化工学报 ›› 2020, Vol. 71 ›› Issue (6): 2628-2642.DOI: 10.11949/0438-1157.20200043
张亚婷1,2( ),张博超1,张建兰1,李可可1,党永强1,段瑛峰1
),张博超1,张建兰1,李可可1,党永强1,段瑛峰1
收稿日期:2020-01-13
修回日期:2020-04-08
出版日期:2020-06-05
发布日期:2020-06-05
通讯作者:
张亚婷
作者简介:张亚婷(1972—),女,教授,基金资助:
Yating ZHANG1,2( ),Bochao ZHANG1,Jianlan ZHANG1,Keke LI1,Yongqiang DANG1,Yingfeng DUAN1
),Bochao ZHANG1,Jianlan ZHANG1,Keke LI1,Yongqiang DANG1,Yingfeng DUAN1
Received:2020-01-13
Revised:2020-04-08
Online:2020-06-05
Published:2020-06-05
Contact:
Yating ZHANG
摘要:
纳米石墨烯是组成石墨烯结构的一部分,尺寸一般介于1~100 nm,可以作为结构单元构筑石墨烯、碳纳米管和富勒烯等功能碳材料。纳米石墨烯具有一定的量子效应、边缘效应和界面效应,在新型分子电子器件、传感器等领域有着巨大的应用潜力。本文重点介绍“自下而上”化学合成纳米石墨烯的方法、含七元环或八元环特殊结构的纳米石墨烯、杂原子掺杂的纳米石墨烯以及纳米石墨烯的边缘修饰。探讨了不同合成方法的优势和特点,介绍了不同结构纳米石墨烯的性能及应用前景,概括了“自下而上”合成纳米石墨烯存在的问题及未来的发展趋势。
中图分类号:
张亚婷, 张博超, 张建兰, 李可可, 党永强, 段瑛峰. “自下而上”化学合成纳米石墨烯的研究进展[J]. 化工学报, 2020, 71(6): 2628-2642.
Yating ZHANG, Bochao ZHANG, Jianlan ZHANG, Keke LI, Yongqiang DANG, Yingfeng DUAN. Research progress in “bottom-up” chemical synthesis of nanographenes[J]. CIESC Journal, 2020, 71(6): 2628-2642.
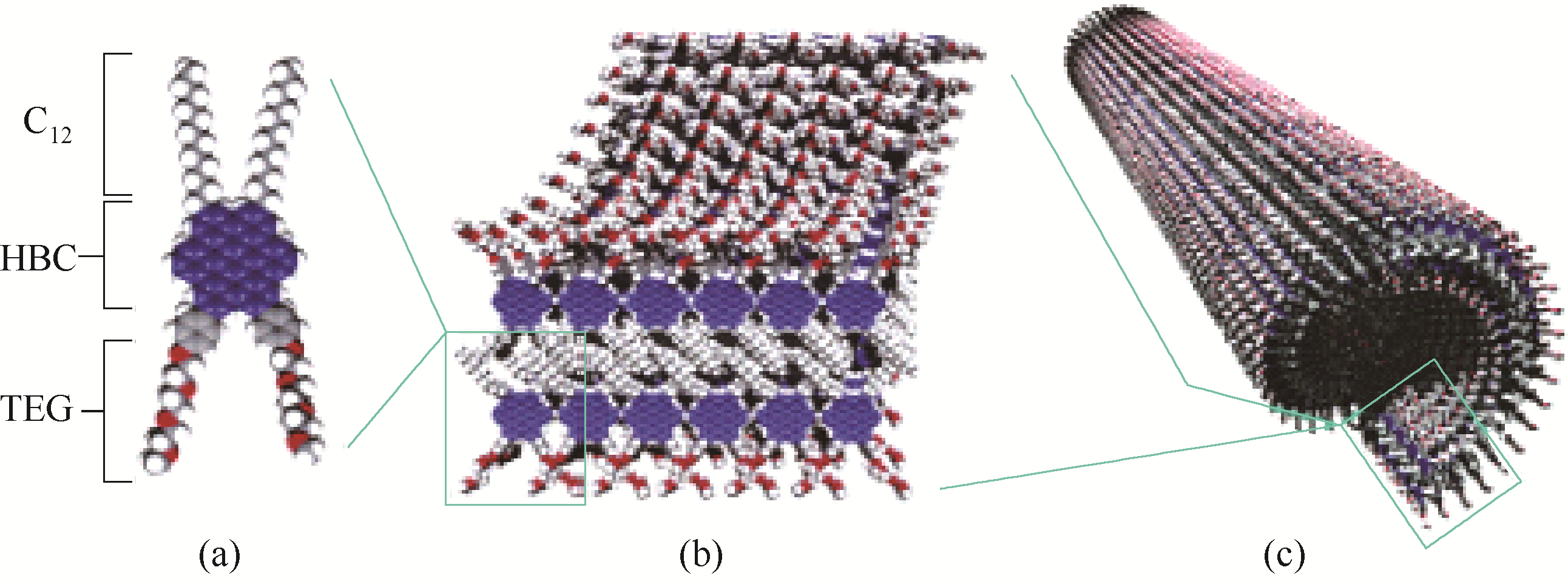
图21 HBC两亲物的结构示意图(a),自组装双层带(b)及石墨纳米管(c) [76]
Fig.21 Schematic diagram of HBC amphiphile (a), self-assembled double-layered belt (b) and graphite nanotubes (c) [76]
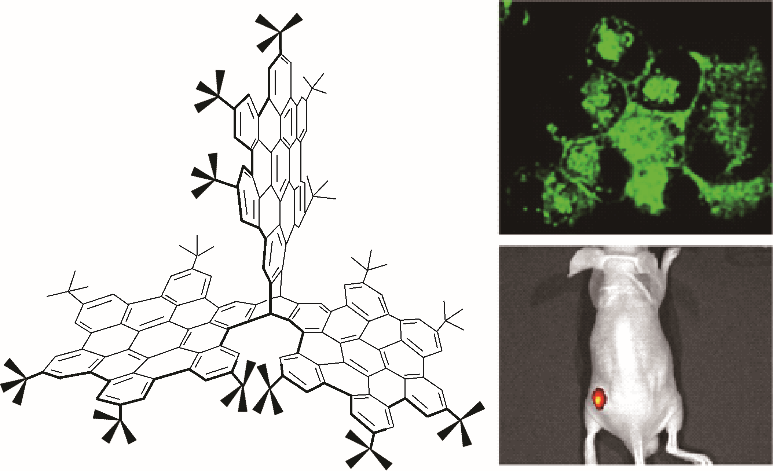
图22 含有三个HBC结构的三维纳米石墨烯在细胞荧光成像中的应用[77]
Fig.22 Application of three-dimensional nanographenes containing three HBC structures in cellular fluorescence imaging [77]
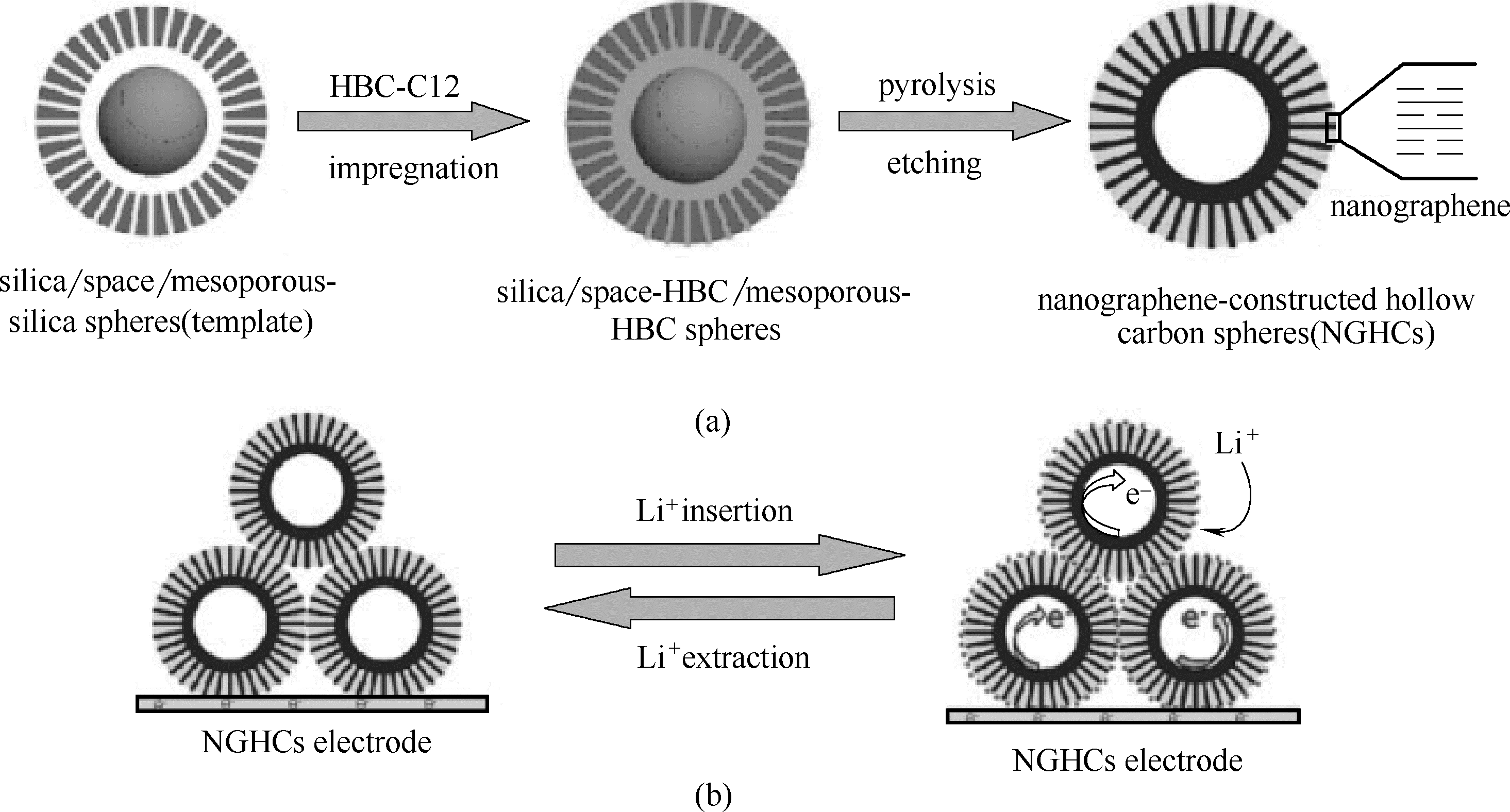
图23 NGHC的构造示意图(a)及在NGHCs电极的放电和充电过程中锂离子和电子的扩散(b) [81]
Fig.23 Schematic diagram of structure of NGHC (a) and diffusion of lithium ions and electrons during discharge and charging of NGHCs electrodes (b) [81]
| 1 | Novoselov K S, Geim A K, Morozov S V, et al. Two-dimensional gas of massless Dirac fermions in graphene[J]. Nature, 2005, 438: 197-200. |
| 2 | Westervelt R M. Graphene nanoelectronics[J]. Science, 2008, 320(5874): 324-325. |
| 3 | Schedin F, Geim A K, Morozov S V, et al. Detection of individual gas molecules adsorbed on graphene[J]. Nature Materials, 2007, 6(9): 652-655. |
| 4 | Dua V, Surwade S P, Ammu S, et al. All‐organic vapor sensor using inkjet‐printed reduced graphene oxide[J]. Angewandte Chemie, 2010, 122(12): 2200-2203. |
| 5 | Scheuermann G M, Rumi L, Steurer P, et al. Palladium nanoparticles on graphite oxide and its functionalized graphene derivatives as highly active catalysts for the Suzuki-Miyaura coupling reaction[J]. Journal of the American Chemical Society, 2009, 131(23): 8262-8270. |
| 6 | 张亚婷, 李可可, 任邵昭, 等. 煤基石墨烯宏观体的制备及其在CO2光催化还原过程中的应用[J]. 新型炭材料, 2015, 30(6): 539-544. |
| Zhang Y T, Li K K, Ren S Z, et al. Fabrication and electrochemical capacitive performance of PANI/coal-based three-dimensional graphene[J]. New Carbon Materials, 2015, 30(6): 539-544. | |
| 7 | Dreyer D R, Bielawski C W. Carbocatalysis: heterogeneous carbons finding utility in synthetic chemistry[J]. Chemical Science, 2011, 2(7): 1233-1240. |
| 8 | Pumera M. Graphene-based nanomaterials for energy storage[J]. Energy & Environmental Science, 2011, 4(3): 668-674. |
| 9 | Zhang Y T, Zhang K B, Jia K L, et al. Preparation of coal-based graphene quantum dots/α-Fe2O3 nanocomposites and their lithium-ion storage properties[J]. Fuel, 2019, 241: 646-652. |
| 10 | Sun Y, Wu Q, Shi G. Graphene based new energy materials[J]. Energy & Environmental Science, 2011, 4(4): 1113-1132. |
| 11 | Xu W, Ling X, Xiao J, et al. Surface enhanced Raman spectroscopy on a flat graphene surface[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(24): 9281-9286. |
| 12 | 张亚婷, 李景凯, 刘国阳, 等. MnO2/煤基石墨烯纳米复合材料的制备及其电化学性能[J]. 新型炭材料, 2016, 31(5): 545-549. |
| Zhang Y T, Li J K, Liu G Y, et al. Preparation of MnO2/coal-based graphene composites for supercapacitors[J]. New Carbon Materials, 2016, 31(5): 545-549. | |
| 13 | Schwierz F. Graphene transistors[J]. Nature Nanotechnology, 2010, 5(7): 487-496. |
| 14 | Chen L, Hernandez Y, Feng X, et al. From nanographene and graphene nanoribbons to graphene sheets: chemical synthesis[J]. Angewandte Chemie International Edition, 2012, 51(31): 7640-7654. |
| 15 | Rieger R, Müllen K. Forever young: polycyclic aromatic hydrocarbons as model cases for structural and optical studies[J]. Journal of Physical Organic Chemistry, 2010, 23(4): 315-325. |
| 16 | Liu J, Nilantha P, Shi Z Q, et al. Molecular-based design and emerging applications of nanoporous carbon spheres[J]. Nature Materials, 2015, 14: 763–774. |
| 17 | Dai Y, Liu Y, Ding K, et al. A short review of nanographenes: structures, properties and applications[J]. Molecular Physics, 2018, 116(7/8): 987-1002. |
| 18 | Narita A, Wang Y X, Feng X L, et al. New advances in nanographene chemistry[J]. Chemical Society Reviews, 2015, 44: 6616-6643. |
| 19 | Wang X Y, Yao X L, Müllen K. Polycyclic aromatic hydrocarbons in the graphene era[J]. Science China Chemistry, 2019, 62(9): 1099-1144 |
| 20 | Zhang Y T, Li K K, Ren S Z, et al. Coal-derived graphene quantum dots produced by ultrasonic physical tailoring and their capacity for Cu(Ⅱ) detection[J]. ACS Sustainable Chemistry & Engineering, 2019,7(11): 9793-9799. |
| 21 | Helge S, Balagi P, David J J, et al. Hexa-peri-hexabenzocoronene in organic electronics[J]. Pure and Applied Chemistry, 2012, 84(4): 1047-1067. |
| 22 | Clar E, Ironside C T, Zander M. The electronic interaction between benzenoid rings in condensed aromatic hydrocarbons. 1: 12-2: 3-4: 5-6: 7-8: 9-10: 11-hexabenzocoronene, 1: 2-3: 4-5: 6-10: 11-tetrabenzoanthanthrene, and 4: 5-6: 7-11: 12-13: 14-tetrabenzoperopyrene[J]. Journal of the Chemical Society, 1959: 142-147. |
| 23 | Halleux A, Martin R H, King G S D. Synthèses dans la série des dérivés polycycliques aromatiques hautement condensés. L'hexabenzo‐1, 12; 2, 3; 4, 5; 6, 7; 8, 9; 10, 11‐coronène, le tétrabenzo‐4, 5; 6, 7; 11, 12; 13, 14‐péropyrène et le tétrabenzo‐1, 2; 3, 4; 8, 9; 10, 11‐bisanthène[J]. Helvetica Chimica Acta, 1958, 41(5): 1177-1183. |
| 24 | Hendel W, Khan Z H, Schmidt W. Hexa-peri-benzocoronene, a candidate for the origin of the diffuse interstellar visible absorption bands[J]. Tetrahedron, 1986, 42(4): 1127-1134. |
| 25 | Wu J, Pisula W, Müllen K. Graphenes as potential material for electronics[J]. Chemical Reviews, 2007, 107(3): 718-747. |
| 26 | Yang X, Dou X, Müllen K. Efficient synthesis of symmetrically and unsymmetrically substituted hexaphenylbenzene analogues by Suzuki–Miyaura coupling reactions[J]. Chemistry-An Asian Journal, 2008, 3(4): 759-766. |
| 27 | Feng X, Wu J, Enkelmann V, et al. Hexa-peri-hexabenzocoronenes by efficient oxidative cyclodehydrogenation: the role of the oligophenylene precursors[J]. Organic Letters, 2006, 8(6): 1145-1148. |
| 28 | Weiss K, Beernink G, Dötz F, et al. Templateffekte bei der Herstellung polycyclischer aromatischer Kohlenwasserstoffe: Cyclodehydrierung und Planarisierung eines Hexaphenylbenzols an einer Kupferoberfläche[J]. Angewandte Chemie, 1999, 111(24): 3974-3978. |
| 29 | Simpson C D, Mattersteig G, Martin K, et al. Nanosized molecular propellers by cyclodehydrogenation of polyphenylene dendrimers[J]. Journal of the American Chemical Society, 126(10): 3139-3147. |
| 30 | Dötz F, Brand J D, Ito S, et al. Synthesis of large polycyclic aromatic hydrocarbons: variation of size and periphery[J]. Journal of the American Chemical Society, 2000, 122(32): 7707-7717. |
| 31 | Simpson C D, Brand J D, Berresheim A J, et al. Synthesis of a giant 222 carbon graphite sheet[J]. Chemical-A Eurpean Journal, 2002, 8(6): 1424-1429. |
| 32 | Rodríguez-Lojo D, Peña D, Pérez D, et al. Aryne-mediated syntheses of structurally related acene derivatives[J]. Organic Biomolecular Chemistry, 2010, 8(15): 3386-3388. |
| 33 | Schuler B, Collazos S, Gross L, et al. From perylene to a 22-ring aromatic hydrocarbon in one-pot[J]. Angewandte Chemie, 2014, 126(34): 9150-9152. |
| 34 | Romero C, Peña D, Pérez D, et al. Synthesis of extended triphenylenes by palladium‐catalyzed [2+2+2] cycloaddition of triphenylynes[J]. Chemical-A Eurpean Journal, 2006, 12(22): 5677-5684. |
| 35 | Rüdiger E C, Porz M, Schaffroth M, et al. Synthesis of soluble, alkyne-substituted trideca- and hexadeca-starphenes[J]. Chemical-A Eurpean Journal, 2014, 20(40): 12725-12728. |
| 36 | Xiao S, Myers M, Miao Q, et al. Molecular wires from contorted aromatic compounds[J]. Angewandte Chemie International Edition, 2005, 44(45): 7390-7394. |
| 37 | Feng X, Pisula W, Müllen K. Large polycyclic aromatic hydrocarbons: synthesis and discotic organization[J]. Pure and Applied Chemistry, 2009, 81(12): 2203-2224. |
| 38 | Huang P Y, Ruiz-Vargas C S, van der Zande A M, et al. Grains and grain boundaries in single-layer graphene atomic patchwork quilts[J]. Nature, 2011, 469(7330): 389-392. |
| 39 | Kurasch S, Kotakoski J, Lehtinen O, et al. Atom-by-atom observation of grain boundary migration in graphene[J]. Nano Letters, 2012, 12(6): 3168-3173. |
| 40 | Scott L T. Methods for the chemical synthesis of fullerenes[J]. Angew. Chem. Int. Ed., 2004, 43, 4994–5007. |
| 41 | Yamamoto K, Harada T, Nakazaki M, et al. Synthesis and characterization of [7]circulene[J]. Journal of the American Chemical Society, 1983, 105(24): 7171-7172. |
| 42 | Yamamoto K, Saitho Y, Iwaki D, et al. [7.7]circulene, a molecule shaped like a figure of eight [J]. Angewandte Chemie International Edition, 1991, 30(9): 1173-1174. |
| 43 | Luo J, Xu X, Mao R, et al. Curved polycyclic aromatic molecules that are π-isoelectronic to hexabenzocoronene[J]. Journal of the American Chemical Society, 2012, 134(33): 13796-13803. |
| 44 | Kawasumi K, Zhang Q, Segawa Y, et al. A grossly warped nanographene and the consequences of multiple odd-membered-ring defects[J]. Nature Chemistry, 2013, 5(9): 739-744. |
| 45 | Feng C N, Kuo M Y, Wu Y T. Synthesis, structural analysis, and properties of [8]circulenes[J]. Angewandte Chemie, 2013, 125(30): 7945-7948. |
| 46 | Sakamoto Y, Suzuki T. Tetrabenzo [8]circulene: aromatic saddles from negatively curved graphene [J]. Journal of the American Chemical Society, 2013, 135(38): 14074-14077. |
| 47 | Jiang W, Li Y, Wang Z. Heteroarenes as high performance organic semiconductors[J]. Chemical Society Reviews, 2013, 42(14): 6113-6127. |
| 48 | Stępień M, Gońka E, Żyła M, et al. Heterocyclic nanographenes and other polycyclic heteroaromatic compounds: synthetic routes, properties, and applications[J]. Chemical Reviews. 2017, 117: 3479-3716. |
| 49 | Wang X Y, Yao X L, Narita A, et al. Heteroatom-doped nanographenes with structural precision[J]. Accounts of Chemical Research, 2019, 52: 2491-2505. |
| 50 | Takase M, Enkelmann V, Sebastiani D, et al. Annularly fused hexapyrrolohexaazacoronenes: an extended π system with multiple interior nitrogen atoms displays stable oxidation states[J]. Angewandte Chemie, 2007, 119(29): 5620-5623. |
| 51 | Takase M, Narita T, Fujita W, et al. Pyrrole-fused azacoronene family: the influence of replacement with dialkoxybenzenes on the optical and electronic properties in neutral and oxidized states[J]. Journal of the American Chemical Society, 2013, 135(21): 8031-8040. |
| 52 | Draper S M, Gregg D J, Madathil R. Heterosuperbenzenes: a new family of nitrogen-functionalized, graphitic molecules[J]. Journal of the American Chemical Society, 2002, 124(14): 3486-3487. |
| 53 | Wei J, Han B, Guo Q, et al. 1, 5, 9‐Triazacoronenes: a family of polycyclic heteroarenes synthesized by a threefold Pictet-Spengler reaction[J]. Angewandte Chemie International Edition, 2010, 49(44): 8209-8213. |
| 54 | Berger R, Giannakopoulos A, Ravat P, et al. Synthesis of nitrogen-doped zigzag‐edge peripheries: dibenzo-9a-azaphenalene as repeating unit[J]. Angewandte Chemie International Edition, 2014, 53(39): 10520-10524. |
| 55 | Martin C J, Gil B, Perera S D, et al. Thienyl directed polyaromatic C—C bond fusions: S-doped hexabenzocoronenes[J]. Chemical Communications, 2011, 47(12): 3616-3618. |
| 56 | Chiu C Y, Kim B, Gorodetsky A A, et al. Shape-shifting in contorted dibenzotetrathienocoronenes[J]. Chemical Science, 2011, 2: 1480-1486. |
| 57 | Chen L, Puniredd S R, Tan Y Z, et al. Hexathienocoronenes: synthesis and self-organization[J]. Journal of the American Chemical Society, 2012, 134(43): 17869-17872. |
| 58 | Chernichenko K Y, Sumerin V V, Shpanchenko R V, et al. “Sulflower”: a new form of carbon sulfide[J]. Angewandte Chemie International Edition, 2006, 45(44): 7367-7370. |
| 59 | Lu R Q, Zhou Y N, Yan X Y, et al. Thiophene-fused bowl-shaped polycyclic aromatics with a dibenzo [a, g] corannulene core for organic field-effect transistors[J]. Chemical Communications, 2015, 51(9): 1681-1684. |
| 60 | Feng X, Wu J, Ai M, et al. Triangle-shaped polycyclic aromatic hydrocarbons[J]. Angewandte Chemie, 2007, 119(17): 3093-3096. |
| 61 | Escande A, Ingleson M J. Fused polycyclic aromatics incorporating boron in the core: fundamentals and applications[J]. Chemical Communications, 2015, 51(29): 6257-6274. |
| 62 | Wang X Y, Wang J Y, Pei J. BN heterosuperbenzenes: synthesis and properties[J]. Chemical-A Eurpean Journal, 2015, 21(9): 3528-3539. |
| 63 | Pisula W, Feng X, Müllen K. Charge-carrier transporting graphene-type molecules[J]. Chemistry of Materials, 2010, 23(3): 554-567. |
| 64 | Sergeyev S, Pisula W, Geerts Y H. Discotic liquid crystals: a new generation of organic semiconductors[J]. Chemical Society Reviews, 2007, 36(12): 1902-1929. |
| 65 | Ito S, Wehmeier M, Brand J D, et al. Synthesis and self‐assembly of functionalized hexa‐peri‐hexabenzocoronenes[J]. Chemical-A Eurpean Journal, 2000, 6(23): 4327-4342. |
| 66 | Wu J, Watson M D, Müllen K. The versatile synthesis and self-assembly of star-type hexabenzocoronenes[J]. Angewandte Chemie International Edition, 2003, 42(43): 5329-5333. |
| 67 | Wu J, Watson M D, Zhang L, et al. Hexakis (4-iodophenyl)-peri-hexabenzocoronene—a versatile building block for highly ordered discotic liquid crystalline materials[J]. Journal of the American Chemical Society, 2004, 126(1): 177-186. |
| 68 | Wu J, Li J, Kolb U, et al. A water-soluble hexa-peri-hexabenzocoronene: synthesis, self-assembly and role as template for porous silica with aligned nanochannels[J]. Chemical Communications, 2006, (1): 48-50. |
| 69 | Wu J, Baumgarten M, Debije M G, et al. Arylamine-substituted hexa-peri-hexabenzocoronenes: facile synthesis and their potential applications as “coaxial” hole-transport materials[J]. Angewandte Chemie International Edition, 2004, 43: 5331-5335. |
| 70 | Tan Y Z, Yang B, Parvez K, et al. Atomically precise edge chlorination of nanographenes and its application in graphene nanoribbons[J]. Nature Communications, 2013, 4: 2646. |
| 71 | Mkhalid I A I, Barnard J H, Marder T B, et al. C- H activation for the construction of C- B bonds[J]. Chemical Reviews, 2009, 110(2): 890-931. |
| 72 | Yamaguchi R, Hiroto S, Shinokubo H. Synthesis of oxygen-substituted hexa-peri-hexabenzocoronenes through Ir-catalyzed direct borylation[J]. Organic Letters, 2012, 14(10): 2472-2475. |
| 73 | Yamaguchi R, Ito S, Lee B S, et al. Functionalization of hexa-peri-hexabenzocoronenes: investigation of the substituent effects on a superbenzene[J]. Chemistry-An Asian Journal, 2013, 8(1): 178-190. |
| 74 | He Y, Yamamoto Y, Jin W, et al. Hexabenzocoronene graphitic nanotube appended with dithienylethene pendants: photochromism for the modulation of photoconductivity[J]. Advanced Materials, 2010, 22: 829. |
| 75 | Hill J P, Jin W, Kosaka A, et al. Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube[J]. Science, 2004, 304: 1481-1483. |
| 76 | Jin W, Fukushima T, Kosaka A, et al. Controlled self-assembly triggered by olefin metathesis: cross-linked graphitic nanotubes from an amphiphilic hexa-peri-hexabenzocoronene[J]. Journal of the American Chemical Society, 2005, 127: 8284-8285 |
| 77 | Zhang C, Liu Y, Xiong X Q, et al. Three-dimensional nanographene based on triptycene: synthesis and its application in fluorescence imaging[J]. Organic Letters, 2012, 23: 5912-5915. |
| 78 | Yin M, Shen J, Pisula W, et al. Functionalization of self-assembled hexa-peri-hexabenzocoronene fibers with peptides for bioprobing[J]. Journal of the American Chemical Society, 2009, 131: 14618. |
| 79 | Hill J P, Jin W, Kosaka A, et al. Self-assembled hexa-peri-hexabenzocoronene graphitic nanotube[J]. Science, 2004, 304(5676): 1481-1483. |
| 80 | Yamamoto Y, Fukushima T, Suna Y, et al. Photoconductive coaxial nanotubes of molecularly connected electron donor and acceptor layers[J]. Science, 2006, 314(5806): 1761-1764. |
| 81 | Yang S B, Feng X L, Zhi L J, et al. Nanographene-constructed hollow carbon spheres and their favorable electroactivity with respect to lithium storage[J]. Advanced Materails, 2010, 22: 838-842 |
| 82 | Amann A, Smith D. Breath analysis for clincal diagnosis and therapeutic monitoring[C]//Conference Breath Gas Analysis for Medical Diagnostics. Dornbirn, Austria, 2004. |
| 83 | Amann A, Spanel P, Smith D. Breath analysis: the approach towards clinical applications[J]. Mini-reviews in Medicinal Chemistry, 2007, 7: 115. |
| 84 | Zilberman Y, Tisch U, Pisula W, et al. Spongelike structures of hexa-peri-hexabenzocoronene derivatives enhance the sensitivity of chemiresistive carbon nanotubes to nonpolar volatile organic compounds of cancer[J]. Langmuir, 2009, 25: 5411-5416. |
| [1] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [2] | 王晓波,赵青山,程智年,张浩然,胡涵,王路海,吴明铂. 高性能碳基储能材料的设计、合成与应用[J]. 化工学报, 2020, 71(6): 2660-2677. |
| [3] | 董灵玉, 葛睿, 原亚飞, 唐宋元, 郝广平, 陆安慧. 多孔炭基二氧化碳电催化材料研究进展[J]. 化工学报, 2020, 71(6): 2492-2509. |
| [4] | 周宇, 王宇新. 杂原子掺杂碳基氧还原反应电催化剂研究进展[J]. 化工学报, 2017, 68(2): 519-534. |
| [5] | 卢慧丽, 林东强, 姚善泾. 亲和仿生层析及在抗体纯化中的应用[J]. 化工学报, 2016, 67(9): 3523-3535. |
| [6] | 戚玉娇, BRIDIER Arnaud, DESMOND LE QUEMENER Elie, 吕凡, 何品晶, BOUCHEZ Théodore. 甲烷化抑制剂在微生物电化学合成乙酸系统中的生物抑制效应[J]. 化工学报, 2016, 67(5): 2033-2040. |
| [7] | 孙旭辉,郑文平,庹万权,于海辉,王冬. 电化学合成法制备高铁酸盐的研究进展[J]. 化工进展, 2014, 33(06): 1380-1386. |
| [8] | 王维大1,2,李浩然2,冯雅丽1,唐新华3,杜竹玮2,杜云龙1. 微生物燃料电池的研究应用进展[J]. 化工进展, 2014, 33(05): 1067-1076. |
| [9] | 马强强,赵广荣. 左旋多巴合成研究进展[J]. 化工进展, 2013, 32(06): 1367-1371. |
| [10] | 马淳安,储诚普,徐颖华,张虹. 3,6-二氯吡啶甲酸合成方法进展 [J]. CIESC Journal, 2011, 62(9): 2398-2405. |
| [11] | 马 烽,杨晓勇,陈明辉. 微波膨化钾-四氢呋喃-石墨层间化合物制备膨胀石墨 [J]. CIESC Journal, 2010, 29(9): 1715-. |
| [12] | 齐 济1,宁桂玲2,刘俊龙3,王 琛1. 二氧化钒粉体研究的新进展 [J]. CIESC Journal, 2010, 29(8): 1513-. |
| [13] | 马淳安, 徐颖华, 褚有群, 毛信表, 赵峰鸣, 朱英红. 3,6-二氯吡啶甲酸电化学合成及其工业化生产 [J]. 化工学报, 2010, 61(3): 699-703. |
| [14] | 董广新,蒋稼欢. 基于微流动混合的微纳米粒子合成进展 [J]. CIESC Journal, 2010, 29(11): 2026-. |
| [15] | 李成未;齐涛;王福安;张懿;陈根生. 铬酸酐电化学合成中阳极液在钛基多元金属氧化物复合电极上的电化学研究 [J]. CIESC Journal, 2008, 59(3): 670-672. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号