化工学报 ›› 2020, Vol. 71 ›› Issue (6): 2612-2627.DOI: 10.11949/0438-1157.20200103
收稿日期:2020-02-03
修回日期:2020-04-10
出版日期:2020-06-05
发布日期:2020-06-05
通讯作者:
陈爱兵
作者简介:宗爽(1984—),女,硕士研究生,基金资助:
Shuang ZONG1,2( ),Xinying LIU2,Aibing CHEN1(
),Xinying LIU2,Aibing CHEN1( )
)
Received:2020-02-03
Revised:2020-04-10
Online:2020-06-05
Published:2020-06-05
Contact:
Aibing CHEN
摘要:
金属-有机框架( metal-organic frameworks,MOFs)衍生的0维材料,具有比表面积大、孔隙率高、孔径可调等特点,近年来广泛用于锂离子电池、燃料电池和超级电容器等储能器件中。电极材料是决定超级电容器电化学性能的关键因素。MOFs衍生的0维材料在超级电容器中的应用具有广阔的前景。综述了MOFs衍生的0维材料在超级电容器中的研究进展,探讨了目前在该领域中存在的问题,并对其未来的发展前景进行了展望。
中图分类号:
宗爽, 刘歆颖, 陈爱兵. 金属有机框架衍生的0维材料在超级电容器中的应用[J]. 化工学报, 2020, 71(6): 2612-2627.
Shuang ZONG, Xinying LIU, Aibing CHEN. Metal-organic frameworks-derived zero-dimensional materials for supercapacitors[J]. CIESC Journal, 2020, 71(6): 2612-2627.
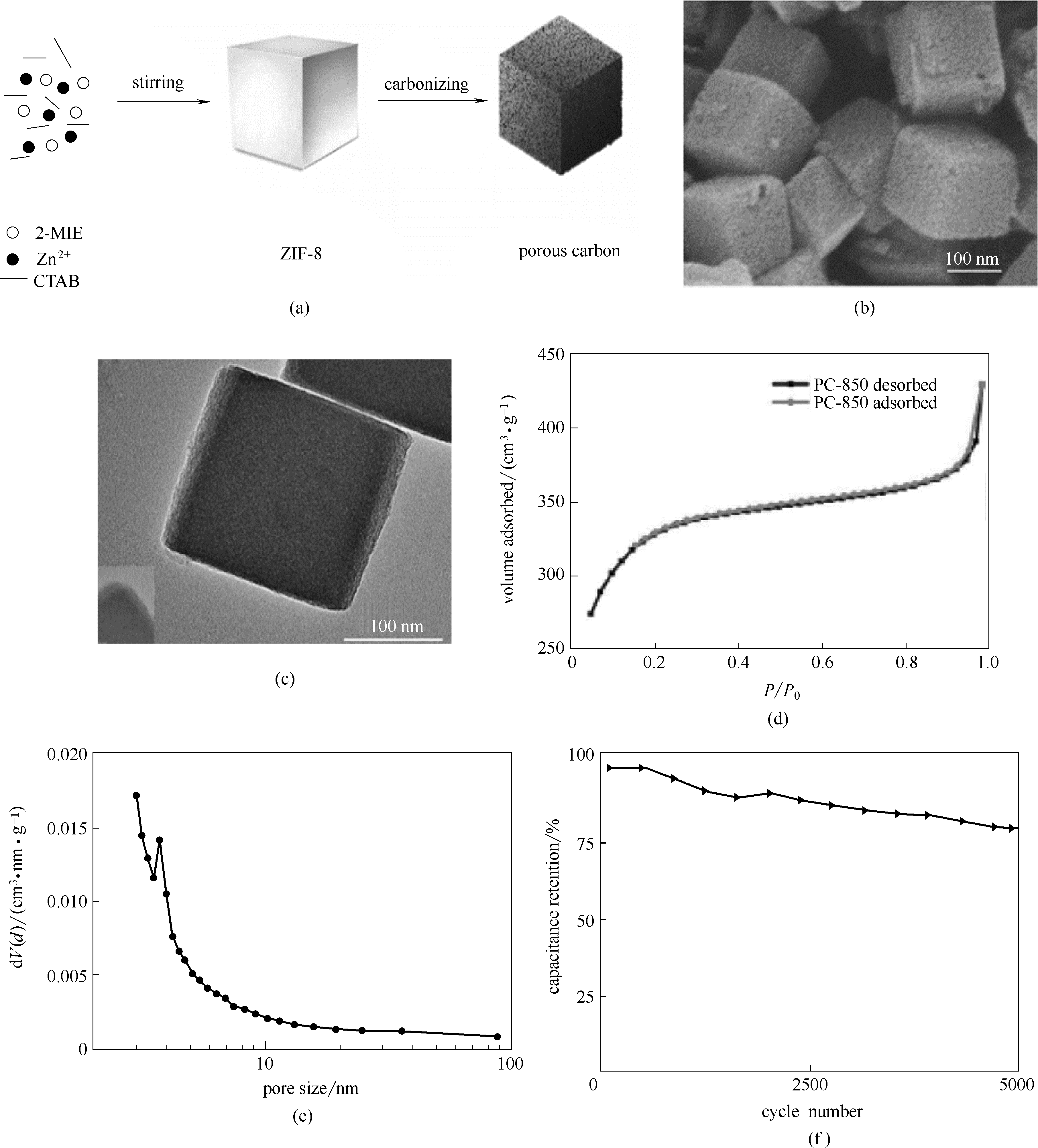
图1 ZIF-8衍生的纳米六面体多孔碳的合成(a); 纳米六面体多孔碳的结构表征[(b)~(e)]; 纳米六面体多孔碳的电化学性能(f) [46]
Fig.1 The preparation procedure of nano-hexahedron porous carbon (a); structural characterization of the nano-hexahedron porous carbon[(b)—(e)]; electrochemical performance of the nano-hexahedron porous carbon electrode (f) [46]

图2 双壳Zn-Co-S RDCs形成示意图(a); 双壳Zn-Co-S RDCs的结构表征[(b)~(e)];单壳及双壳Zn-Co-S RDCs的电化学性能[(f)~(h)][47]
Fig.2 Schematic illustration of the formation process of double-shelled Zn-Co-S RDCs (a); structural characterization of the single-shelled Zn-Co-S RDCs[(b)—(e)]; electrochemical performance of the double-shelled and single-shelled Zn-Co-S RDCs [(f)—(h)][47]
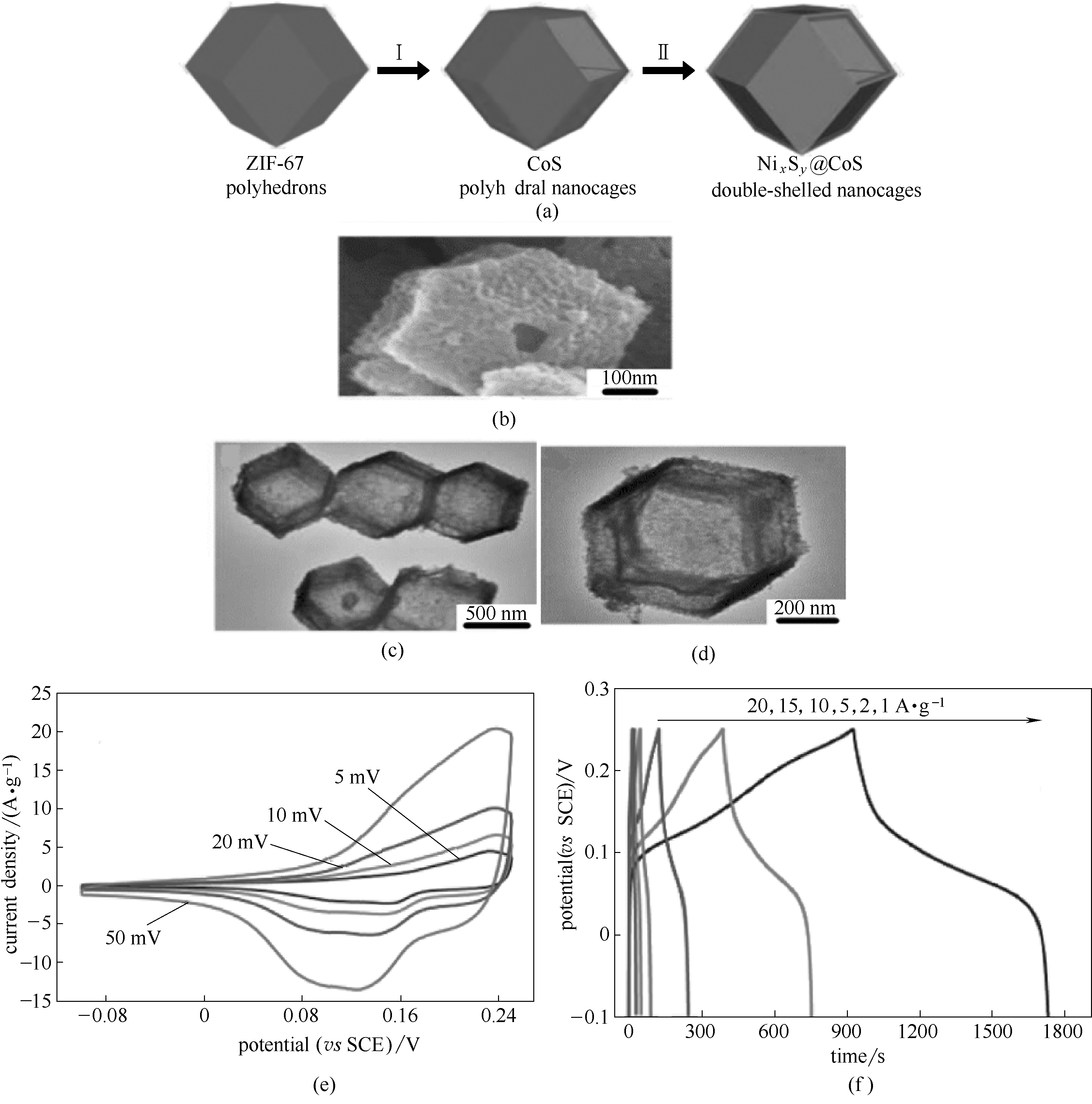
图3 NixSy@CoS双壳纳米笼的合成示意图(a); NixSy@CoS双壳纳米笼的结构表征[(b)~(d)]; NixSy@CoS双壳纳米笼的电化学性能[(e)、(f)] [48]
Fig.3 Schematic illustration of the formation process of NixSy@CoS double-shelled nanocages (a); structural characterization of the NixSy@CoS double-shelled nanocages[(b)—(d)]; electrochemical performance of the NixSy@CoS double-shelled nanocages[(e),(f)] [48]
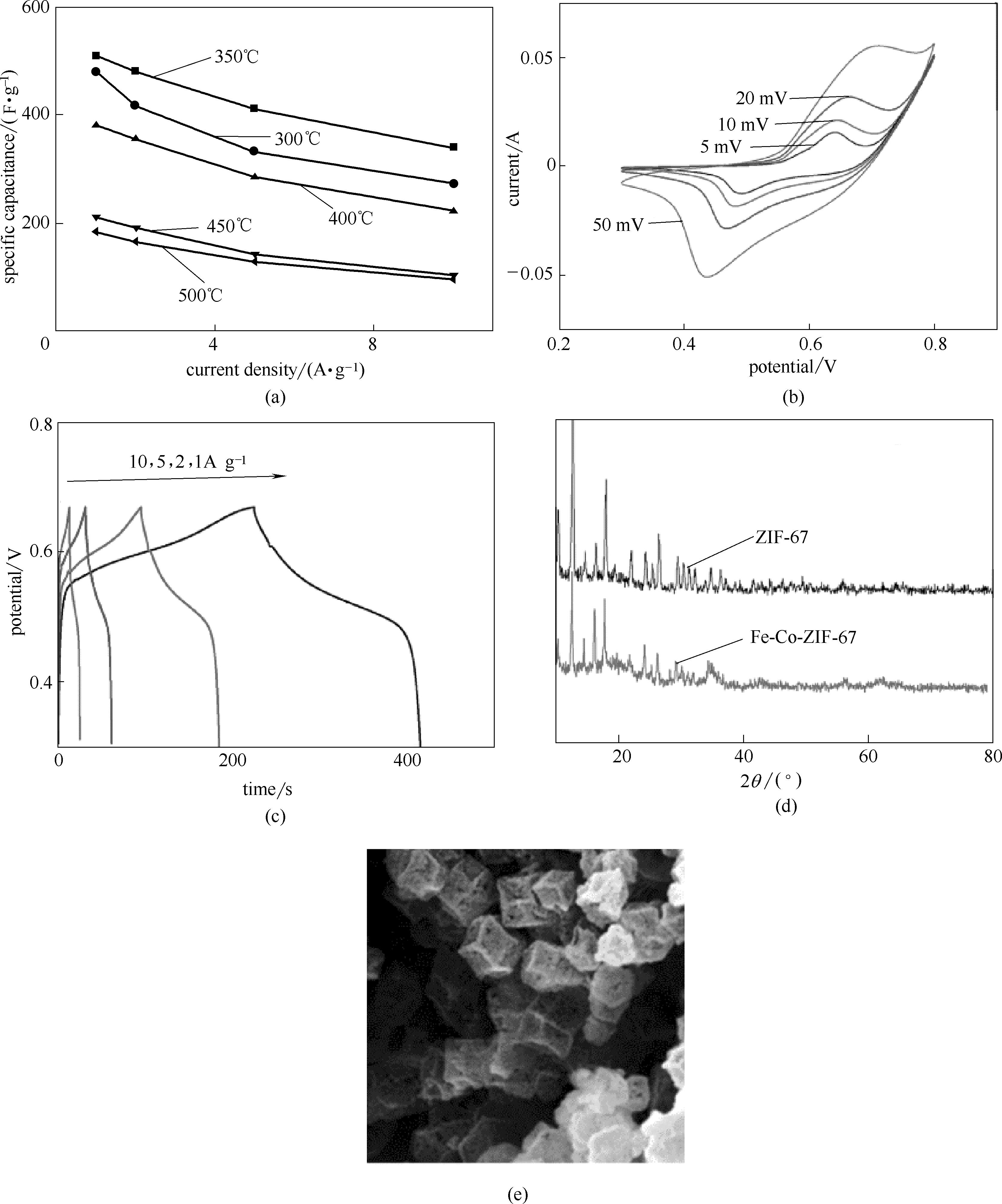
图4 FeCo2O4电化学性能[(a)~(c)]; 纯ZIF-67、铁-钴-ZIF-67 (d)、FeCo2O4的结构表征(e) [49]
Fig.4 Electrochemical performance of the FeCo2O4 samples[(a)—(c)]; structural characterization of the pure ZIF-67, Fe-Co-ZIF-67(d) and FeCo2O4 (e) samples[49]

图5 Co3O4-CeO2中空多面体的扫描电镜图(a)和透射电镜图(b); Co3O4-CeO2中空多面体的恒流充放电曲线(c); Co3O4-CeO2中空多面体的Ragone曲线(d) [50]
Fig.5 SEM (a) and TEM (b) images of the hybrid porous Co3O4-CeO2 hollow polyhedrons; GCD curves (c) and Ragone plots (d) of the hybrid porous Co3O4-CeO2 hollow polyhedrons [50]
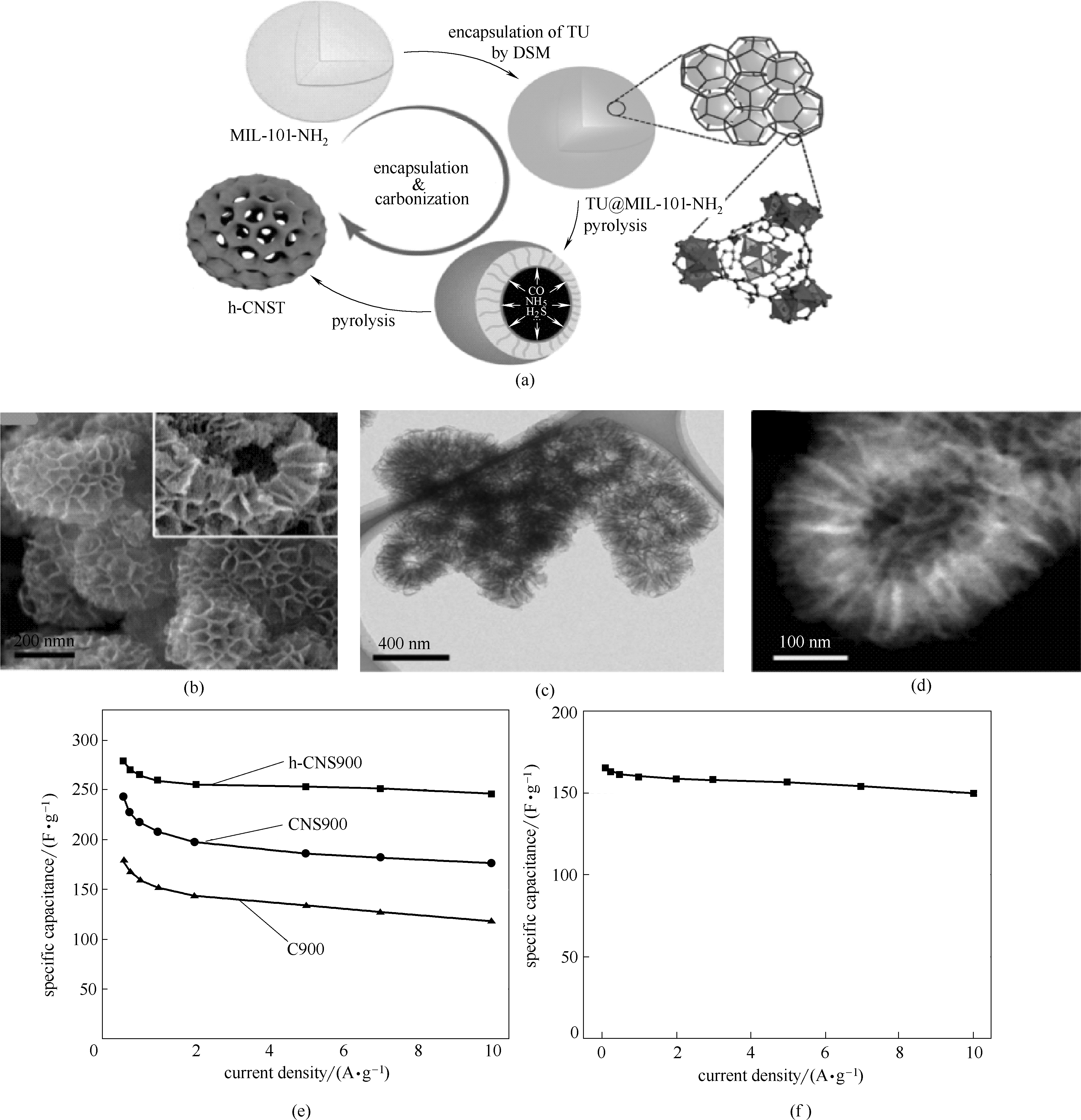
图6 N,S-共掺杂的中空多孔碳纳米胶囊(h-CNST)制备路线 (a); h-CNS900的扫描电镜图(b); h-CNS900的透射电镜图(c); h-CNS900的场发射高倍扫描电镜图(d); 1 mol·L-1 H2SO4电解液中h-CNS900, CNS900, C900 电容随电流密度的变化曲线(e); h-CNS900在离子液体电解液中电容随电流密度变化的曲线(900代表900℃下得到的产物)(f) [55]
Fig.6 Schematic diagram of the formation of the N and S co-doped hollow cellular carbon nanocapsules (h-CNST) (a); SEM images of the h-CNS900 hollow cellular carbon nanocapsules (b); TEM images of the h-CNS900 hollow cellular carbon nanocapsules (c); HAADF-STEM images of the h-CNS900 hollow cellular carbon nanocapsules (d); variation of specific capacitance as a function of current density for h-CNS900, CNS900 and C900 at 1 mol·L-1 H2SO4 electrolyte (e); variation of specific capacitance as a function of current densities at IL electrolyte (f) [55]
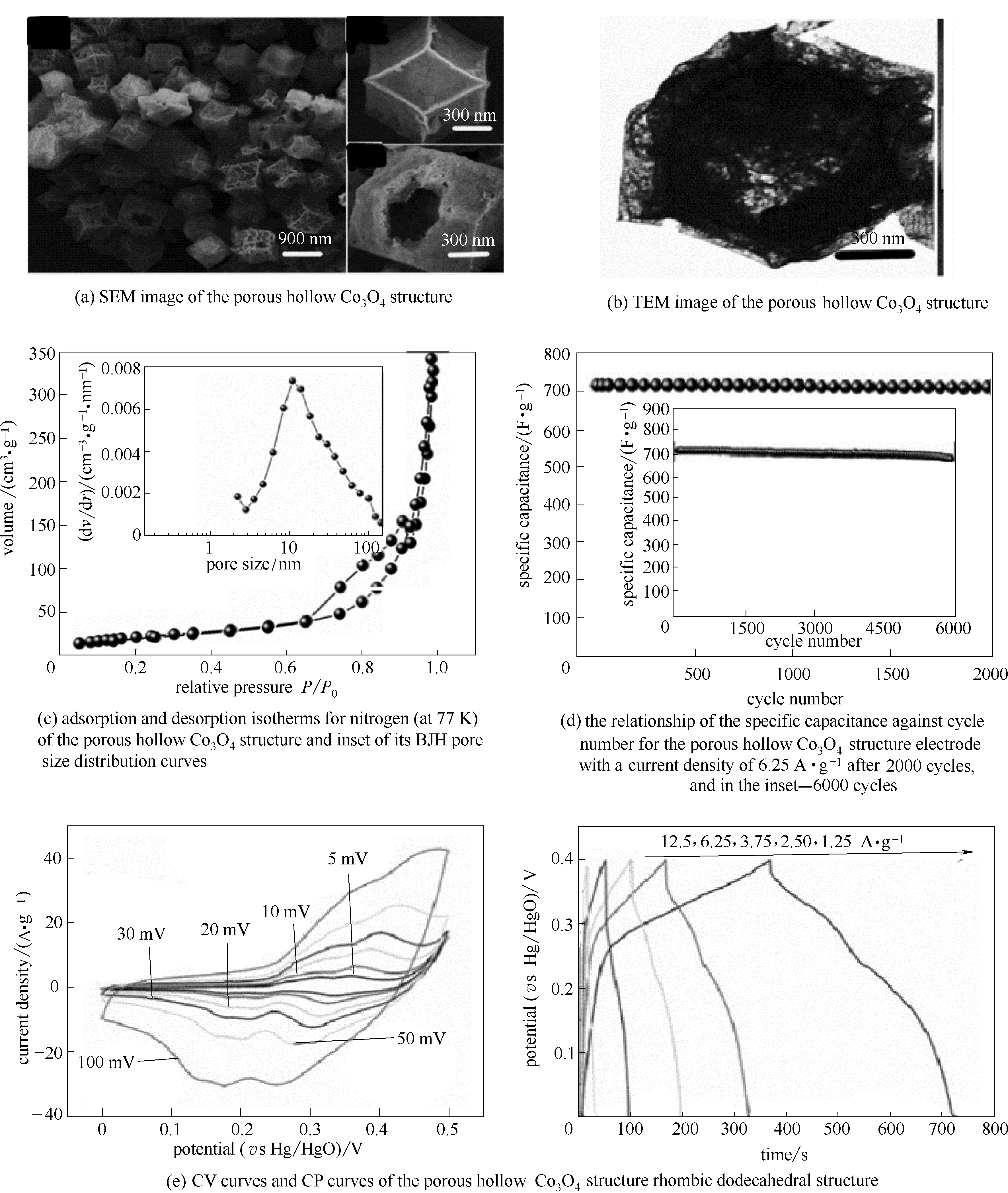
图7 多孔中空氧化钴的结构表征[(a)~(c)]; 多孔中空氧化钴的电化学性能[(d)、(e)] [56]
Fig.7 Structural characterization of the porous hollow Co3O4 structure(a—c); electrochemical performance of the porous hollow Co3O4[(d),(e)][56]
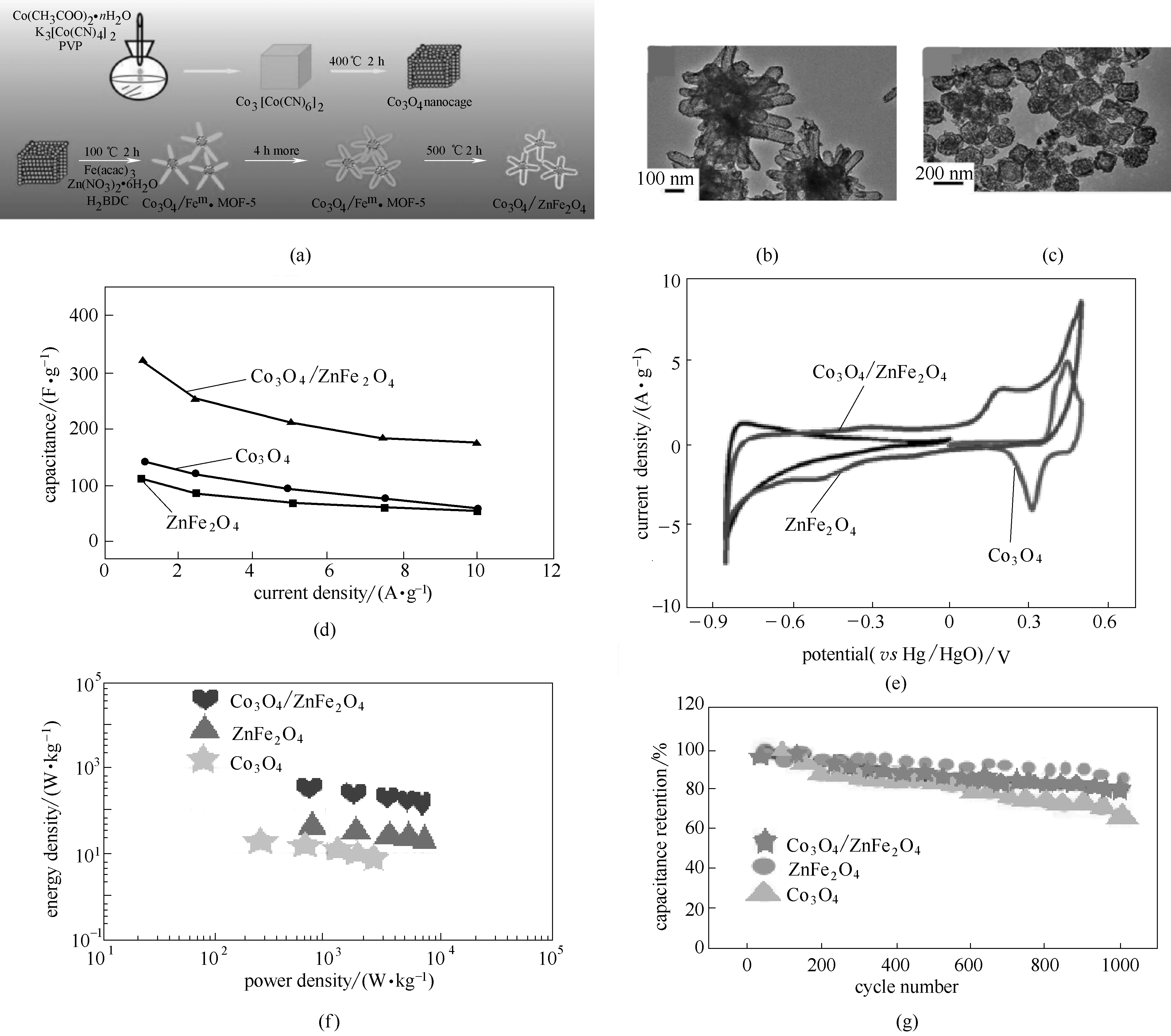
图8 海星状中空多孔Co3O4/ZnFe2O4合成示意图(a); 海星状中空多孔Co3O4/ZnFe2O4的透射电镜图(b); Co3O4纳米笼的透射电镜图(c); Co3O4、ZnFe2O4、Co3O4/ZnFe2O4的电化学性能[(d)~(g)] [57]
Fig.8 Schematic illustration of the synthesis procedure of the starfish-shaped porous Co3O4/ZnFe2O4 hollow nanocomposite (a); TEM image of Co3O4/ZnFe2O4 hollow nanocomposites (b); TEM images of Co3O4 nanocages (c); electrochemical performance of Co3O4, ZnFe2O4, Co3O4/ZnFe2O4 [(d)—(g)] [57]
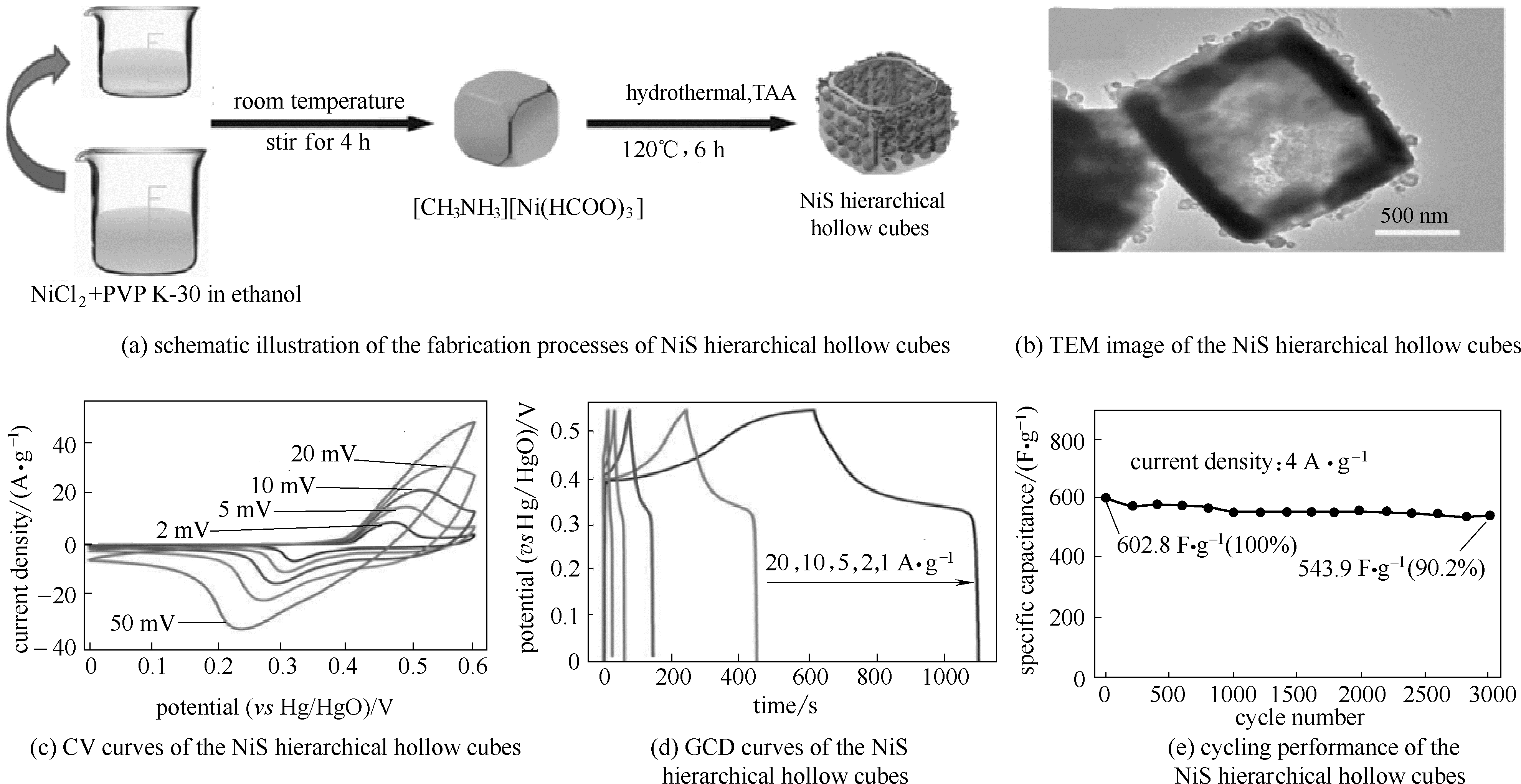
图9 硫化镍中空立方体的合成(a); 硫化镍中空立方体透射电镜图(b); 硫化镍中空立方体的电化学性能[(c)~(e)] [64]
Fig.9 Schematic illustration of the fabrication processes of NiS hierarchical hollow cubes (a); TEM image of the NiS hierarchical hollow cubes (b); electrochemical performance of the NiS hierarchical hollow cubes[(c)—(e)] [64]
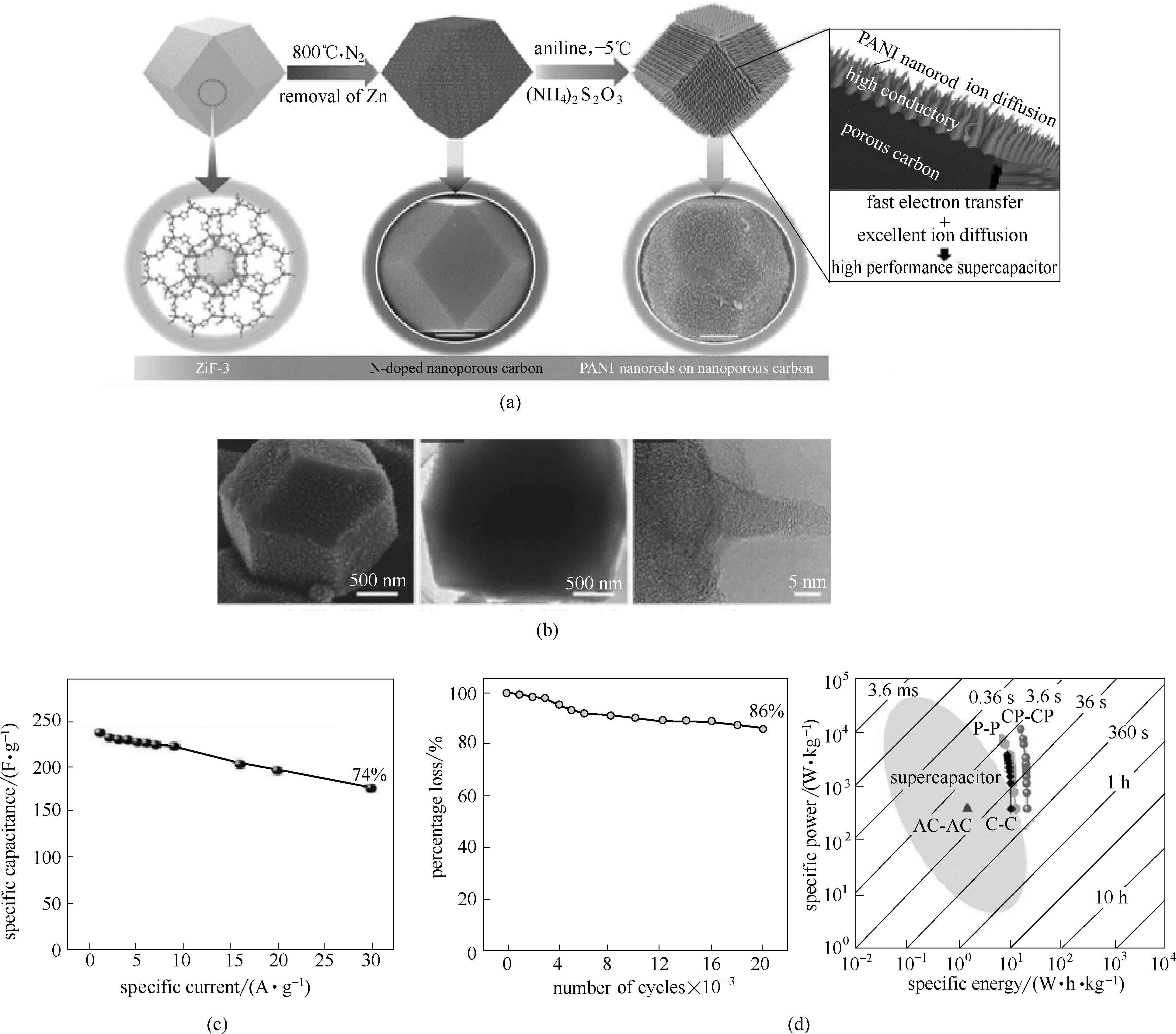
图10 合成核壳多孔碳-PANI复合物的流程图(a);核壳多孔碳-PANI复合物的结构表征(b) [66];核壳多孔碳-PANI复合物的电化学性能[(c)、(d)]
Fig.10 Schematic illustration of synthetic process for the attainment of nanoporous carbon-PANI core-shell nanocomposite materials(a); structural characterization of the nanoporous carbon-PANI core-shell nanocomposite materials (b)[66]; electrochemical performance of the carbon-PANI (CP-CP) nanocomposite[(c), (d)]
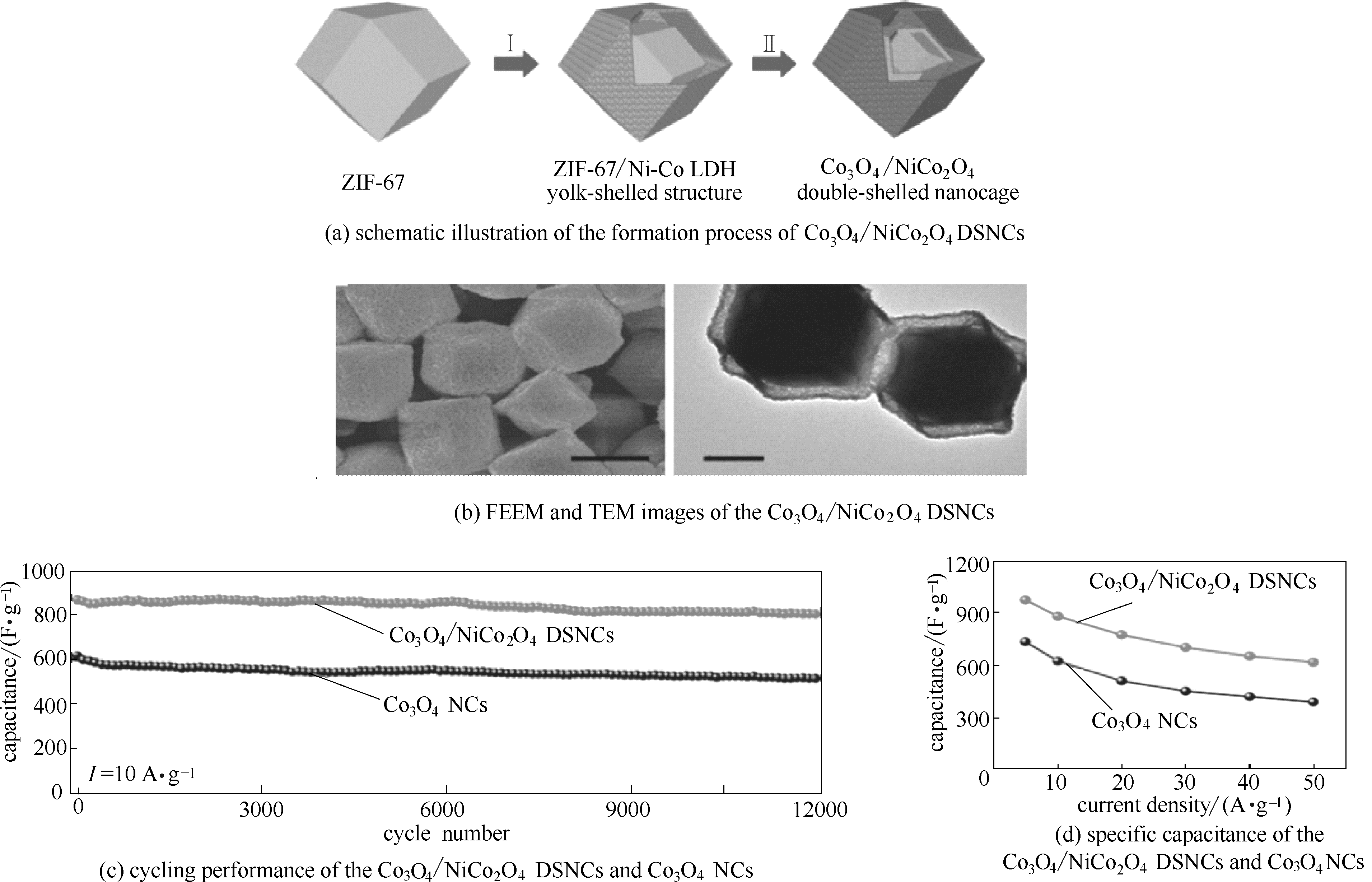
图11 合成Co3O4/NiCo2O4双壳结构的纳米笼制备的示意图(a); Co3O4/NiCo2O4双壳结构的纳米笼的结构表征(b); Co3O4/NiCo2O4双壳结构的纳米笼和氧化钴纳米粒子的电化学性能[(c)、(d)] [67]
Fig.11 Schematic illustration of the formation process of Co3O4/NiCo2O4 DSNCs (a); structural characterization of the Co3O4/NiCo2O4 DSNCs and Co3O4 NCs (b); electrochemical performance of Co3O4/NiCo2O4 DSNCs and Co3O4 NCs [(c), (d)] [67]
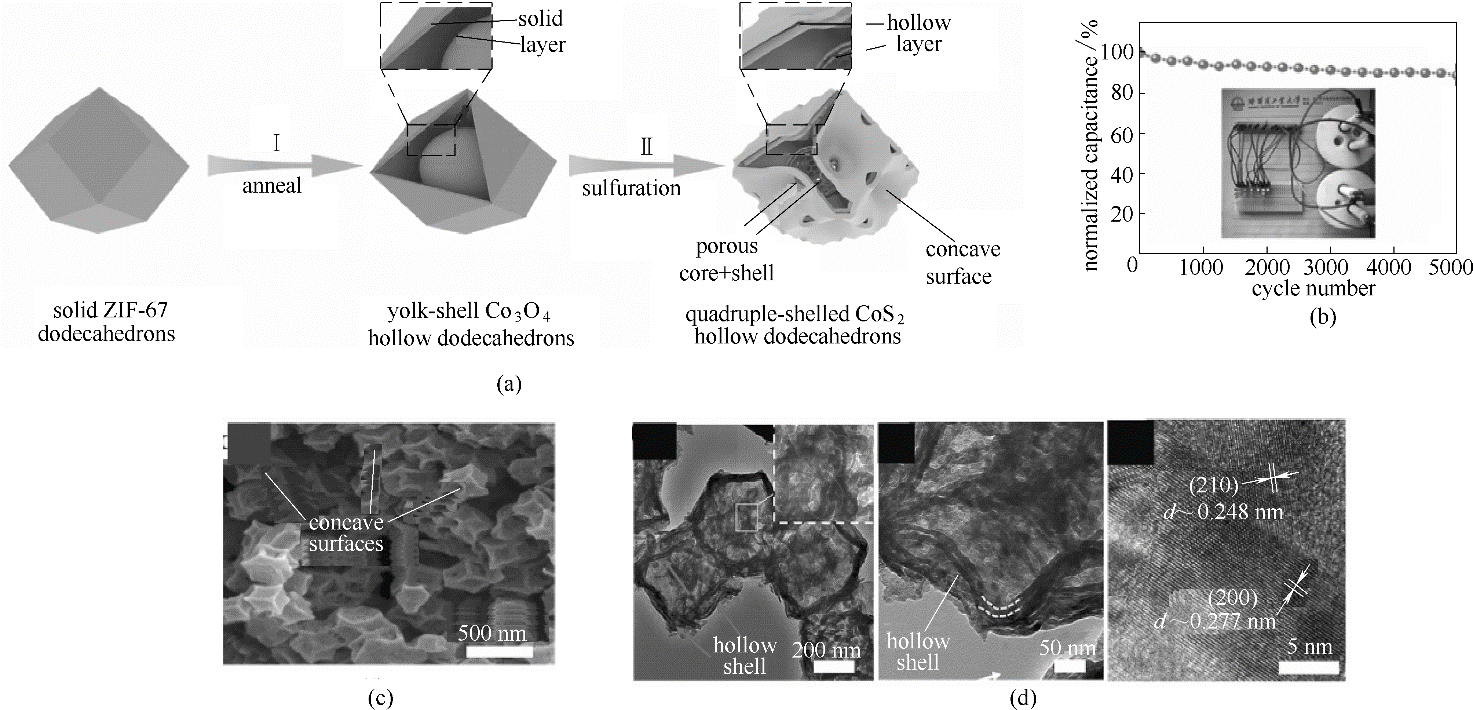
图12 四壳中空CoS2十二面体合成流程图(a); 四壳中空CoS2十二面体的电化学性能(b); 四壳中空CoS2十二面体的扫描电镜、透射电镜以及高分辨透射电镜图[(c)、(d)] [68]
Fig.12 Schematic illustration for the fabrication of quadruple-shelled CoS2 hollow dodecahedrons electrodes by stepwise synthesis approach (a); electrochemical performance of the quadruple-shelled CoS2 hollow dodecahedrons (b); SEM, TEM and HRTEM images of quadruple-shelled CoS2 hollow dodecahedrons [(c),(d)][68]
| 类别 | MOF前体 | 电解质 | 电流密度/(A·g-1) | 电化学性能/(F·g-1) | 参考文献 |
|---|---|---|---|---|---|
| 双金属MOF | Ni/Co-MOF | 3 mol·L-1 KOH | 1 | 1067 | [ |
| Co/Fe-MOF | 1 mol·L-1 LiOH | 1 | 319.5 | [ | |
| ZIF-CoZn | 3 mol·L-1 KCl | 1 | 340.7 | [ | |
| 多面体 | ZIF-8 | 2 mol·L-1 KOH | 0.5 | 187 | [ |
| Zn/Co-ZIF | 6 mol·L-1 KOH | 1 | 1266 | [ | |
| ZIF-67 | 6 mol·L-1 KOH | 1 | 2291.4 | [ | |
| Fe-Co-ZIF | 1 mol·L-1 KOH | 1 | 510 | [ | |
| ZIF-67 | 3 mol·L-1 KOH | 2.5 | 1288.3 | [ | |
| 中空结构 | MIL-101-NH2 | 1 mol·L-1 H2SO4 | 0.1 | 261 | [ |
| ZIF-67 | 3 mol·L-1 KOH | 5.55 F·cm-2 | 1110 | [ | |
| Co3O4/FeIII-MOF-5 | 6 mol·L-1 KOH | 1 | 326.7 | [ | |
| [CH3NH3][Ni(HCOO)3] | 2 mol·L-1 KOH | 1 | 874.5 | [ | |
| 核壳结构 | ZIF-8 | 1 mol·L-1 H2SO4 | 5 mV·s-1 | 1100 | [ |
| ZIF-67 | 1 mol·L-1 KOH | 5 | 972 | [ | |
| ZIF-67 | 2 mol·L-1 KOH | 1 | 375.2 | [ |
表1 MOFs和MOFs衍生的0维材料的电化学性能
Table 1 Electrochemical performance of MOFs and MOFs-derived zero-dimensional materials
| 类别 | MOF前体 | 电解质 | 电流密度/(A·g-1) | 电化学性能/(F·g-1) | 参考文献 |
|---|---|---|---|---|---|
| 双金属MOF | Ni/Co-MOF | 3 mol·L-1 KOH | 1 | 1067 | [ |
| Co/Fe-MOF | 1 mol·L-1 LiOH | 1 | 319.5 | [ | |
| ZIF-CoZn | 3 mol·L-1 KCl | 1 | 340.7 | [ | |
| 多面体 | ZIF-8 | 2 mol·L-1 KOH | 0.5 | 187 | [ |
| Zn/Co-ZIF | 6 mol·L-1 KOH | 1 | 1266 | [ | |
| ZIF-67 | 6 mol·L-1 KOH | 1 | 2291.4 | [ | |
| Fe-Co-ZIF | 1 mol·L-1 KOH | 1 | 510 | [ | |
| ZIF-67 | 3 mol·L-1 KOH | 2.5 | 1288.3 | [ | |
| 中空结构 | MIL-101-NH2 | 1 mol·L-1 H2SO4 | 0.1 | 261 | [ |
| ZIF-67 | 3 mol·L-1 KOH | 5.55 F·cm-2 | 1110 | [ | |
| Co3O4/FeIII-MOF-5 | 6 mol·L-1 KOH | 1 | 326.7 | [ | |
| [CH3NH3][Ni(HCOO)3] | 2 mol·L-1 KOH | 1 | 874.5 | [ | |
| 核壳结构 | ZIF-8 | 1 mol·L-1 H2SO4 | 5 mV·s-1 | 1100 | [ |
| ZIF-67 | 1 mol·L-1 KOH | 5 | 972 | [ | |
| ZIF-67 | 2 mol·L-1 KOH | 1 | 375.2 | [ |
| 1 | Deng J, Li M M, Wang Y. Biomass-derived carbon: synthesis and applications in energy storage and conversion[J]. Green Chemistry, 2016, 18(18): 4824-4854. |
| 2 | Yu D B, Wu B, Ge L, et al. Decorating nanoporous ZIF-67-derived NiCo2O4 shells on a Co3O4 nanowire array core for battery-type electrodes with enhanced energy storage performance[J]. Journal of Materials Chemistry A, 2016, 4(28): 10878-10884. |
| 3 | Guo N N, Li M, Sun X K, et al. Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitors in ionic liquids with high power and energy densities[J]. Green Chemistry, 2017, 19(11): 2595-2602. |
| 4 | Xia W, Mahmood A, Zou R Q, et al. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion[J]. Energy & Environmental Science, 2015, 8(7): 1837-1866. |
| 5 | Wang L, Han Y Z, Feng X, et al. Metal-organic frameworks for energy storage: batteries and supercapacitors[J]. Coordination Chemistry Reviews, 2016, 307(2): 361-381. |
| 6 | Liang H Y, Lin J H, Jia H N, et al. Hierarchical NiCo-LDH@NiOOH core-shell heterostructure on carbon fiber cloth as battery-like electrode for supercapacitor[J]. Journal of Power Sources, 2018, 378: 248-254. |
| 7 | Huang M, Zhang Y X, Li F, et al. Merging of Kirkendall growth and Ostwald ripening: CuO@MnO2 core-shell architectures for asymmetric supercapacitors[J]. Scientific Reports, 2014, 4: 4518. |
| 8 | Wang G P, Zhang L, Zhang J J. A review of electrode materials for electrochemical supercapacitors[J]. Chemical Society Reviews, 2012, 41(2): 797-828. |
| 9 | Ma W J, Chen S H, Yang S Y, et al. Hierarchical MnO2 nanowire/graphene hybrid fibers with excellent electrochemical performance for flexible solid-state supercapacitors[J]. Journal of Power Sources, 2016, 306: 481-488. |
| 10 | Wang D W, Fang G L, Xue T, et al. A melt route for the synthesis of activated carbon derived from carton box for high performance symmetric supercapacitor applications[J]. Journal of Power Sources, 2016, 307: 401-409. |
| 11 | Wu S L, Zhu Y W. Highly densified carbon electrode materials towards practical supercapacitor devices[J]. Science China Materials, 2017, 60(1): 25-38. |
| 12 | Gamby J, Taberna P L, Simon P, et al. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors[J]. Journal of Power Sources, 2001, 101(1): 109-116. |
| 13 | Portet C, Taberna P L, Simon P, et al. High power density electrodes for carbon supercapacitor applications[J]. Electrochimica Acta, 2005, 50(20): 4174-4181. |
| 14 | Hsieh T F, Chuang C C, Liu M Y, et al. A new way to manufacture a carbon nanotubes supercapacitor[C]//Advanced Materials Research. Trans. Tech. Publications Ltd., 2009, 79: 47-50. |
| 15 | Pan H, Li J Y, Feng Y P. Carbon nanotubes for supercapacitor[J]. Nanoscale Research Letters, 2010, 5(3): 654-668. |
| 16 | González A, Goikolea E, Barrena J A, et al. Review on supercapacitors: technologies and materials[J]. Renewable and Sustainable Energy Reviews, 2016, 58: 1189-1206. |
| 17 | Wang Y, Shi Z Q, Huang Y, et al. Supercapacitor devices based on graphene materials[J]. The Journal of Physical Chemistry C, 2009, 113(30): 13103-13107. |
| 18 | Huang Y, Liang J J, Chen Y S. An overview of the applications of graphen-based materials in supercapacitors[J]. Small, 2012, 8(12): 1805-1834. |
| 19 | Wang J G, Kang F Y, Wei B Q. Engineering of MnO2-based nanocomposites for high-performance supercapacitors[J]. Progress in Materials Science, 2015, 74: 51-124. |
| 20 | Liu M K, Du Y F, Miao Y E, et al. Anisotropic conductive films based on highly aligned polyimide fibers containing hybrid materials of graphene nanoribbons and carbon nanotubes[J]. Nanoscale, 2015, 7(3): 1037-1046. |
| 21 | Wang K, Zhang X, Sun X Z, et al. Conducting polymer hydrogel materials for high-performance flexible solid-state supercapacitors[J]. Science China Materials, 2016, 59(6): 412-420. |
| 22 | Huang C, Young N P, Grant P S. Spray processing of TiO2 nanoparticle/ionomer coatings on carbon nanotube scaffolds for solid-state supercapacitors[J]. Journal of Materials Chemistry A, 2014, 2(29): 11022-11028. |
| 23 | Zhang H B, Nai J W, Yu L, et al. Metal-organic-framework-based materials as platforms for renewable energy and environmental applications[J]. Joule, 2017, 1(1): 77-107. |
| 24 | Trickett C A, Helal A, Al-Maythalony B A, et al. The chemistry of metal-organic frameworks for CO2 capture, regeneration and conversion[J]. Nature Reviews Materials, 2017, 2(8): 1-16. |
| 25 | Meng W, Zeng Y F, Liang Z B, et al. Tuning expanded pores in metal-organic frameworks for selective capture and catalytic conversion of carbon dioxide[J]. ChemSusChem, 2018, 11(21): 3751-3757. |
| 26 | Wu C D, Zhao M. Incorporation of molecular catalysts in metal-organic frameworks for highly efficient heterogeneous catalysis[J]. Advanced Materials, 2017, 29(14): 1605446. |
| 27 | Liang J, Liang Z B, Zou R Q, et al. Heterogeneous catalysis in zeolites, mesoporous silica, and metal-organic frameworks[J]. Advanced Materials, 2017, 29(30): 1701139. |
| 28 | Yuan H Y, Aljneibi S A A A, Yuan J R, et al. Biosensors: ZnO nanosheets abundant in oxygen vacancies derived from metal-organic frameworks for ppb-level gas sensing [J]. Advanced Materials, 2019, 31(11): 1970076. |
| 29 | Wang M J, Shen Z R, Zhao X D, et al. Rational shape control of porous Co3O4 assemblies derived from MOF and their structural effects on n-butanol sensing[J]. Journal of Hazardous Materials, 2019, 371: 352-361. |
| 30 | Wu M X, Yang Y W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy[J]. Advanced Materials, 2017, 29(23): 1606134. |
| 31 | Zhao S F, Zeng L Z, Cheng G, et al. Ni/Co-based metal-organic frameworks as electrode material for high performance supercapacitors[J]. Chinese Chemical Letters, 2019, 30(3): 605-609. |
| 32 | Yu H N, Xia H C, Zhang J N, et al. Fabrication of Fe-doped Co-MOF with mesoporous structure for the optimization of supercapacitor performances[J]. Chinese Chemical Letters, 2018, 29(6): 834-836. |
| 33 | Dang S, Zhu Q L, Xu Q. Nanomaterials derived from metal-organic frameworks[J]. Nature Reviews Materials, 2018, 3(1): 17075. |
| 34 | Liang Z B, Zhao R, Qiu T J, et al. Metal-organic framework-derived materials for electrochemical energy applications[J]. Energy Chem., 2019, 1(1): 100001. |
| 35 | Zhu C M, He Y, Liu Y J, et al. ZnO@MOF@PANI core-shell nanoarrays on carbon cloth for high-performance supercapacitor electrodes[J]. Journal of Energy Chemistry, 2019, 35: 124-131. |
| 36 | Liu B, Shioyama H, Akita T, et al. Metal-organic framework as a template for porous carbon synthesis[J]. Journal of the American Chemical Society, 2008, 130(16): 5390-5391. |
| 37 | Hu H, Guan B Y, Xia B Y, et al. Designed formation of Co3O4/NiCo2O4 double-shelled nanocages with enhanced pseudocapacitive and electrocatalytic properties[J]. Journal of the American Chemical Society, 2015, 137(16): 5590-5595. |
| 38 | Pachfule P, Shinde D, Majumder M, et al. Fabrication of carbon nanorods and graphene nanoribbons from a metal-organic framework[J]. Nature Chemistry, 2016, 8(7): 718-724. |
| 39 | Cao X H, Tan C L, Sindoro M, et al. Hybrid micro-/nano-structures derived from metal-organic frameworks: preparation and applications in energy storage and conversion[J]. Chemical Society Reviews, 2017, 46(10): 2660-2677. |
| 40 | Liang Z B, Qu C, Guo W H, et al. Pristine metal-organic frameworks and their composites for energy storage and conversion[J]. Advanced Materials, 2018, 30(37): 1702891. |
| 41 | Wu H B, Xia B Y, Yu L, et al. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production[J]. Nature Communications, 2015, 6(1): 1-8. |
| 42 | Chen Y Z, Wang C M, Wu Z Y, et al. From bimetallic metal-organic framework to porous carbon: high surface area and multicomponent active dopants for excellent electrocatalysis[J]. Advanced Materials, 2015, 27(34): 5010-5016. |
| 43 | Zhang J T, Li Z, Chen Y, et al. Nickel-iron layered double hydroxide hollow polyhedrons as a superior sulfur host for lithium-sulfur batteries[J]. Angewandte Chemie, 2018, 57(34): 10944-10948. |
| 44 | Gu H C, Kong L J, Cui H J, et al. Fabricating high-performance sodium ion capacitors with P2-Na0.67Co0.5Mn0.5O2 and MOF-derived carbon[J]. Journal of Energy Chemistry, 2018, 28: 79-84. |
| 45 | You B, Jiang N, Sheng M L, et al. High-performance overall water splitting electrocatalysts derived from cobalt-based metal-organic frameworks[J]. Chemistry of Materials, 2015, 27(22): 7636-7642. |
| 46 | Wu J, Zhang X P, Wei F X, et al. Controllable synthesis of ZIF-derived nano-hexahedron porous carbon for supercapacitor electrodes[J]. Materials Letters, 2020, 258: 126761. |
| 47 | Zhang P, Guan B Y, Yu L, et al. Formation of double-shelled zinc-cobalt sulfide dodecahedral cages from bimetallic zeolitic imidazolate frameworks for hybrid supercapacitors[J]. Angewandte Chemie International Edition, 2017, 56(25): 7141-7145. |
| 48 | Gao R S, Zhang Q G, Soyekwo F, et al. Novel amorphous nickel sulfide@CoS double-shelled polyhedral nanocages for supercapacitor electrode materials with superior electrochemical properties[J]. Electrochimica Acta, 2017, 237: 94-101. |
| 49 | Liu X, Wei F X, Sui Y W, et al. Polyhedral ternary oxide FeCo2O4: a new electrode material for supercapacitors[J]. Journal of Alloys and Compounds, 2018, 735: 1339-1343. |
| 50 | Wei C Z, Liu K F, Tao J, et al. Self-template synthesis of hybrid porous Co3O4-CeO2 hollow polyhedrons for high-performance supercapacitors[J]. Chemistry-An Asian Journal, 2018, 13(1): 111-117. |
| 51 | Shang L, Yu H J, Huang X, et al. Well-dispersed ZIF-derived Co, N-Co-doped carbon nanoframes through mesoporous-silica-protected calcination as efficient oxygen reduction electrocatalysts[J]. Advanced Materials, 2016, 28(8): 1668-1674. |
| 52 | Li X, Sun Q, Liu J, et al. Tunable porous structure of metal organic framework derived carbon and the application in lithium–sulfur batteries[J]. Journal of Power Sources, 2016, 302: 174-179. |
| 53 | Yu L, Wu H B, Lou X W D. Self-templated formation of hollow structures for electrochemical energy applications[J]. Accounts of Chemical Research, 2017, 50(2): 293-301. |
| 54 | Tang Y F, Chen S J, Mu S C, et al. Synthesis of capsule-like porous hollow nanonickel cobalt sulfides via cation exchange based on the Kirkendall effect for high-performance supercapacitors[J]. ACS Applied Materials & Interfaces, 2016, 8(15): 9721-9732. |
| 55 | Zhu Q L, Pachfule P, Strubel P, et al. Fabrication of nitrogen and sulfur co-doped hollow cellular carbon nanocapsules as efficient electrode materials for energy storage[J]. Energy Storage Materials, 2018, 13: 72-79. |
| 56 | Zhang Y Z, Wang Y, Xie Y L, et al. Porous hollow Co3O4 with rhombic dodecahedral structures for high-performance supercapacitors[J]. Nanoscale, 2014, 6(23): 14354-14359. |
| 57 | Hu X W, Liu S, Qu B T, et al. Starfish-shaped Co3O4/ZnFe2O4 hollow nanocomposite: synthesis, supercapacity, and magnetic properties[J]. ACS Applied Materials & Interfaces, 2015, 7(18): 9972-9981. |
| 58 | Rui X H, Tan H T, Yan Q Y. Nanostructured metal sulfides for energy storage[J]. Nanoscale, 2014, 6(17): 9889-9924. |
| 59 | Wang T S, Hu P, Zhang C J, et al. Nickel disulfide-graphene nanosheets composites with improved electrochemical performance for sodium ion battery[J]. ACS Applied Materials & Interfaces, 2016, 8(12): 7811-7817. |
| 60 | Li W J, Han C, Chou S L, et al. Graphite-nanoplate-coated Bi2S3 composite with high-volume energy density and excellent cycle life for room-temperature sodium-sulfide batteries[J]. Chemistry-A European Journal, 2016, 22(2): 590-597. |
| 61 | Yang W L, Wang H, Liu T T, et al. A Bi2S3@CNT nanocomposite as anode material for sodium ion batteries[J]. Materials Letters, 2016, 167: 102-105. |
| 62 | Choi J H, Ha C W, Choi H Y, et al. Sb2S3 embedded in amorphous P/C composite matrix as high-performance anode material for sodium ion batteries[J]. Electrochimica Acta, 2016, 210: 588-595. |
| 63 | He P L, Yu X Y, Lou X W. Carbon-incorporated nickel-cobalt mixed metal phosphide nanoboxes with enhanced electrocatalytic activity for oxygen evolution[J]. Angewandte Chemie International Edition, 2017, 56(14): 3897-3900. |
| 64 | Ma X, Zhang L, Xu G H, et al. Facile synthesis of NiS hierarchical hollow cubes via Ni formate frameworks for high performance supercapacitors[J]. Chemical Engineering Journal, 2017, 320: 22-28. |
| 65 | Nai J W, Tian Y, Guan X, et al. Pearson s principle inspired generalized strategy for the fabrication of metal hydroxide and oxide nanocages[J]. Journal of the American Chemical Society, 2013, 135(43): 16082-16091. |
| 66 | Salunkhe R R, Tang J, Kobayashi N, et al. Ultrahigh performance supercapacitors utilizing core-shell nanoarchitectures from a metal-organic framework-derived nanoporous carbon and a conducting polymer[J]. Chemical Science, 2016, 7(9): 5704-5713. |
| 67 | Hu H, Guan B Y, Xia B Y, et al. Designed formation of Co3O4/NiCo2O4 double-shelled nanocages with enhanced pseudocapacitive and electrocatalytic properties[J]. Journal of the American Chemical Society, 2015, 137(16): 5590-5595. |
| 68 | Jia H N, Wang Z Y, Zheng X H, et al. Controlled synthesis of MOF-derived quadruple-shelled CoS2 hollow dodecahedrons as enhanced electrodes for supercapacitors[J]. Electrochimica Acta, 2019, 312: 54-61. |
| [1] | 李靖, 沈聪浩, 郭大亮, 李静, 沙力争, 童欣. 木质素基碳纤维复合材料在储能元件中的应用研究进展[J]. 化工学报, 2023, 74(6): 2322-2334. |
| [2] | 徐东, 田杜, 陈龙, 张禹, 尤庆亮, 胡成龙, 陈韶云, 陈建. 聚苯胺/二氧化锰/聚吡咯复合纳米球的制备及其电化学储能性[J]. 化工学报, 2023, 74(3): 1379-1389. |
| [3] | 陈健鑫, 朱瑞杰, 盛楠, 朱春宇, 饶中浩. 纤维素基生物质多孔炭的制备及其超级电容器性能研究[J]. 化工学报, 2022, 73(9): 4194-4206. |
| [4] | 顾仁杰, 张加威, 靳雪阳, 文利雄. 微撞击流反应器制备镍钴复合氢氧化物超级电容器材料及其性能研究[J]. 化工学报, 2022, 73(8): 3749-3757. |
| [5] | 刘学安, 汤丽怡, 覃健, 唐大江, 童张法, 曲慧颖. 热解Ni/Co-ZIF-8制备碳纳米管桥连多孔碳及其在超级电容器中的应用[J]. 化工学报, 2022, 73(7): 3287-3297. |
| [6] | 焦帅, 杨磊, 武婷婷, 李宏强, 吕辉鸿, 何孝军. 混合盐模板法制备超级电容器用氮掺杂分级多孔碳纳米片[J]. 化工学报, 2021, 72(5): 2869-2877. |
| [7] | 叶珍珍, 陈鑫祺, 汪剑, 李博凡, 崔超婕, 张刚, 钱陆明, 金鹰, 骞伟中. 离子液体型超级电容器软包高温老化性能评测研究[J]. 化工学报, 2021, 72(12): 6351-6360. |
| [8] | 徐晓倩, 程俊霞, 朱亚明, 高丽娟, 赖仕全, 赵雪飞. 针状焦基电容器碳质电极材料的制备及电化学性能研究[J]. 化工学报, 2020, 71(6): 2830-2839. |
| [9] | 杨乐, 余金河, 付蓉, 谢远洋, 于畅, 邱介山. 超级电容器用solvent-in-salt型电解液的研究进展[J]. 化工学报, 2020, 71(6): 2457-2465. |
| [10] | 周锋, 田利军, 高磊, 吴忠帅. 电化学阴极剥离制备少层石墨烯及其微型超级电容器[J]. 化工学报, 2020, 71(6): 2724-2734. |
| [11] | 王晓波,赵青山,程智年,张浩然,胡涵,王路海,吴明铂. 高性能碳基储能材料的设计、合成与应用[J]. 化工学报, 2020, 71(6): 2660-2677. |
| [12] | 赵少飞, 刘鹏, 李婉萍, 曾小红, 钟远红, 余林, 曾华强. 一步电沉积法制备硫化镍/泡沫镍材料及其赝电容性能研究[J]. 化工学报, 2020, 71(4): 1836-1843. |
| [13] | 李鑫健,王保禄,高天,王旗,王学斌. 三维筋撑石墨烯负载氧化锰的超级电容器[J]. 化工学报, 2020, 71(11): 5025-5034. |
| [14] | 赵杰,郭月,沈桢,杨立军,吴强,王喜章,胡征. 高倍率容量层状双金属氢氧化物超级电容材料的研究进展[J]. 化工学报, 2020, 71(11): 4851-4872. |
| [15] | 胡兵兵, 杨束, 李彦, 徐川岚, 陈鹏, 于晶晶, 余丹梅, 陈昌国. 免黏结剂V2O5和Fe2O3柔性电极的构建及在超级电容器中的应用[J]. 化工学报, 2020, 71(10): 4836-4846. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号