia[J]. Zeolites, 1991, 11(1): 93-94. |
| 31 | Katada N, Igi H, Kim J. Determination of the acidic properties of zeolite by theoretical analysis of temperature-programmed desorption of ammonia based on adsorption equilibrium[J]. Journal of Physical Chemistry B, 1997, 101(31): 5969-5977. |
| 32 | Costa C, Lopes J M, Lemos F, et al. Activity-acidity relationship in zeolite Y(part Ⅲ): Application of Brönsted type equations[J]. Journal of Molecular Catalysis A: Chemical, 1999, 144(1): 233-238. |
| 33 | Costa C, Lopes J, Lemos F, et al. Acidity-activity relationship in zeolite Y: a preliminary study for n-heptane transformation[J]. Catalysis Letters, 1997, 44(3): 255-257. |
| 34 | Borges P, Ramos P R, Lemos A, et al. Activity-acidity relationship for alkane cracking over zeolites: n-hexane cracking over HZSM-5[J]. Journal of Molecular Catalysis A: Chemical, 2005, 229(1/2): 127-135. |
| 35 | Costa C, Dzikh I P, Lopes J M, et al. Activity-acidity relationship in zeolite ZSM-5. Application of Brönsted-type equations[J]. Journal of Molecular Catalysis A: Chemical, 2000, 154(1): 193-201. |
| 36 | Jin F, Fan Y, Wu G, et al. Modified Brönsted type equation with ammonia as probe molecule: quantitative acidity-activity relationship for pyridine synthesis with ZSM-5 catalyst[J]. Reaction Kinetics, Mechanisms and Catalysis, 2018, 123(2): 517-527. |
| 37 | Wang C M, Brogaard R Y, Weckhuysen B M, et al. Reactivity descriptor in solid acid catalysis: predicting turnover frequencies for propene methylation in zeotypes[J]. The Journal of Physical Chemistry Letters, 2014, 5(9): 1516-1521. |
| 38 | Masuda T, Fujikata Y, Ikeda H, et al. A method for calculating the activation energy distribution for desorption of ammonia using a TPD spectrum obtained under desorption control conditions[J]. Appl. Catal. A, 1997, 162(1): 29-40. |
| 39 | Karge H, Dondur V. Investigation of the distribution of acidity in zeolites by temperature-programmed desorption of probe molecules(Ⅰ): Dealuminated mordenites[J]. The Journal of Physical Chemistry, 1990, 94(2): 765-772. |
| 40 | Wang C, Wang L, Wu G, et al. Quantitative relationship between activity and acid site distribution in the oligomerization of ethylene over MCM-41 catalyst[J]. Catalysis Letters, 2020, 150: 429-437. |
| 41 | Jin F, Fan Y, Yuan M, et al. Single-event kinetic modeling of ethene oligomerization on ZSM-5[J]. Catalysis Today, 2018, 316: 129-141. |
| [1] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [4] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [5] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [6] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [7] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [8] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [9] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [10] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [11] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [12] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [13] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [14] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [15] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号